Characterization and Determination of the Toxicological Risk of Biochar Using Invertebrate Toxicity Tests in the State of Aguascalientes, México
Abstract
:1. Introduction
2. Materials and Methods
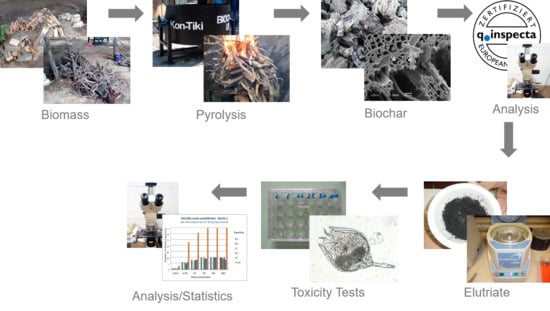
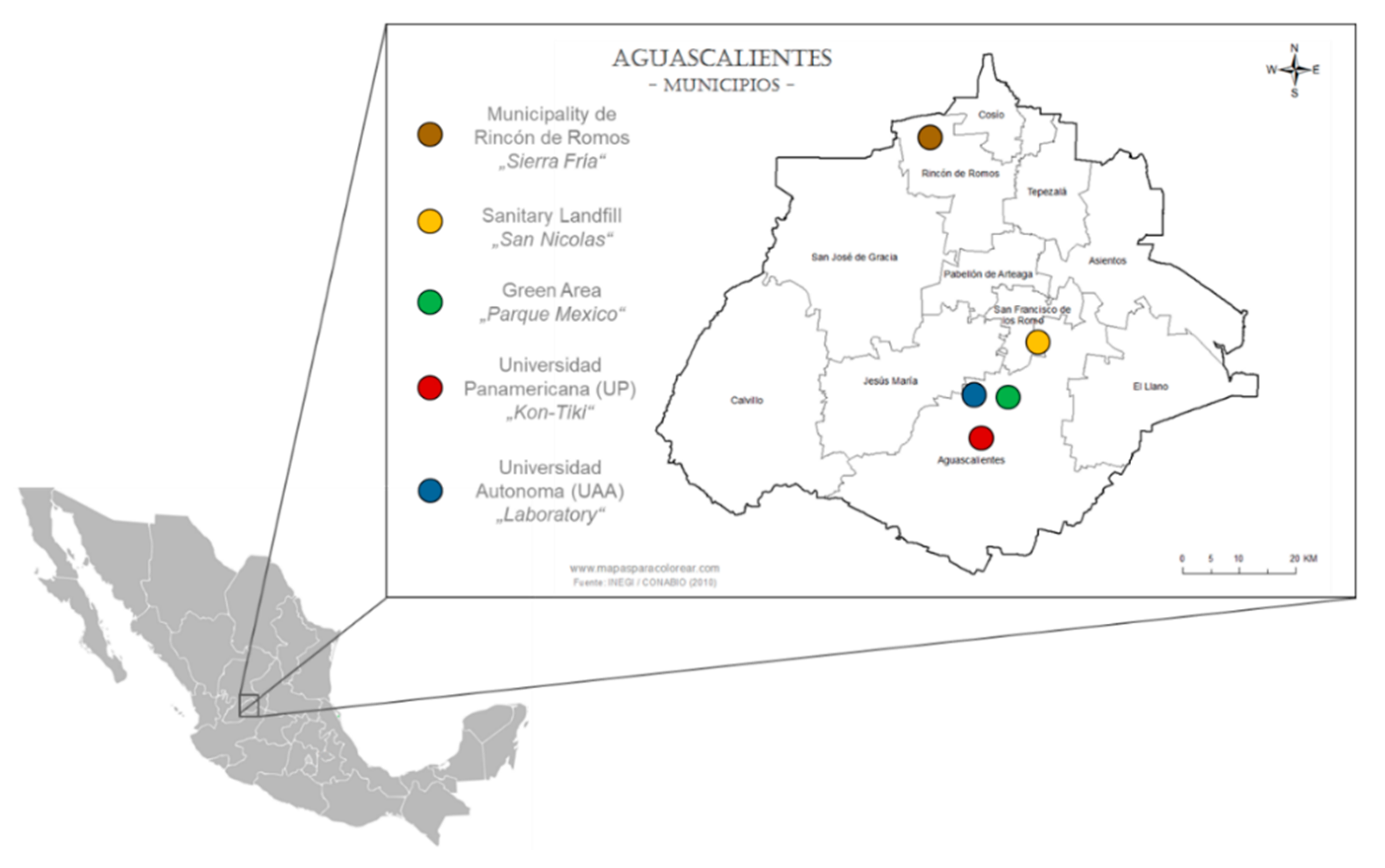
2.1. Study Site, Feedstock, and Biochar Generation
2.2. Biochar Analysis and Elutriate Preparation
2.3. Toxicity Tests
2.3.1. Paramecium caudatum 24 h Acute Toxicity Test
2.3.2. Lecane quadridentata 48 h Acute Toxicity Test
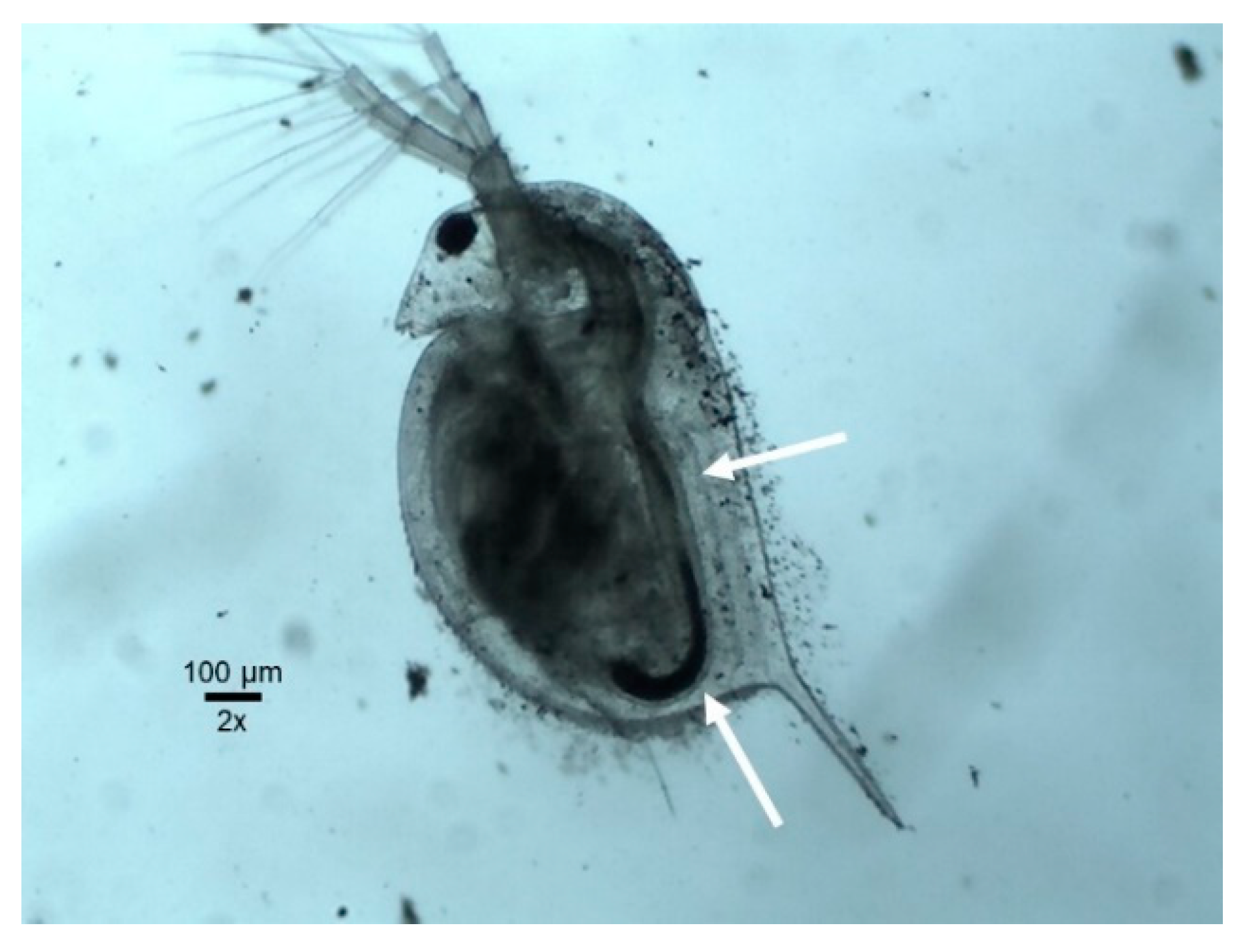
2.3.3. Daphnia magna 48 h Acute Toxicity Test
2.3.4. Moina macrocopa 48 h Acute Toxicity Test
2.3.5. Paramecium caudatum Growth Inhibition Test
- N = total number of P. caudatum organisms alive after 96-h
- t = treatment
- c = control
2.3.6. Chronic Five-Day Toxicity Tests (Growth Inhibition) with Lecane quadridentata
2.3.7. Chronic Seven-Day Toxicity Test (Growth Inhibition) with Moina macrocopa
2.4. Statistical Analysis for the 48h Acute Toxicity Test
3. Results
3.1. Physical and Chemical Parameters of the Biochars
3.2. Paramecium caudatum 24 h Acute and Sublethal Toxicity Tests
3.3. Lecane quadridentata 48 h Acute and Sublethal Toxicity Tests
3.4. Daphnia magna 48 h Acute Toxicity Test
3.5. Moina macrocopa 48 h Acute Toxicity Test
3.6. Soil–Biochar Mixture Elutriate Tests
4. Discussion
4.1. Toxicity of Biochar Elutriates to Aquatic Invertebrates
4.2. Toxic Mechanism, Actuator, and Relevance to the Environment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Substance | Unit | BC-1 | BC-2 | BC-3 | BC-4 | EBC |
|---|---|---|---|---|---|---|
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin | ng kg−1 DM | <0.1 | <0.1 | <0.1 | <0.1 | |
| 1,2,3,7,8-Pentachlorodibenzo-p-dioxin | ng kg−1 DM | <0.15 | <0.15 | <0.15 | <0.15 | |
| 1,2,3,4,7,8-Hexachlorodibenzo-p-dioxin | ng kg−1 DM | <0.15 | <0.15 | <0.15 | <0.15 | |
| 1,2,3,6,7,8-Hexachlorodibenzo-p-dioxin | ng kg−1 DM | <0.15 | <0.15 | <0.15 | <0.15 | |
| 1,2,3,7,8,9-Hexachlorodibenzo-p-dioxin | ng kg−1 DM | <0.15 | <0.15 | <0.15 | <0.15 | |
| 1,2,3,4,6,7,8,-Heptachlorodibenzo-p-dioxin | ng kg−1 DM | 0.57 | 0.39 | 0.25 | 0.55 | |
| Octachlorodibenzo-p-dioxin | ng kg−1 DM | 1.6 | 1.6 | 0.9 | 1.5 | |
| 2,3,7,8-Tetrachlorodibenzofuran | ng kg−1 DM | 0.17 | 0.12 | 0.17 | 0.17 | |
