Focality of the Induced E-Field Is a Contributing Factor in the Choice of TMS Parameters: Evidence from a 3D Computational Model of the Human Brain
Abstract
1. Introduction
2. Methods
2.1. E-Field Modeling
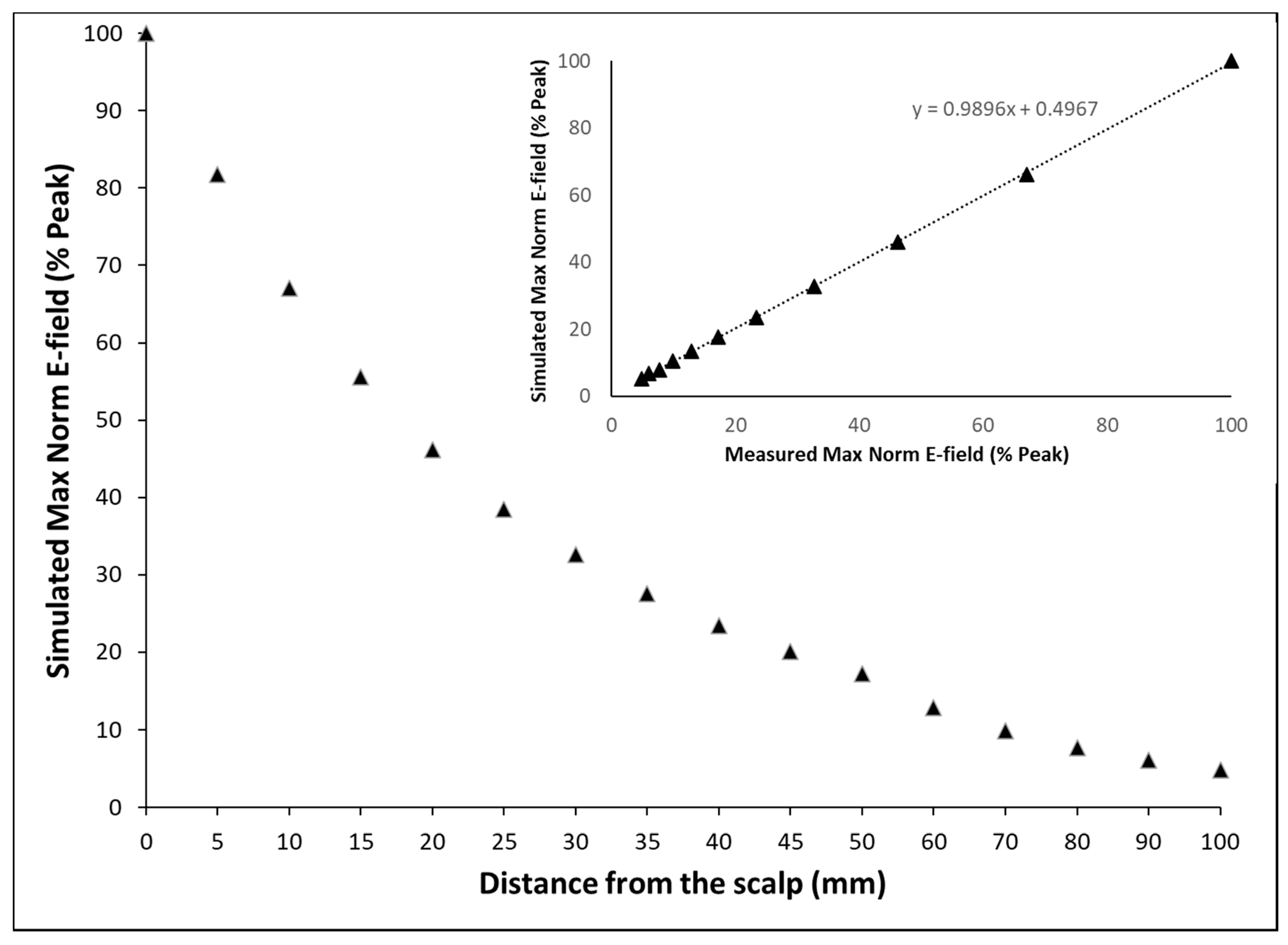
2.2. Model Verification
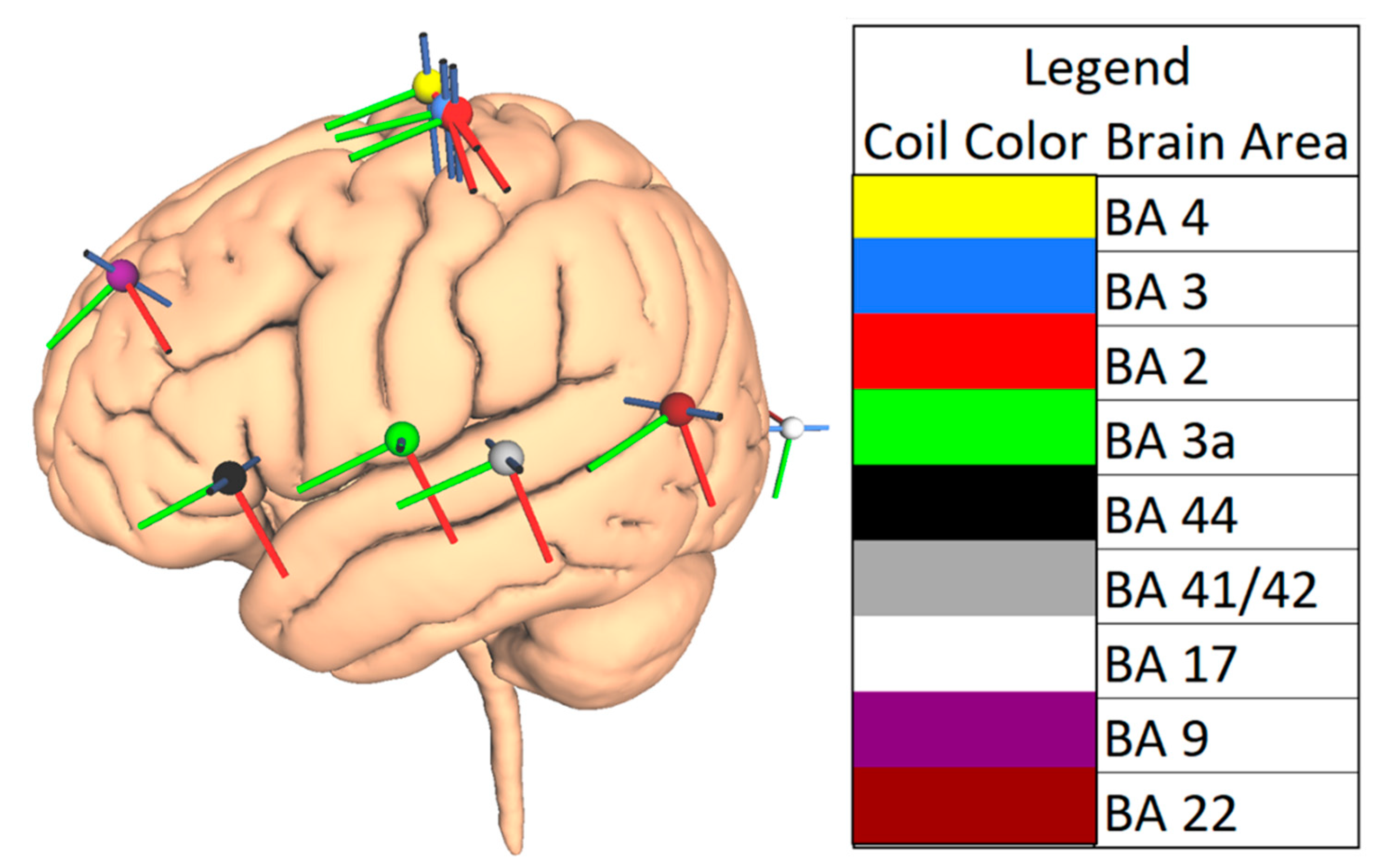
2.3. Choice of Brain Areas
2.4. TMS Parameters
3. Results
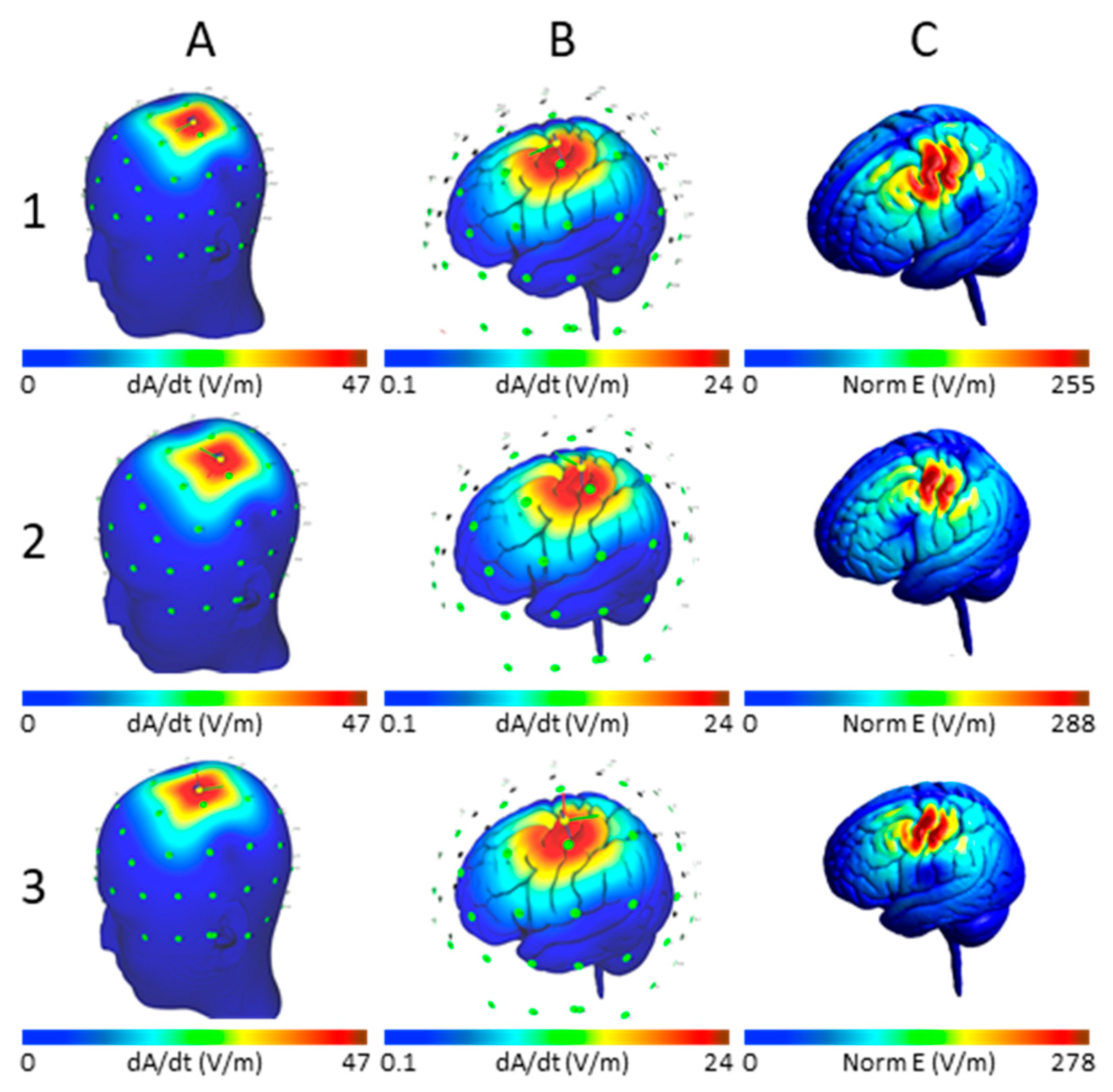
3.1. Maximum E-Field at Different Cortical Locations
3.2. Volume of Cortex Activated at Different Cortical Locations
3.3. Effect of Varying TMS Intensity on the Maximum E-Field and Its Distribution
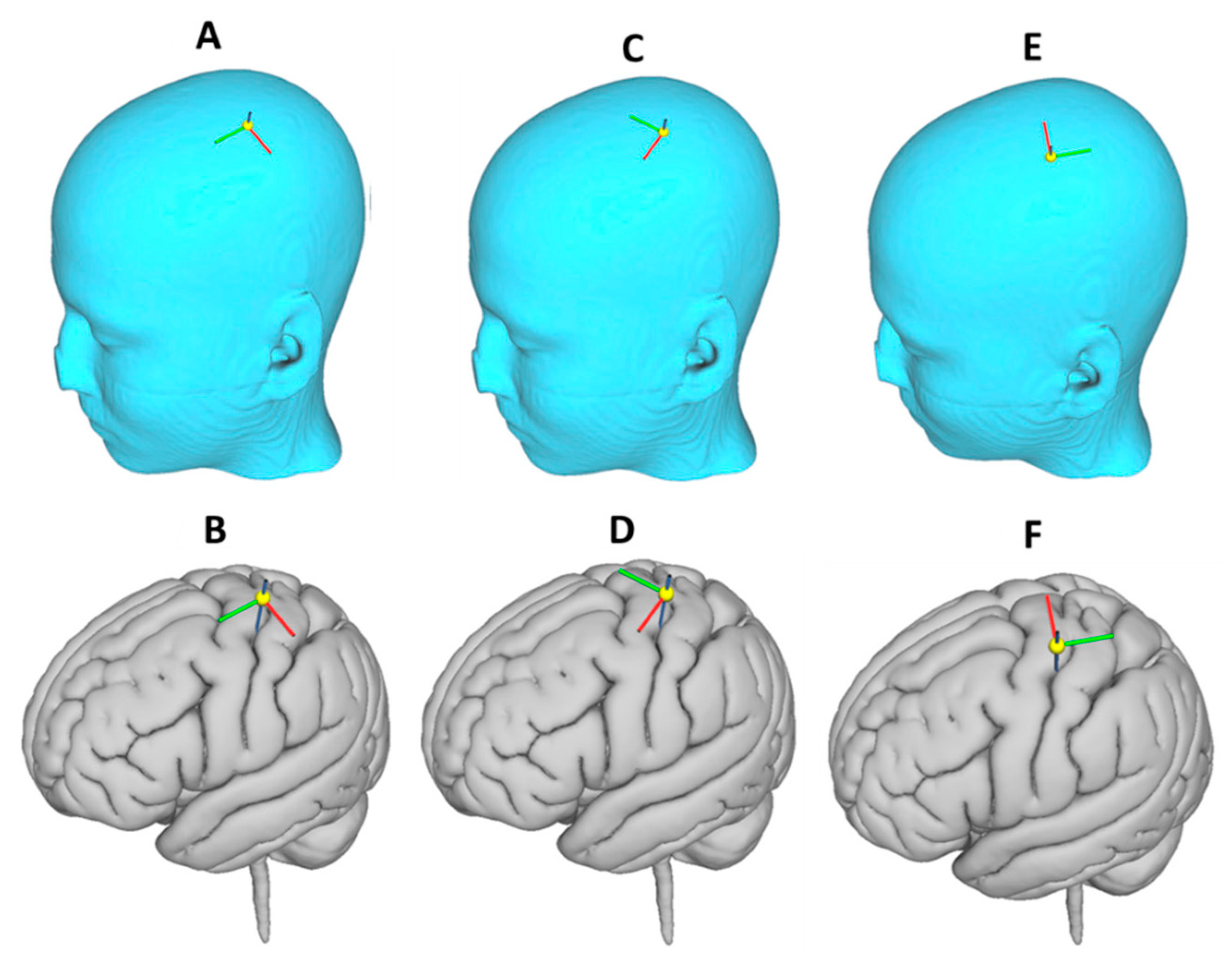
3.4. Effect of Varying TMS Coil Orientation on the Maximum E-Field and Its Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BA | Brodmann area |
| CSF | Cerebrospinal fluid |
| E-field | Electrical field |
| EEG | Electroencephalogram |
| FCz | Electrode position in the 10-10 EEG system at the vertex |
| FDA | US Food and Drug Administration |
| FEM | Finite element method |
| GM | Gray matter |
| LH | Left hemisphere |
| MNI | Montreal Neurological Institute |
| Nz | Electrode position in the 10-10 EEG system at the nose |
| Oz | Electrode position in the 10-10 EEG system at the occipital pole |
| RH | Right hemisphere |
| SimNIBS | Simulation for non-invasive brain stimulation |
| TMS | Transcranial Magnetic Stimulation |
| WM | White matter |
References
- Ilmoniemi, R.J.; Ruohonen, J.; Karhu, J. Transcranial magnetic stimulation—A new tool for functional imaging of the brain. Crit. Rev. Biomed. Eng. 1999, 27, 241–284. [Google Scholar] [PubMed]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.; Papanicolaou, A.C.; McGregor, A.; Boop, F.A.; Wheless, J.W. Clinical Applications of Transcranial Magnetic Stimulation in Pediatric Neurology. J. Child Neurol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, E.; Bestmann, S. On tickling brains to investigate minds. Cortex A J. Devoted Study Nerv. Syst. Behav. 2009, 45, 1021–1024. [Google Scholar] [CrossRef]
