Three-Motorized-Stage Cyclic Stretching System for Cell Monitoring Based on Chamber Local Displacement Waveforms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Required Specifications
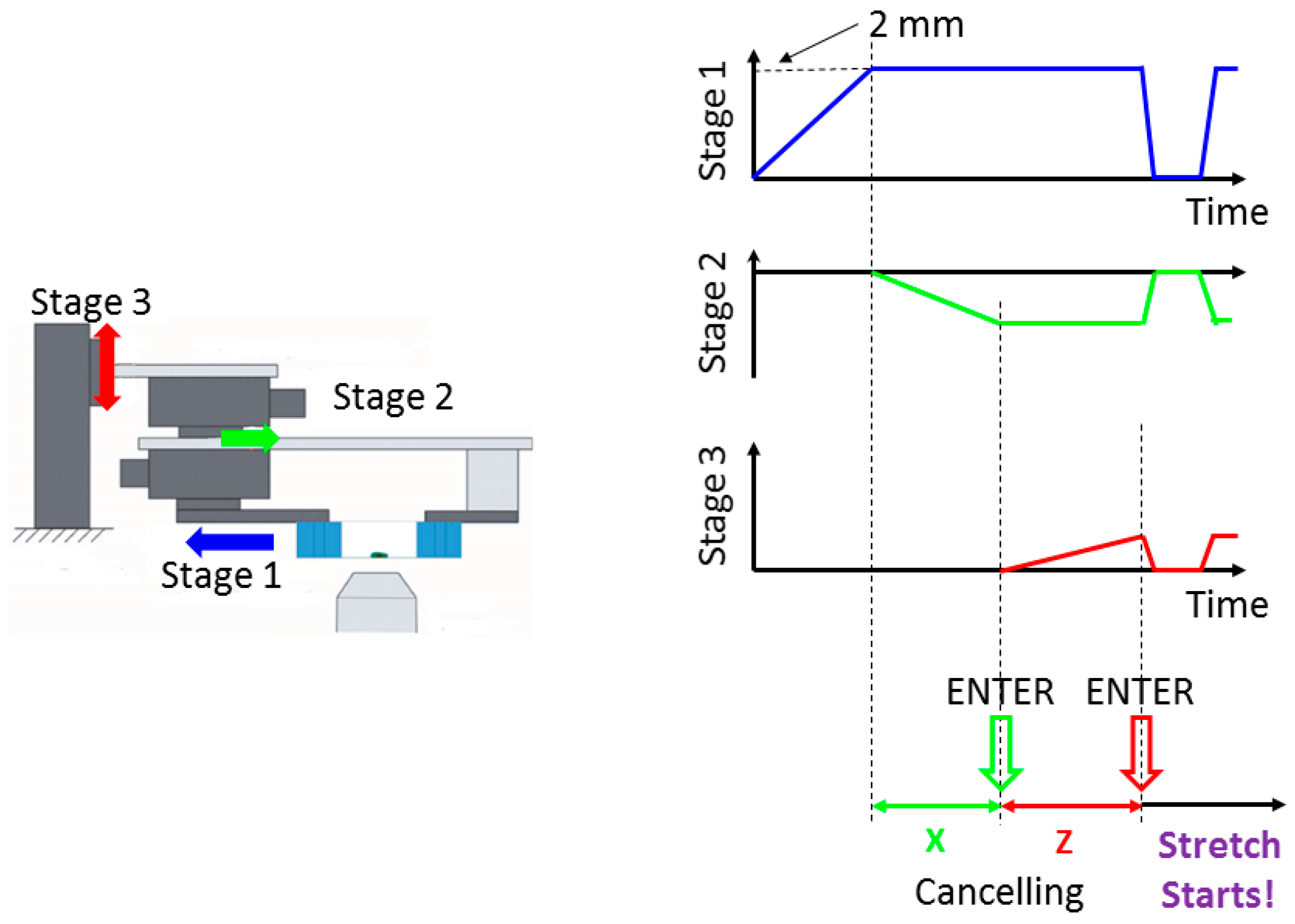
2.2. Design of the Cyclic Stretch System