| 1,2,3,7,8-Pentachlorodibenzofuran | ng kg−1 DM | <0.1 | <0.1 | <0.1 | <0.1 | |
| 2,3,4,7,8-Pentachlorodibenzofuran | ng kg−1 DM | <0.1 | <0.1 | <0.1 | <0.1 | |
| 1,2,3,4,7,8-Hexachlorodibenzofuran | ng kg−1 DM | 0.13 | <0.1 | <0.1 | 0.12 | |
| 1,2,3,6,7,8-Hexachlorodibenzofuran | ng kg−1 DM | <0.1 | <0.1 | <0.1 | <0.1 | |
| 1,2,3,7,8,9-Hexachlorodibenzofuran | ng kg−1 DM | <0.1 | <0.1 | <0.1 | <0.1 | |
| 2,3,4,6,7,8-Hexachlorodibenzofuran | ng kg−1 DM | <0.1 | <0.1 | <0.1 | <0.1 | |
| 1,2,3,4,6,7,8-Heptachlorodibenzofuran | ng kg−1 DM | 0.21 | 0.14 | 0.16 | 0.25 | |
| 1,2,3,4,7,8,9-Heptachlorodibenzofuran | ng kg−1 DM | <0.1 | <0.1 | <0.1 | <0.1 | |
| Octachlorodibenzofuran | ng kg−1 DM | <0.2 | 0.2 | <0.2 | 0.2 | |
| Ʃ WHO(2005) PCDD(7)/F(10) (TEQ) [excl. LOQ] | ng kg−1 DM | 0.04 | 0.02 | 0.02 | 0.04 | |
| Ʃ WHO(2005) PCDD(7)/F(10) (TEQ) [incl. LOQ] | ng kg−1 DM | 0.40 | 0.39 | 0.39 | 0.40 | 20 |
| Ʃ WHO(2005) PCDD(7)/F(10) (TEQ) [incl. LOQ] | ng kg−1 88%DM | 0.35 | 0.34 | 0.34 | 0.35 | 0.75 |
| 3,3’,4,4’-Tetrachlorobiphenyl | ng kg−1 DM | 3 | 1.6 | 1.3 | 1.6 | |
| 3,4,4’,5-Tetrachlorobiphenyl | ng kg−1 DM | <0.2 | <0.2 | <0.2 | <0.2 | |
| 2,3,3’,4,4’-Pentachlorobiphenyl | ng kg−1 DM | 13 | 6.5 | 6.4 | 7.5 | |
| 2,3,4,4’,5-Pentachlorobiphenyl | ng kg−1 DM | 29 | 15 | 14 | 18 | |
| 2,3’,4,4’,5-Pentachlorobiphenyl | ng kg−1 DM | <3 | <3 | <3 | <3 | |
| 2,3’,4,4’,5’-Pentachlorobiphenyl | ng kg−1 DM | <2 | <2 | <2 | <2 | |
| 3,3’,4,4’,5-Pentachlorobiphenyl | ng kg−1 DM | <0.3 | <0.3 | <0.3 | <0.3 | |
| 2,3,3’,4,4’,5-Hexachlorobiphenyl | ng kg−1 DM | 4.2 | 2.3 | <2 | 2.4 | |
| 2,3,3’,4,4’,5’-Hexachlorobiphenyl | ng kg−1 DM | <2 | <2 | <2 | <2 | |
| 2,3’,4,4’,5,5’-Hexachlorobiphenyl | ng kg−1 DM | <2 | <2 | <2 | <2 | |
| 3,3’,4,4’,5,5’-Hexachlorobiphenyl | ng kg−1 DM | <0.3 | <0.3 | <0.3 | <0.3 | |
| 2,3,3’,4,4’,5,5’-Heptachlorobiphenyl | ng kg−1 DM | <3 | <3 | <3 | <3 | |
| Ʃ WHO(2005) PCB(12) (TEQ) [excl. LOQ] | ng kg−1 DM | 0.00169 | 0.00087 | 0.00074 | 0.00100 | |
| Ʃ WHO(2005) PCB(12) (TEQ) [incl. LOQ] | ng kg−1 DM | 0.04111 | 0.04029 | 0.04022 | 0.04042 | 0.35 |
| Ʃ WHO(2005) PCB(12) (TEQ) [incl. LOQ] | ng kg−1 88%DM | 0.03617 | 0.03546 | 0.03540 | 0.03557 | |
| Ʃ WHO(2005) PCDD(7)/F(10) + PCB (TEQ) [incl. LOQ] | ng kg−1 DM | 0.43845 | 0.42713 | 0.43065 | 0.43693 | |