- Sparing, R.; Hesse, M.D.; Fink, G.R. Neuronavigation for transcranial magnetic stimulation (TMS): Where we are and where we are going. Cortex J. Devoted Study Nerv. Syst. Behav. 2010, 46, 118–120. [Google Scholar] [CrossRef]
- Salinas, F.S.; Lancaster, J.L.; Fox, P.T. 3D modeling of the total electric field induced by transcranial magnetic stimulation using the boundary element method. Phys. Med. Biol. 2009, 54, 3631–3647. [Google Scholar] [CrossRef]
- Madsen, K.H.; Ewald, L.; Siebner, H.R.; Thielscher, A. Transcranial Magnetic Stimulation: An Automated Procedure to Obtain Coil-specific Models for Field Calculations. Brain Stimul. 2015, 8, 1205–1208. [Google Scholar] [CrossRef]
- Aberra, A.S.; Wang, B.; Grill, W.M.; Peterchev, A.V. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. 2020, 13, 175–189. [Google Scholar] [CrossRef]
- Chen, M.; Mogul, D.J. A structurally detailed finite element human head model for simulation of transcranial magnetic stimulation. J. Neurosci. Methods 2009, 179, 111–120. [Google Scholar] [CrossRef]
- Salinas, F.S.; Lancaster, J.L.; Fox, P.T. Detailed 3D models of the induced electric field of transcranial magnetic stimulation coils. Phys. Med. Biol. 2007, 52, 2879–2892. [Google Scholar] [CrossRef]
- Krieg, T.D.; Salinas, F.S.; Narayana, S.; Fox, P.T.; Mogul, D.J. Computational and experimental analysis of TMS-induced electric field vectors critical to neuronal activation. J. Neural Eng. 2015, 12, 046014. [Google Scholar] [CrossRef] [PubMed]