| Ʃ WHO(2005) PCDD(7)/F(10) + PCB(12) (TEQ) [incl. LOQ] | ng kg−1 88%DM | 0.38583 | 0.37588 | 0.37897 | 0.38450 | 1.25 |
| 2,4,4’-Trichlorobiphenyl | µg kg−1 88%DM | 0.080 | <0.055 | <0.050 | <0.050 | |
| 2,2’,5,5’-Tetrachlorobiphenyl | µg kg−1 88%DM | 0.130 | 0.075 | 0.074 | 0.078 | |
| 2,2’,4,5,5’-Pentachlorobiphenyl | µg kg−1 88%DM | 0.082 | 0.044 | 0.047 | 0.047 | |
| 2,2’,3,4,4’,5’-Hexachlorobiphenyl | µg kg−1 88%DM | 0.050 | 0.028 | 0.026 | 0.031 | |
| 2,2’,4,4’,5,5’-Hexachlorobiphenyl | µg kg−1 88%DM | 0.054 | 0.03 | 0.028 | 0.032 | |
| 2,2’,3,4,4’,5,5’-Heptachlorobiphenyl | µg kg−1 88%DM | <0.020 | <0.020 | <0.020 | <0.0020 | |
| Ʃ WHO(2005) Indicator PCB(6) [excl. LOQ] | µg kg−1 88%DM | 0.400 | 0.230 | 0.018 | 0.024 | 10 |
References
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. The “Terra Preta” Phenomenon: A Model for Sustainable Agriculture in the Humid Tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Birk, J.J. State of the Scientific Knowledge on Properties and Genesis of Anthropogenic Dark Earths in Central Amazonia (Terra Preta de Índio). Geochim. Cosmochim. Acta. 2012, 82, 39–51. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-Energy in the Black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the Pyrolysis Platform for Coproducing Bio-Oil and Biochar. Biofuels Bioprod. Biorifining 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One Step Forward toward Characterization: Some Important Material Properties to Distinguish Biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.P.; Gerber, H. Biochar Organic Fertilizers from Natural Resources as Substitute for Mineral Fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef]
- Bucheli, T.D.; Hilber, I.; Schmidt, H.-P. Polycyclic Aromatic Hydrocarbons and Polychlorinated Aromatic Compounds in Biochar. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2015; pp. 595–624. [Google Scholar]
- European Biochar Certificate. Guidelines for a Sustainable Production of Biochar. Eur. Biochar Found (EBC) Arbaz Switzerland 2013. [Google Scholar] [CrossRef]
- Ministerstvo Zemědělství. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil, v. 1.1. Int. Biochar Initiat., 2016. No. January 2011, 134. Available online: https://doi.org/http://www.biochar-international.org/characterizationstandard (accessed on 22 March 2019).
- Cornelissen, G.; Pandit, N.R.; Taylor, P.; Pandit, B.H.; Sparrevik, M.; Schmidt, H.P. Emissions and Char Quality of Flame-Curtain “Kon Tiki” Kilns for Farmer-Scale Charcoal/Biochar Production. PLoS ONE 2016, 11, e0154617. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; He, Y.; Tang, J.; Hecker, M.; Liu, Q.; Jones, P.D.; Codling, G.; Giesy, J.P. Effect of Pyrolysis Temperature on Potential Toxicity of Biochar If Applied to the Environment. Environ. Pollut. 2016, 218, 1–7. [Google Scholar] [CrossRef]
- Hamid, R.; Liedtke, V.; Schwanninger, M.; Soja, G.; Dellantonio, A.; Ottner, F.; Zehetner, F.; Kloss, S.; Gerzabek, M.H. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of Phytoremediation and Biochar to Remediate Heavy Metal Polluted Soils: A Review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of Biochar and Greenwaste Compost Amendments on Mobility, Bioavailability and Toxicity of Inorganic and Organic Contaminants in a Multi-Element Polluted Soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Naidu, R.; Bolan, N.S.; Megharaj, M.; Choppala, G.K. The Influence of Biochar and Black Carbon on Reduction and Bioavailability of Chromate in Soils. J. Environ. Qual. 2012, 41, 1175–1184. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Kuśmierz, M. Biochar Properties Regarding to Contaminants Content and Ecotoxicological Assessment. J. Hazard. Mater. 2013, 260, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Pranagal, J.; Kuśmierz, M.; Oleszczuk, P.; Ligęza, S.; Jośko, I.; Futa, B.; Wielgosz, E. Microbiological, Biochemical and Ecotoxicological Evaluation of Soils in the Area of Biochar Production in Relation to Polycyclic Aromatic Hydrocarbon Content. Geoderma 2013, 213, 502–511. [Google Scholar] [CrossRef]
- Rand, G.M.; Petrocelli, S.R. Fundamentals of Aquatic Toxicology: Methods and Applications, 5th ed.; Hemisphere Publ.: New York, NY, USA, 1989. [Google Scholar]
- Villamar, F.F. Bioensayo de Toxicidad (CL50) Del Dispersante de Petroleo BP 1100 WD, Con Fintoplancton Marino (Tetraselmis sp). Acta Oceanografica del Pacifico 1996, 8, 67–73. [Google Scholar]
- Snell, T.W.; Janssen, C.R. Microscale Toxicity Testing with Rotifers. In Microscale testing in Aquatic Toxicology; Wells, P.G., Lee, K.L., Blaise, C., Eds.; CRC Press: Boca Raton, FL, USA, 1998; pp. 409–421. [Google Scholar]
- Wallace, R.L.; Snell, T.W.; Ricci, C. Rotifera, Part 1: Biology, Ecology and Systematics. In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World, 23; Segers, H., Dumont, H., Eds.; Kenobi Productions and Backhuys Academic Publishing: Ghent, Belgium, 2006; p. 299. [Google Scholar]
- Denekamp, N.Y.; Thorne, M.A.S.; Clark, M.S.; Kube, M.; Reinhardt, R.; Lubzens, E. Discovering Genes Associated with Dormancy in the Monogonont Rotifer Brachionus plicatilis. BMC Genom. 2009, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Denekamp, N.Y.; Suga, K.; Hagiwara, A.; Reinhardt, R.; Lubzens, E. A Role for Molecular Studies in Unveiling the Pathways for Formation of Rotifer Resting Eggs and Their Survival during Dormancy. In Dormancy and Resistance in Harsh Environments. Topics in Current Genetics; Lubzens, E., Cerda, J., Clark, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 21, 300p. [Google Scholar]
- Gilbert, J.J. Rotifera. In Fertilization, Development, and Parental Care. Reproductive Biology of Invertebrates; Oxford & IBH Publishing Corporation, PVT, Ltd.: Oxford, UK, 1989; pp. 179–199. [Google Scholar]
- Marcial, H.S.; Hagiwara, A.; Snell, T.W. Effect of Some Pesticides on Reproduction of Rotifer Brachionus plicatilis Müller. Hydrobiologia 2005, 546, 569–575. [Google Scholar] [CrossRef]
- Yaobin, Q.I. Estudos Sobre a Variação Temporal Da Composição de Macroalgas Marinhas Em Uma Baía Poluída—O Caso de Santos; Universidade de São Paulo: Litoral de São Paulo, Brazil, 1999. [Google Scholar]
- Fenchel, T. Ecology of Protozoa. The Biology of Free-Living Phagotrophic Protists; Brock, T.D., Ed.; Springer: Berlin, Germany, 1987. [Google Scholar] [CrossRef]
- Sakakura, Y.; Hagiwara, A.; Mark Welch, D.; Suga, K.; Tanaka, Y. Analysis of Expressed Sequence Tags of the Cyclically Parthenogenetic Rotifer Brachionus plicatilis. PLoS ONE 2007, 2, e671. [Google Scholar] [CrossRef]
- Hueck-Van Der Plas, E.H. Experiences with an Inventory of Ecological Tests Based on an Enquiry by the OECD Chemicals Group. In Tests for the Ecological Effects of Chemicals; Erich Schmidt Verlag: Berlin, Germany, 1978; pp. 63–73. [Google Scholar]
- Baudo, R. Ecotoxicological Testing with Daphnia. In Daphnia; Peters, R.H., de Bernardi, R., Eds.; Mem. Ist. Ital. Idrobiol.: Verbania, Italy, 1987; pp. 45, 461–482. [Google Scholar]
- Rico-Martínez, R.; Velázquez-Rojas, C.A.; Pérez-Legaspi, I.A.; Santos-Medrano, G.E. The Use of Aquatic Invertebrate Toxicity Tests and Invertebrate Enzyme Biomarkers to Assess Toxicity in the States of Aguascalientes and Jalisco, Mexico. In Biomonitors and Biomarkers as Indicators of Environmental Change; Butterworth, F.M., Gunatilake, A., Gonsebatt Bonaparte, M.E., Eds.; Plenum Press: New York, NY, USA, 2000; in press. [Google Scholar]
- Snell, T.W.; Dusenbery, D.; Dunn, L.; Walls, N. Biomarkers for Managing Water Resources; ERC 02-93; Georgia Institute of Technology, Envrionmental Resources Center, Publication: Atlanta, GE, USA, 1993. [Google Scholar]
- Pérez-Legaspi, I.A.; Rico-Martínez, R. Acute Toxicity Tests on Three Species of the Genus lecane (Rotifera: Monogononta). Hydrobiologia 2001, 446–447, 375–381. [Google Scholar] [CrossRef]
- INEGI. Estudio Hidrológico Del Estado de Aguascalientes; INEGI: Aguascalientes, Mexico, 1993. [Google Scholar]
- Cheng, C.H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of Black Carbon by Biotic and Abiotic Processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating Physical and Chemical Properties of Highly Weathered Soils in the Tropics with Charcoal—A Review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Joseph, S.; Downie, A.; Chan, K.Y.; Van Zwieten, L.; Meszaros, I. Agronomic Values of Greenwaste Biochar as a Soil Amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar]
- EBC. Guidelines for a Sustainable Production of Biochar. Eur. Biochar Found. 2016, 1–22. [Google Scholar] [CrossRef]
- ASTM. Standard Guide for Collection, Storage, Characterization, and Manipulation of Sediments for Toxicological Testings, E1391-94. In ASTM Standards on Environmental Sampling; American Society for Testing and Materials International: West Conshohocken, PA, USA, 1996. [Google Scholar]
- USEPA/USACE. Dredged Material Proposed for Discharge in Waters of the U.S. (Inland Testing Manual); USEPA/USACE: Washington, DC, USA, 1998.