- Laakso, I.; Murakami, T.; Hirata, A.; Ugawa, Y. Where and what TMS activates: Experiments and modeling. Brain Stimul. 2018, 11, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.-D.; Lisanby, S.H.; Peterchev, A.V. Electric field depth-focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimul. 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.R.; Boniface, S.J.; Schubert, M. Magnetic brain stimulation with a double coil: The importance of coil orientation. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 17–21. [Google Scholar] [CrossRef]
- Brasil-Neto, J.P.; McShane, L.M.; Fuhr, P.; Hallett, M.; Cohen, L.G. Topographic mapping of the human motor cortex with magnetic stimulation: Factors affecting accuracy and reproducibility. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 9–16. [Google Scholar] [CrossRef]
- Janssen, A.M.; Oostendorp, T.F.; Stegeman, D.F. The coil orientation dependency of the electric field induced by TMS for M1 and other brain areas. J. Neuroeng. Rehabil. 2015, 12, 47. [Google Scholar] [CrossRef]
- Gomez, L.J.; Dannhauer, M.; Koponen, L.M.; Peterchev, A.V. Conditions for numerically accurate TMS electric field simulation. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2020, 13, 157–166. [Google Scholar] [CrossRef]
- Gomez-Tames, J.; Hamasaka, A.; Laakso, I.; Hirata, A.; Ugawa, Y. Atlas of optimal coil orientation and position for TMS: A computational study. Brain Stimul. 2018, 11, 839–848. [Google Scholar] [CrossRef]
- Day, B.L.; Dressler, D.; De Noordhout, A.M.; Marsden, C.D.; Nakashima, K.; Rothwell, J.C.; Thompson, P.D. Electric and magnetic stimulation of human motor cortex: Surface EMG and single motor unit responses. J. Physiol. 1989, 412, 449–473. [Google Scholar] [CrossRef]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet Lond. Engl. 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Fox, P.T.; Narayana, S.; Tandon, N.; Sandoval, H.; Fox, S.P.; Kochunov, P.; Lancaster, J.L. Column-based model of electric field excitation of cerebral cortex. Hum. Brain Mapp. 2004, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.G.; Rastogi, P.; Hadimani, R.L.; Jiles, D.C.; Camprodon, J.A. Impact of non-brain anatomy and coil orientation on inter- and intra-subject variability in TMS at midline. Clin. Neurophysiol. 2018, 129, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.J.; Dannhauer, M.; Peterchev, A.V. Fast computational optimization of TMS coil placement for individualized electric field targeting. Bioengineering 2020. preprint. [Google Scholar] [CrossRef]
- Weise, K.; Numssen, O.; Thielscher, A.; Hartwigsen, G.; Knösche, T.R. A novel approach to localize cortical TMS effects. NeuroImage 2020, 209, 116486. [Google Scholar] [CrossRef]
- Thielscher, A.; Antunes, A.; Saturnino, G.B. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 222–225. [Google Scholar] [CrossRef]
- Nielsen, J.D.; Madsen, K.H.; Puonti, O.; Siebner, H.R.; Bauer, C.; Madsen, C.G.; Saturnino, G.B.; Thielscher, A. Automatic skull segmentation from MR images for realistic volume conductor models of the head: Assessment of the state-of-the-art. NeuroImage 2018, 174, 587–598. [Google Scholar] [CrossRef]
- Windhoff, M.; Opitz, A.; Thielscher, A. Electric field calculations in brain stimulation based on finite elements: An optimized processing pipeline for the generation and usage of accurate individual head models. Hum. Brain Mapp. 2013, 34, 923–935. [Google Scholar] [CrossRef]
- Wagner, T.A.; Zahn, M.; Grodzinsky, A.J.; Pascual-Leone, A. Three-dimensional head model Simulation of transcranial magnetic stimulation. IEEE Trans. Biomed. Eng. 2004, 51, 1586–1598. [Google Scholar] [CrossRef]
- Thielscher, A.; Kammer, T. Linking Physics with Physiology in TMS: A Sphere Field Model to Determine the Cortical Stimulation Site in TMS. NeuroImage 2002, 17, 1117–1130. [Google Scholar] [CrossRef]
- Thielscher, A.; Kammer, T. Electric field properties of two commercial figure-8 coils in TMS: Calculation of focality and efficiency. Clin. Neurophysiol. 2004, 115, 1697–1708. [Google Scholar] [CrossRef]