- United States Environmental Protection Agency (EPA). Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analyses: Technical Manual; EPA 823-B-01-002; U.S. Environ. Prot. Agency, Off. Water: Washington, DC, USA, 2001; pp. 1–208.
- DÜV. Verordnung über Die Anwendung von Düngemitteln Nach Den Grundsätzen Der Guten Fachlichen Praxis Beim Düngen (Düngeverordnung—DÜV); DÜV: Bundesgesetzblatt I: Bonn, Germany, 2006; pp. 221–247. [Google Scholar]
- Bundesgesetzblatt. Gesetz Zum Schutz Vor Schädlichen Bodenveränderungen Und Zur Sanierung von Altlasten (Bundes-Bodenschutzgesetz); Bundesgesetzblatt I: Bonn, Germany, 2015; pp. 1–12. [Google Scholar]
- Dieckmann, J. An Improved Protargol Impregnation for Ciliates Yielding Reproducible Results. Eur. J. Protistol. 1995, 31, 372–382. [Google Scholar] [CrossRef]
- Sonneborn, T.M. Chapter 12 Methods in Paramecium Research. In Methods in Cell Biology; Academic Press: New York, New York, USA, 1970; pp. 241–339. [Google Scholar] [CrossRef]
- Madoni, P. The Acute Toxicity of Nickel to Freshwater Ciliates. Environ. Pollut. 2000, 109, 53–59. [Google Scholar] [CrossRef]
- USEPA. Technical Support Document for Water Quality-Based Toxic Control. Off. Water Enforc. 1991, 2, 335. [Google Scholar] [CrossRef]
- Secretaría de Economía. Norma Mexicana NMX-AA-087-SCFI-2010 Análisis de Agua-Evaluación de Toxicidad Aguda Con Daphnia Magna, Straus (Crustácea-Cladócera)-MÉTODO DE PRUEBA (Cancela a La NMX-AA-087-SCFI-1995); Secretaría de Economía: Cuauhtémoc, Mexico, 2010; p. 39.
- Cooney, J.D. Freshwater Tests. In Fundamentals of Aquatic Toxicology: Effects, Environmental Fate, and Risk Assessment; Rand, G.M., Petrocelli, S.R., Eds.; Washington Hemisphere Publishing Corporation: Washington, DC, USA, 1995; pp. 71–102. [Google Scholar]
- Miyoshi, N.; Kawano, T.; Tanaka, M.; Kadono, T.; Kosaka, T.; Kunimoto, M.; Takahashi, T.; Hosoya, H. Use of Paramecium Species in Bioassays for Environmental Risk Management: Determination of IC50 Values for Water Pollutants. J. Health Sci. 2003, 49, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Flores, S.; Rico-Martínez, R. Study of the Effects of Pb and Hg Toxicity Using a Chronic Toxicity Reproductive 5-Day Test with the Freshwater Rotifer Lecane Quadridentata. Environ. Toxicol. 2006, 21, 533–540. [Google Scholar] [CrossRef]
- USEPA. Daphnid, Ceriodaphnia Dubia, Survival and Reproduction Test; Chronic Toxicity; No. Method 1002.0; USEPA: Washington, DC, USA, 2002.
- Boateng, A.; Garcia-Perez, M.; Masek, O.; Brown, R.; del Campo, B. Biochar Production Technology. In Biochar for Environmental Management: Science, Technology and Implementation; Lehman, J., Joseph, S., Eds.; Routledge: London, UK, 2015; pp. 63–87. [Google Scholar]
- Conte, P.; Schmidt, H.P. Soil-Water Interactions Unveiled by Fast Field Cycling NMR Relaxometry. eMagRes 2017, 6, 453–464. [Google Scholar] [CrossRef]