- Wang, W.; Eisenberg, S.R. A three-dimensional finite element method for computing magnetically induced currents in tissues. IEEE Trans. Magn. 1994, 30, 5015–5023. [Google Scholar] [CrossRef]
- Saturnino, G.B.; Puonti, O.; Nielsen, J.D.; Antonenko, D.; Madsen, K.H.; Thielscher, A. SimNIBS 2.1: A comprehensive pipeline for individualized electric field modelling for transcranial brain stimulation. In Brain and Human Body Modeling: Computational Human Modeling at EMBC 2018; Makarov, S., Horner, M., Noetscher, G., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.B.; Loo, C.; et al. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Lioumis, P.; Mäkelä, J.P.; Wilenius, J.; Karhu, J.; Hannula, H.; Savolainen, P.; Lucas, C.W.; Seidel, K.; Laakso, A.; et al. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir. 2017, 159, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Wassermann, E.M.; Williams, W.A.; Callahan, A.; Ketter, T.A.; Basser, P.; Hallett, M.; Post, R.M. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995, 6, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Nuwer, M.R. 10-10 electrode system for EEG recording. Clin. Neurophysiol. 2018, 129, 1103. [Google Scholar] [CrossRef] [PubMed]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Whitsel, B.L.; Vierck, C.J.; Waters, R.S.; Tommerdahl, M.; Favorov, O.V. Contributions of Nociresponsive Area 3a to Normal and Abnormal Somatosensory Perception. J. Pain Off. J. Am. Pain Soc. 2019, 20, 405–419. [Google Scholar] [CrossRef]
- Panchuelo, R.M.S.; Eldeghaidy, S.; Marshall, A.; McGlone, F.; Francis, S.T.; Favorov, O. A nociresponsive specific area of human somatosensory cortex within BA3a: BA3c? NeuroImage 2020, 221, 117187. [Google Scholar] [CrossRef]
- Rotem, A.; Moses, E. Magnetic stimulation of curved nerves. IEEE Trans. Biomed. Eng. 2006, 53, 414–420. [Google Scholar] [CrossRef]
- Roth, B.J.; Basser, P.J. A model of the stimulation of a nerve fiber by electromagnetic induction. IEEE Trans. Biomed. Eng. 1990, 37, 588–597. [Google Scholar] [CrossRef]
- Rábago, C.A.; Lancaster, J.L.; Narayana, S.; Zhang, W.; Fox, P.T. Automated-parameterization of the motor evoked potential and cortical silent period induced by transcranial magnetic stimulation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2009, 120, 1577–1587. [Google Scholar] [CrossRef][Green Version]
- Krieg, T.D.; Salinas, F.S.; Narayana, S.; Fox, P.T.; Mogul, D.J. PET-based confirmation of orientation sensitivity of TMS-induced cortical activation in humans. Brain Stimul. 2013, 6, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Raffin, E.; Pellegrino, G.; di Lazzaro, V.; Thielscher, A.; Siebner, H.R. Bringing transcranial mapping into shape: Sulcus-aligned mapping captures motor somatotopy in human primary motor hand area. NeuroImage 2015, 120, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Ille, S.; Obermueller, T.; Negwer, C.; Ringel, F.; Meyer, B.; Krieg, S.M. The impact of repetitive navigated transcranial magnetic stimulation coil positioning and stimulation parameters on human language function. Eur. J. Med. Res. 2015, 20. [Google Scholar] [CrossRef]
- Sollmann, N.; Fuss-Ruppenthal, S.; Zimmer, C.; Meyer, B.; Krieg, S.M. Investigating Stimulation Protocols for Language Mapping by Repetitive Navigated Transcranial Magnetic Stimulation. Front. Behav. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Thielscher, A.; Opitz, A.; Windhoff, M. Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. NeuroImage 2011, 54, 234–243. [Google Scholar] [CrossRef]
- Karabanov, A.; Thielscher, A.; Siebner, H.R. Transcranial brain stimulation: Closing the loop between brain and stimulation. Curr. Opin. Neurol. 2016, 29, 397–404. [Google Scholar] [CrossRef]