- Mejía-Saavedra, J.; Sánchez-Armass, S.; Santos-Medrano, G.E.; González-Amaro, R.; Razo-Soto, I.; Rico-Martínez, R.; Díaz-Barriga, F. Effect of Coexposure to DDT and Manganese on Freshwater Invertebrates: Pore Water from Contaminated Rivers and Laboratory Studies. Environ. Toxicol. Chem. 2005, 24, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Santos-Medrano, G.E.; Ramírez-López, E.M.; Hernández-Flores, S.; Azuara-Medina, P.M.; Rico-Martínez, R. Determination of Toxicity Levels in the San Pedro River Watershed, Aguascalientes, Mexico. J. Environ. Sci. Health Part A 2007, 42, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Torres Guzmán, F.; González, F.J.A.; Rico Martínez, R. Implementing Lecane Quadridentata Acute Toxicity Tests to Assess the Toxic Effects of Selected Metals (Al, Fe and Zn). Ecotoxicol. Environ. Saf. 2010, 73, 287–295. [Google Scholar] [CrossRef]
- Štrojsová, M.; Vrba, J. Direct Detection of Digestive Enzymes in Planktonic Rotifers Using Enzyme-Labelled Fluorescence (ELF). Mar. Freshw. Res. 2005, 56, 189–195. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The Use of Biomarkers in Daphnia Magna Toxicity Testing: I. The Digestive Physiology of Daphnids Exposed to Toxic Stress. Hydrobiologia 1998, 367, 199–209. [Google Scholar] [CrossRef]
- Freddo, A.; Cai, C.; Reid, B.J. Environmental Contextualisation of Potential Toxic Elements and Polycyclic Aromatic Hydrocarbons in Biochar. Environ. Pollut. 2012, 171, 18–24. [Google Scholar] [CrossRef]
- Chen, M.; Soudek, P.; Xia, W.; Luo, F.; Song, J.; Dong, M. Characterization of Contaminants and Evaluation of the Suitability for Land Application of Maize and Sludge Biochars. Environ. Sci. Pollut. Res. 2014, 21, 8707–8717. [Google Scholar] [CrossRef]
- Kim, E.J.; Oh, J.E.; Chang, Y.S. Effects of Forest Fire on the Level and Distribution of PCDD/Fs and PAHs in Soil. Sci. Total Environ. 2003, 311, 177–189. [Google Scholar] [CrossRef]
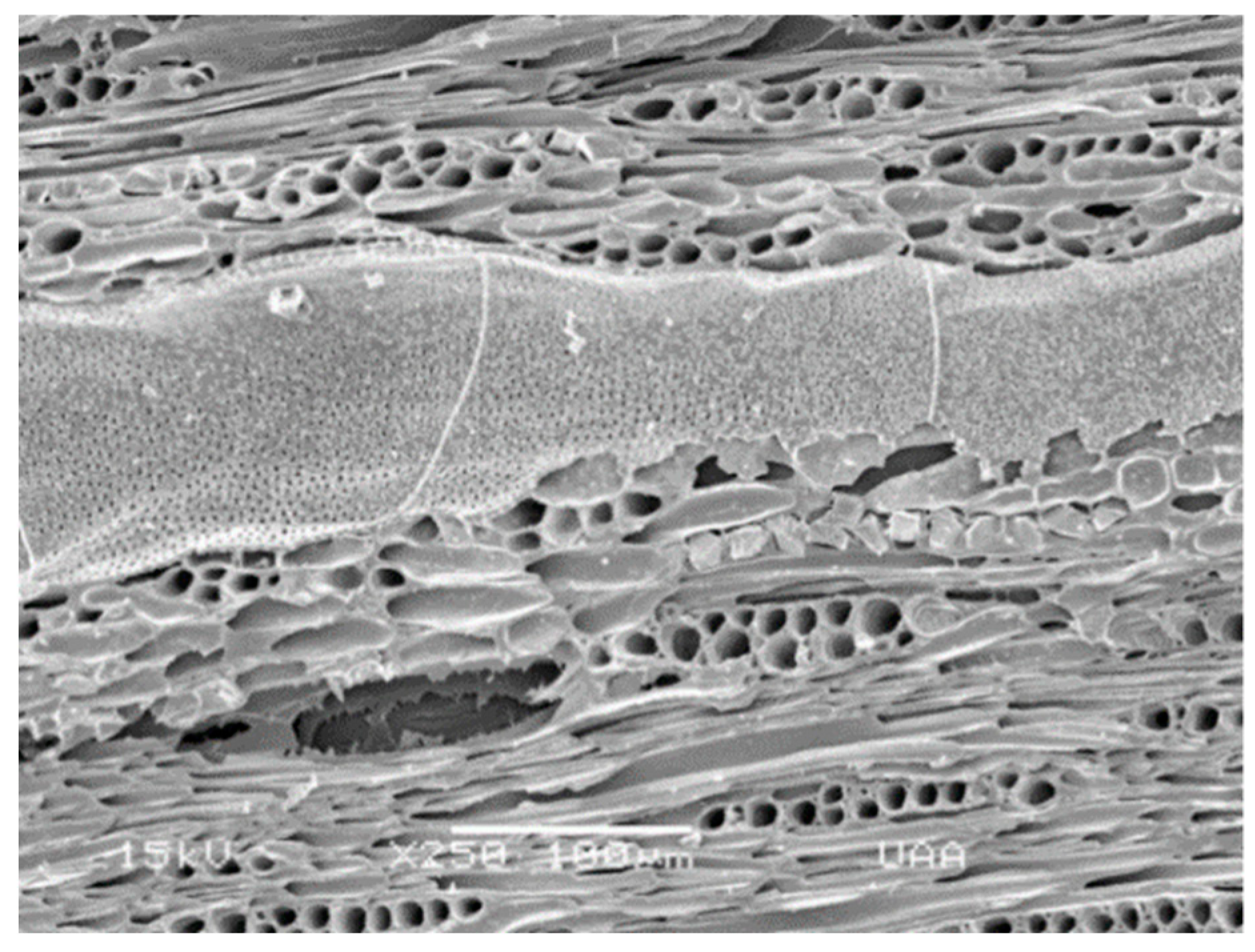
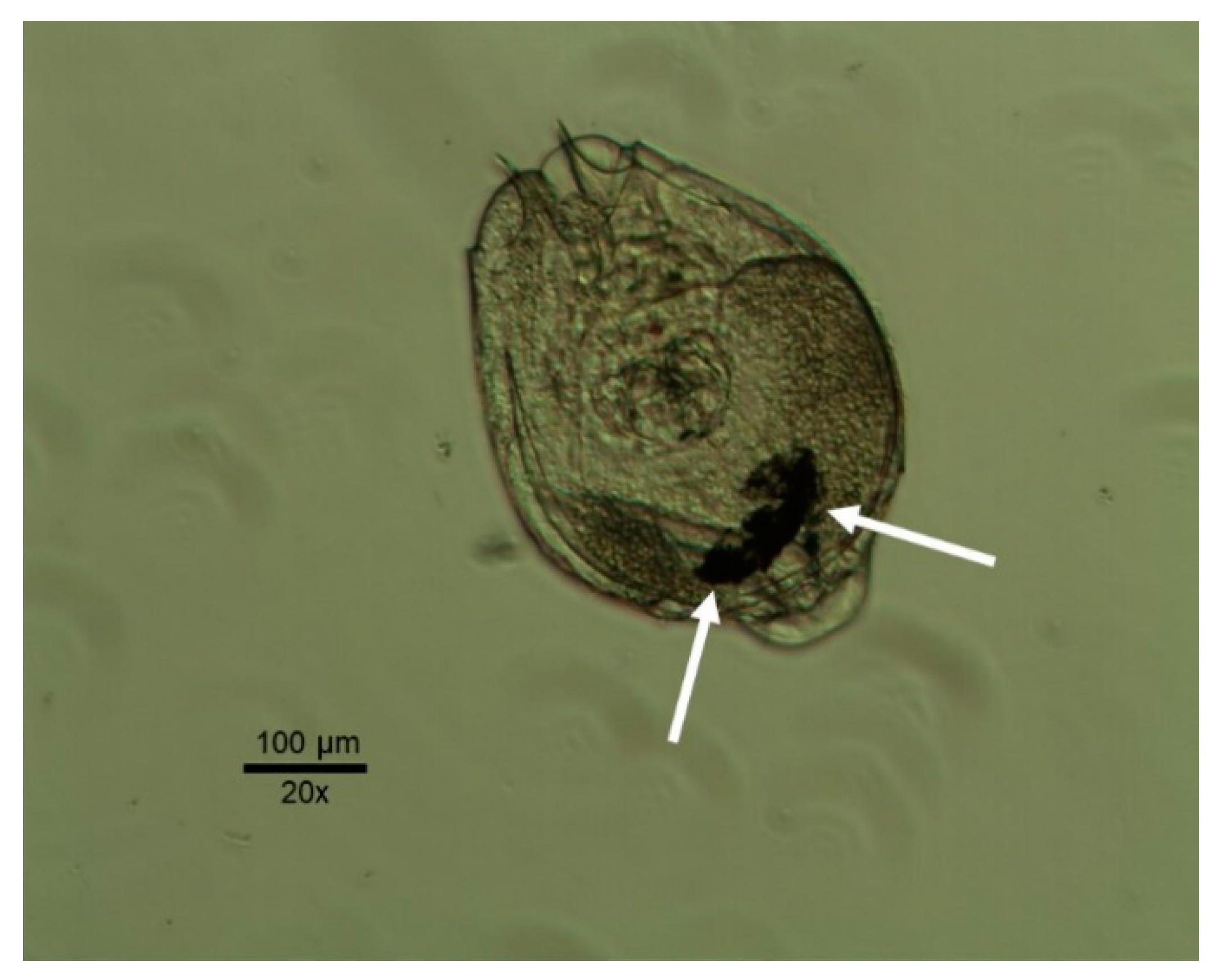

| Timber Species | ||
|---|---|---|
| Common Name | Scientific Name | Content (%) |
| Velvet Mesquite | Prosopis velutina | 18 |
| Ash Tree | Fraxinus excelsiur | 16 |
| Australian Pine | Casuarina equisetifolia | 15 |
| Eucalyptus | Eucalyptus spp. | 15 |
| Ficus Tree | Ficus benjamina | 12 |
| Peruvian Mastic Tree | Schinus molle | 11 |
| Pynion Pine | Pinus cembroides | 8 |
| Bugambilia | Bougainvillea spp. | 4 |
| Palm | Family Arecaceae | 1 |
| Tree Species | ||
|---|---|---|
| Colloquial Name | Scientific Name | Content (%) |
| Velvet Mesquite | Prosopis velutina | 38 |
| Ash Tree | Fraxinus excelsiur | 24 |
| Manchineel Tree | Hippomane mancinella | 21 |
| Australian Pine | Casuarina equisetifolia | 17 |
| Biochar (BC) | BC 1 | BC 2 | BC 3 | BC 4 | EBC Threshold | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Unit | FM | aDM | FM | aDM | FM | aDM | FM | aDM | Premium | Basic |
| Water holding capacity (WHC) | mass-% | 165.8 | 200.0 | 149.1 | 254.2 | ||||||
| Bulk density | kg m−3 | 385 | 504 | 567 | 368 | ||||||
| Specific surface (by BET) | m−2 g | 305 | 140 | 280 | 54 | ||||||
| Particle density | g cm−3 | 1.64 | 1.59 | 1.76 | 1.58 | ||||||
| Total water content | mass-% | 28.4 | 39.8 | 35.9 | 60.7 | ||||||
| Ash content 550 °C | mass-% | 8.6 | 12 | 8.7 | 14.5 | 11.4 | 17.7 | 5.1 | 13.1 | ||
| Hydrogen | mass-% | 0.69 | 0.96 | 1.10 | 1.82 | 0.56 | 0.87 | 0.67 | 1.70 | ||