- Mutz, J.; Edgcumbe, D.R.; Brunoni, A.R.; Fu, C.H.Y. Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: A systematic review and meta-analysis of randomised sham-controlled trials. Neurosci. Biobehav. Rev. 2018, 92, 291–303. [Google Scholar] [CrossRef]
- Puonti, O.; van Leemput, K.; Saturnino, G.B.; Siebner, H.R.; Madsen, K.H.; Thielscher, A. Accurate and robust whole-head segmentation from magnetic resonance images for individualized head modeling. NeuroImage 2020, 219, 117044. [Google Scholar] [CrossRef]


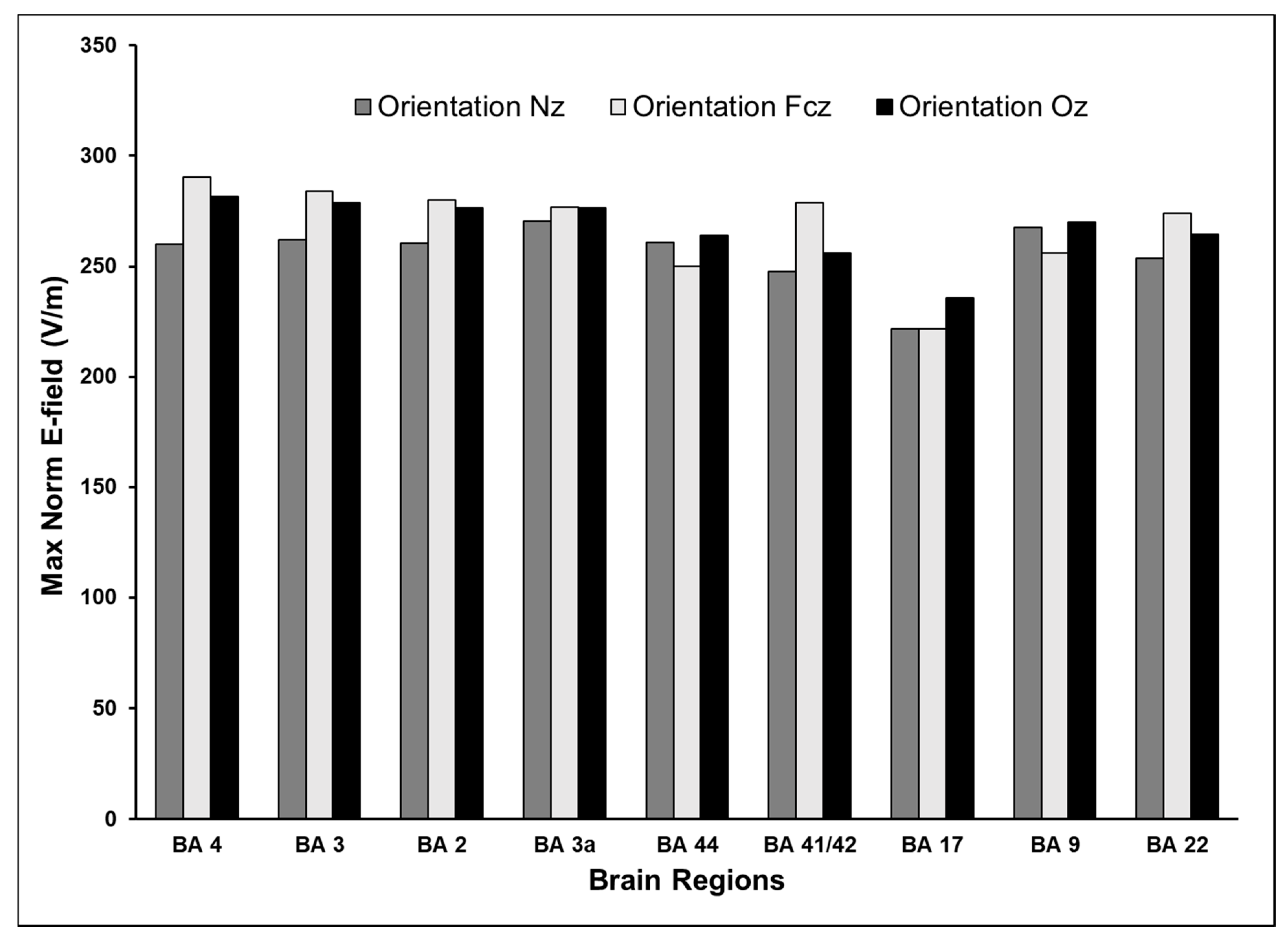
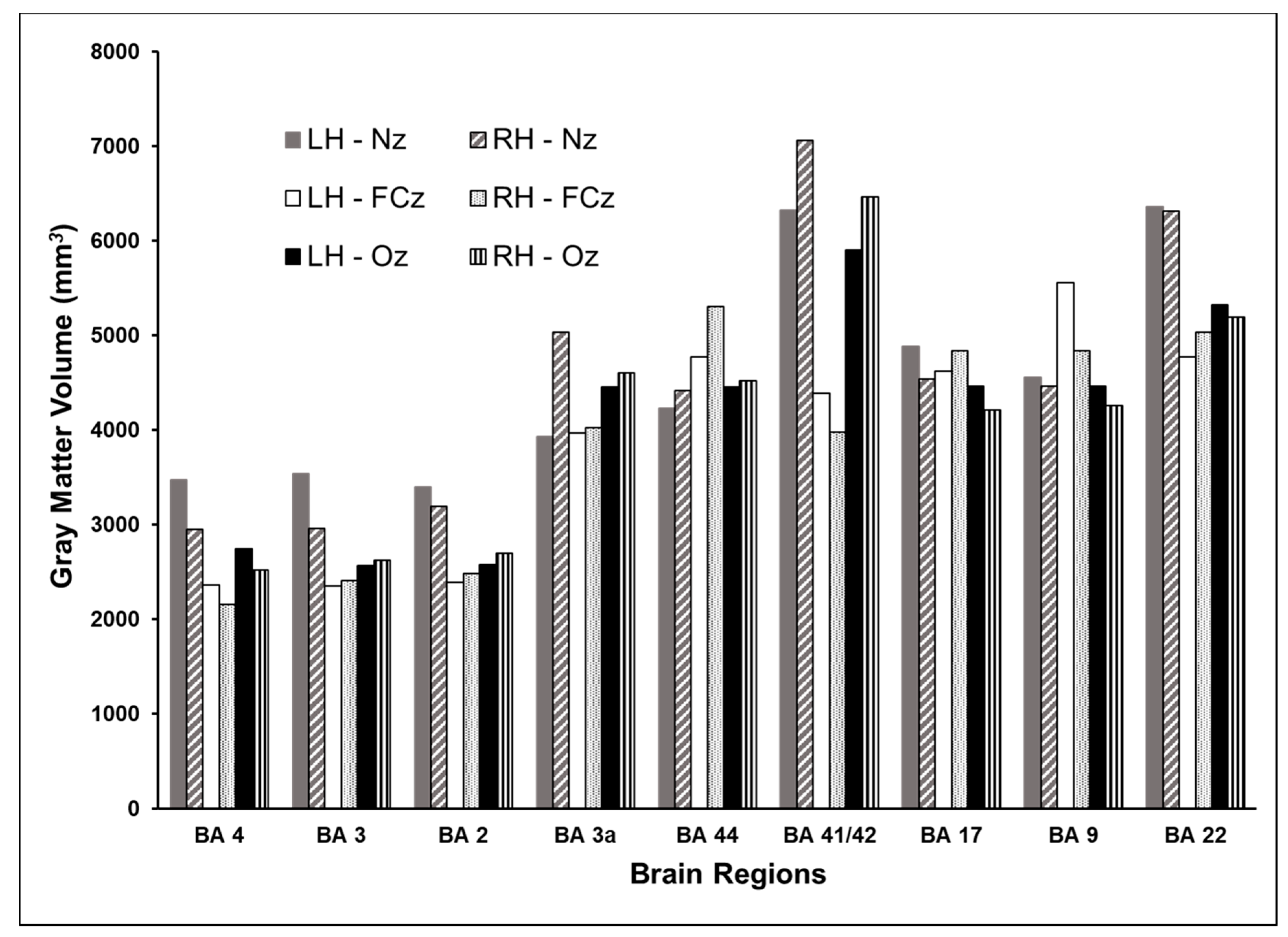
| Hemisphere | Brain Region | Brodmann Area | Coordinates (x,y,z) | dI/dt (A/µs) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 100 | 150 | 200 | 100 | 150 | 200 | ||||
| Max Norm E (V/m) | |||||||||
| Reference: Nz | Reference: Fcz | ||||||||
| Left | Primary motor cortex | BA 4 | (−38,−18.5,54) | 170 | 255 | 340 | 192 | 288 | 384 |
| Left | Primary somatosensory cortex | BA 3 | (−46,−18.5,54) | 174 | 261 | 348 | 187 | 280 | 374 |
| Left | Primary somatosensory cortex | BA 2 | (−48,−20,54) | 174 | 261 | 349 | 186 | 279 | 372 |
| Left | Primary somatosensory cortex | BA 3a | (−64,−6,14) | 182 | 273 | 364 | 184 | 276 | 368 |
| Left | Inferior frontal gyrus | BA 44 | (−46,16,6) | 173 | 259 | 346 | 165 | 248 | 331 |
| Left | Primary auditory cortex | BA 41/42 | (−60,−22,10) | 164 | 246 | 328 | 182 | 274 | 365 |
| Left | Primary visual cortex | BA 17 | (−8,−96,0) | 146 | 219 | 292 | 148 | 221 | 295 |
| Left | Dorsolateral prefrontal cortex | BA 9 | (−42,36,34) | 180 | 269 | 359 | 169 | 254 | 338 |
| Left | Middle temporal gyrus | BA 22 | (−60,−46,18) | 166 | 249 | 332 | 182 | 273 | 364 |
| Right | Primary motor cortex | BA 4 | (38,−18.5,54) | 177 | 265 | 353 | 195 | 293 | 390 |
| Right | Primary somatosensory cortex | BA 3 | (46,−18.5,54) | 175 | 263 | 351 | 192 | 288 | 384 |
| Right | Primary somatosensory cortex | BA 2 | (48,−20,54) | 173 | 260 | 346 | 187 | 281 | 374 |
| Right | Primary somatosensory cortex | BA 3a | (64,−6,14) | 179 | 268 | 357 | 185 | 278 | 371 |
| Right | Inferior frontal gyrus | BA 44 | (46,16,6) | 176 | 263 | 351 | 168 | 252 | 337 |
| Right | Primary auditory cortex | BA 41/42 | (60,−18,6) | 166 | 249 | 332 | 189 | 284 | 378 |
| Right | Primary visual cortex | BA 17 | (8,−96,0) | 149 | 224 | 298 | 148 | 222 | 296 |
| Right | Dorsolateral prefrontal cortex | BA 9 | (42,36,34) | 178 | 266 | 355 | 172 | 258 | 343 |
| Right | Middle temporal gyrus | BA 22 | (60,−46,18) | 172 | 258 | 344 | 182 | 275 | 367 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konakanchi, D.; de Jongh Curry, A.L.; Waters, R.S.; Narayana, S. Focality of the Induced E-Field Is a Contributing Factor in the Choice of TMS Parameters: Evidence from a 3D Computational Model of the Human Brain. Brain Sci. 2020, 10, 1010. https://doi.org/10.3390/brainsci10121010
Konakanchi D, de Jongh Curry AL, Waters RS, Narayana S. Focality of the Induced E-Field Is a Contributing Factor in the Choice of TMS Parameters: Evidence from a 3D Computational Model of the Human Brain. Brain Sciences. 2020; 10(12):1010. https://doi.org/10.3390/brainsci10121010
Chicago/Turabian StyleKonakanchi, Deepika, Amy L. de Jongh Curry, Robert S. Waters, and Shalini Narayana. 2020. "Focality of the Induced E-Field Is a Contributing Factor in the Choice of TMS Parameters: Evidence from a 3D Computational Model of the Human Brain" Brain Sciences 10, no. 12: 1010. https://doi.org/10.3390/brainsci10121010
APA StyleKonakanchi, D., de Jongh Curry, A. L., Waters, R. S., & Narayana, S. (2020). Focality of the Induced E-Field Is a Contributing Factor in the Choice of TMS Parameters: Evidence from a 3D Computational Model of the Human Brain. Brain Sciences, 10(12), 1010. https://doi.org/10.3390/brainsci10121010