| Total carbon (TC) | mass-% | 63.9 | 89.2 | 47.0 | 78.1 | 51.5 | 80.4 | 30.9 | 78.7 | >50 | >50 |
| Total inorganic carbon (TIC) | mass-% | 0.7 | 1.0 | 0.8 | 1.3 | 0.9 | 1.3 | 0.4 | 1.0 | ||
| Nitrogen | mass-% | 0.22 | 0 0.3 | 0.39 | 40.66 | 0.5 | 0.79 | 0.46 | 1.17 | ||
| Oxygen | mass-% | 0.8 | 1.1 | 5.1 | 8.5 | 3.3 | 5.2 | 3.1 | 7.9 | ||
| Carbonate CO2 | mass-% | 2.62 | 3.66 | 2.77 | 4.6 | 3.12 | 4.87 | 1.51 | 3.84 | ||
| Organic carbon | mass-% | 63.2 | 88.2 | 46.2 | 76.8 | 50.6 | 79.1 | 30.5 | 77.7 | ||
| H/C (molar ratio) | 0.13 | 0.13 | 0.28 | 0.28 | 0.13 | 0.13 | 0.26 | 0.26 | <0.6 | <0.6 | |
| H/Corg (molar ratio) | 0.13 | 0.13 | 0.28 | 0.28 | 0.13 | 0.13 | 0.26 | 0.26 | <0.7 | <0.7 | |
| O/C (molar ratio) | 0.01 | 0.009 | 0.08 | 0.082 | 0.05 | 0.05 | 0.08 | 0.08 | <0.4 | <0.4 | |
| pH value (CaCl2) | 7.8 | 8.2 | 8.5 | 8.3 | ≤10 | ≤10 | |||||
| Electric conductivity | µS cm−1 | 336 | 566 | 617 | 580 | ||||||
| Salt content | g kg−1 | 1.77 | 2.48 | 2.99 | 4.96 | 3.26 | 5.09 | 3.06 | 7.79 | ||
| Salt content | g L−1 | 0.68 | 0.95 | 1.51 | 2.50 | 1.85 | 2.88 | 1.16 | |||
| Phosphorous | mg kg−1 | 470 | 1400 | 2300 | 7.79 | ||||||
| Magnesium | mg kg−1 | 1800 | 2500 | 2500 | 2.94 | ||||||
| Calcium | mg kg−1 | 36,000 | 41,000 | 51,000 | 2300 | ||||||
| Potassium | mg kg−1 | 4000 | 11,000 | 9800 | 2900 | ||||||
| Sodium | mg kg−1 | 350 | 1000 | 910 | 32,000 | ||||||
| Iron | mg kg−1 | 460 | 760 | 830 | 12,000 | ||||||
| Silica | mg kg−1 | 6100 | 10,000 | 9200 | 1400 | ||||||
| Sulfur | mg kg−1 | 170 | 680 | 2100 | 1000 | ||||||
| Arsenic | mg kg−1 | <0.8 | <0.8 | <0.8 | <0.8 | <13 | <13 | ||||
| Lead | mg kg−1 | 3 | 3 | <2 | 3 | <120 | <150 | ||||
| Cadmium | mg kg−1 | <0.2 | <0.2 | <0.2 | <0.2 | <1.0 | <1.5 | ||||
| Copper | mg kg−1 | 7 | 13 | 15 | 37 | <100 | <100 | ||||
| Nickel | mg kg−1 | <1 | 1 | <1 | 1 | <30 | <50 | ||||
| Mercury | mg kg−1 | <0.07 | <0.07 | <0.07 | <0.07 | <1.0 | <1 | ||||
| Zinc | mg kg−1 | 61 | 28 | 21 | 53 | <400 | <400 | ||||
| Chromium | mg kg−1 | <1 | 1 | <1 | <1 | < 0 | <90 | ||||
| Boron | mg kg−1 | 15 | 29 | 21 | 51 | ||||||
| Manganese | mg kg−1 | 560 | 350 | 360 | 460 | ||||||
| Total PAH (EPA-16) | mg kg−1 | 4.8 | 5.3 | 0.7 | 8.0 | <4 | <12 | ||||
| pH QW (source water pH 8.1) | n.a. | 10.8 | 13.2 | 10.5 | |||||||
| Biochar (BC) | BC 1 | BC 2 | BC 3 | BC 4 |
|---|---|---|---|---|
| PAH | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 |
| Naphtalin | 2.5 | 2.6 | 0.6 | 3.3 |
| Acenaphthylen | <0.1 | <0.1 | <0.1 | <0.1 |
| Acenaphthen | <0.1 | <0.1 | <0.1 | <0.1 |
| Fluoren | 0.7 | 0.3 | <0.1 | 0.5 |
| Phenanthren | 0.6 | 1.1 | 0.1 | 1.6 |
| Anthracen | 0.1 | 0.3 | <0.1 | 0.3 |
| Fluoranthen | 0.4 | 0.4 | <0.1 | 1.0 |
| Pyren | 0.5 | 0.4 | <0.1 | 1.0 |
| Benz(a)anthraren | <0.1 | 0.1 | <0.1 | 0.1 |
| Chrysen | <0.1 | 0.1 | <0.1 | 0.2 |
| Benzo(b)fluoranthen | <0.1 | <0.1 | <0.1 | <0.1 |
| Benzo(k)fluoranthen | <0.1 | <0.1 | <0.1 | <0.1 |
| Benzo(a)pyren | <0.1 | <0.1 | <0.1 | <0.1 |
| Indeno(1,2,3-cd)pyren | <0.1 | <0.1 | <0.1 | <0.1 |
| Dibenzo(a,h)anthracen | <0.1 | <0.1 | <0.1 | <0.1 |
| Benzo(g,h,i)perylen | <0.1 | <0.1 | <0.1 | <0.1 |
| Total PAH | 4.8 | 5.3 | 0.7 | 8.0 |
| Sum of Substances | Unit | BC 1 | BC 2 | BC 3 | BC 4 | EBC |
|---|---|---|---|---|---|---|
| Ʃ WHO(2005) PCDD(7)/F(10) (TEQ) [incl. LOQ] | ng kg−1 DM | 0.40 | 0.39 | 0.39 | 0.40 | 20 |
| Ʃ WHO(2005) PCDD(7)/F(10) (TEQ) [incl. LOQ] | ng kg−1 88%DM | 0.35 | 0.34 | 0.34 | 0.35 | 0.75 |
| Ʃ WHO(2005) PCB(12) (TEQ) [incl. LOQ] | ng kg−1 DM | 0.041 | 0.040 | 0.040 | 0.040 | 0.35 |
| Ʃ WHO(2005) PCDD(7)/F(10) + PCB(12) (TEQ) [incl. LOQ] | ng kg−1 88%DM | 0.036 | 0.035 | 0.035 | 0.036 | 1.25 |
| Ʃ WHO(2005) Indicator PCB(6) [excl. LOQ] | µg kg−1 88%DM | 0.400 | 0.230 | 0.018 | 0.024 | 10 |
| Treatment | % Inhibition |
|---|---|
| Control | 0 |
| Soil | 1.99 |
| BC 1 | 2.65 |
| BC 2 | 2.65 |
| BC 3 | 0 |
| BC 4 | 1.32 |
| Parameter | BC 1 | BC 2 | BC 3 | BC 4 |
|---|---|---|---|---|
| LC10 | 10.76 | 4.16 | 13.36 | 10.37 |
| LC50 | 19.95 | 8.36 | 25.14 | 20.36 |
| NOEC | 25 | <6.25 | 6.25 | <6.25 |
| LOEC | 50 | 6.25 | 12.50 | 6.25 |
| 95% CL LC50 | 12.36–32.21 | 5.92–11.83 | 17.78–35.48 | 15.85–20.40 |
| CV | 3.8 | 5.7 | 6.5 | 6.8 |
| r² | 0.3855 | 0.7001 | 0.6876 | 0.8110 |
| Parameter | BC 1 | BC 2 | BC 3 | BC 4 |
|---|---|---|---|---|
| LC10 | ND | ND | ND | ND |
| LC50 | ND | 134.87 | 306.33 | ND |
| NOEC | ND | 50 | 50 | ND |
| LOEC | ND | 100 | 100 | ND |
| 95% CL LC50 | ND | 36.16–503.03 | 37.59–2495.74 | ND |
| CV | ND | ND | ND | ND |
| r² | ND | 0.25 | 0.23 | ND |
| Treatment | Mean Value r |
|---|---|
| Control | 0.408 |
| BC 1 | 0.401 |
| BC 4 | 0.401 |
| Soil | 0.406 |
| Treatment | Lecane Mean Value r | Moina Mean Value r | Paramecium % Inhibition |
|---|---|---|---|
| Control | 0.371 | 0.408 | 0 |
| BC 1 + soil | 0.356 | 0.400 | 1.99 |
| BC 2 + soil | 0.358 | 0.402 | 0.66 |
| BC 3 + soil | 0.356 | 0.402 | 2.65 |
| BC 4 + soil | 0.359 | 0.402 | 0.66 |
| Soil | 0.358 | 0.406 | 1.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flesch, F.; Berger, P.; Robles-Vargas, D.; Santos-Medrano, G.E.; Rico-Martínez, R. Characterization and Determination of the Toxicological Risk of Biochar Using Invertebrate Toxicity Tests in the State of Aguascalientes, México. Appl. Sci. 2019, 9, 1706. https://doi.org/10.3390/app9081706
Flesch F, Berger P, Robles-Vargas D, Santos-Medrano GE, Rico-Martínez R. Characterization and Determination of the Toxicological Risk of Biochar Using Invertebrate Toxicity Tests in the State of Aguascalientes, México. Applied Sciences. 2019; 9(8):1706. https://doi.org/10.3390/app9081706
Chicago/Turabian StyleFlesch, Felix, Pia Berger, Daniel Robles-Vargas, Gustavo Emilio Santos-Medrano, and Roberto Rico-Martínez. 2019. "Characterization and Determination of the Toxicological Risk of Biochar Using Invertebrate Toxicity Tests in the State of Aguascalientes, México" Applied Sciences 9, no. 8: 1706. https://doi.org/10.3390/app9081706
APA StyleFlesch, F., Berger, P., Robles-Vargas, D., Santos-Medrano, G. E., & Rico-Martínez, R. (2019). Characterization and Determination of the Toxicological Risk of Biochar Using Invertebrate Toxicity Tests in the State of Aguascalientes, México. Applied Sciences, 9(8), 1706. https://doi.org/10.3390/app9081706