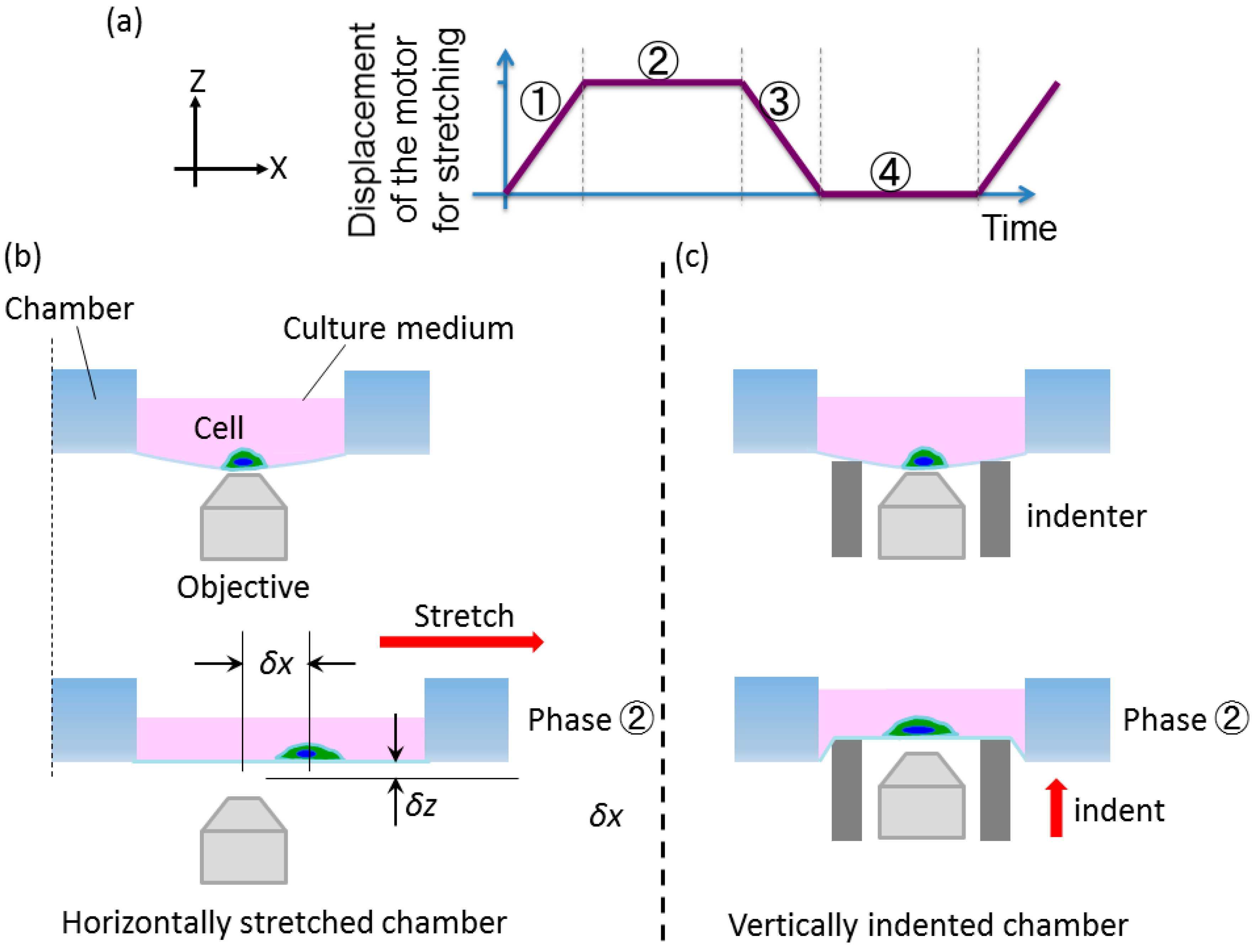
2.2.1. Basic Concept
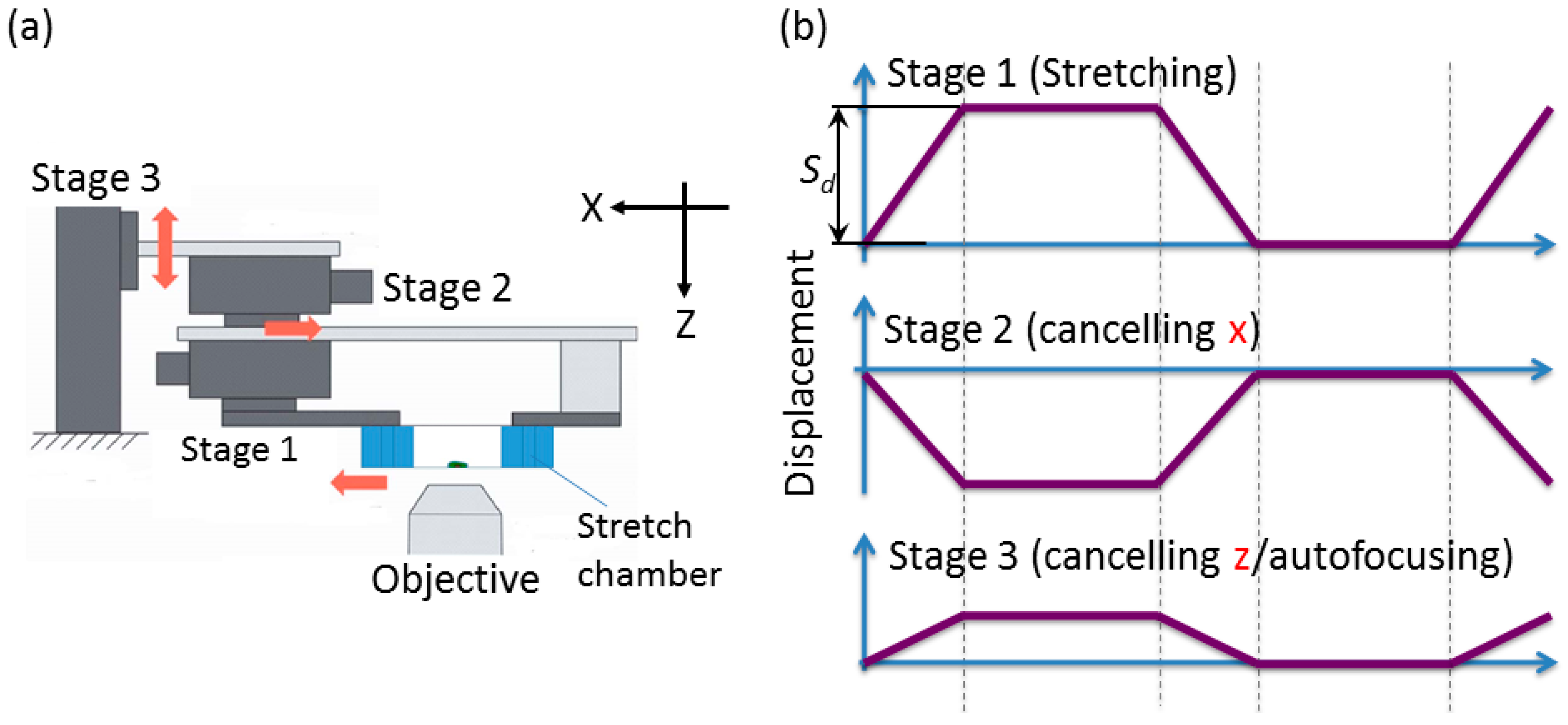
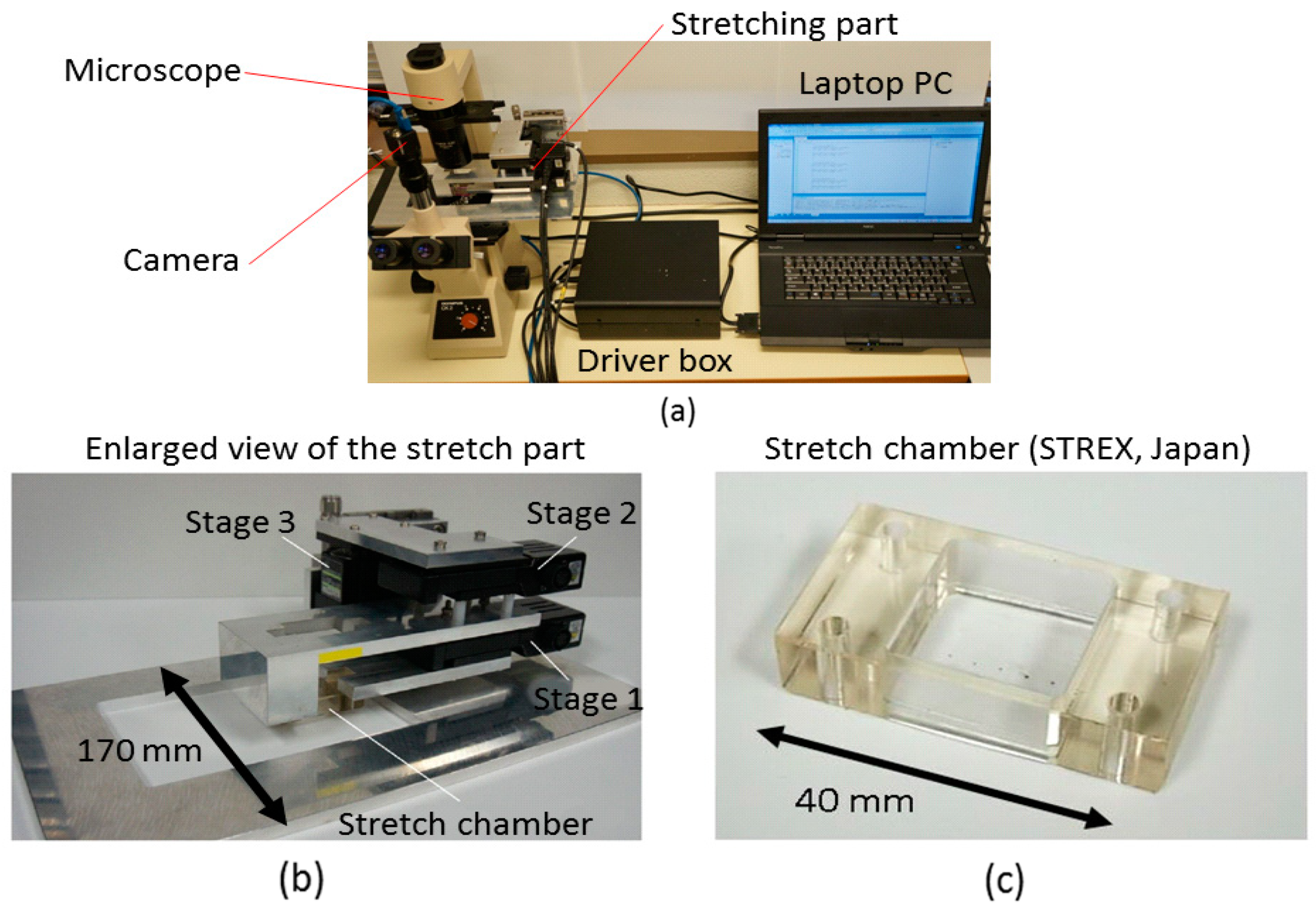
2.2.2. Developed System
- An inverted microscope with 40× objective lenses was used for image and video capture (Olympus Inc., Tokyo, Japan).
- A laptop PC equipped with the interface card slot with 8 bit Digital I/O, with 1.9 GHz CPU, installed physical memory of 2 GB, and Windows 7 OS as the operation system.
- Stage unit: the 5-phase stepping motors with the positioning accuracy of 2 μm/pulse and drive speed of 15 mm/s (SIGMA KOKI Inc., Tokyo, Japan) are required for both stage 1 and stage 2. A stepper motor with the positioning accuracy of 1 μm/pulse and drive speed of 2 mm/s is required for stage 3.
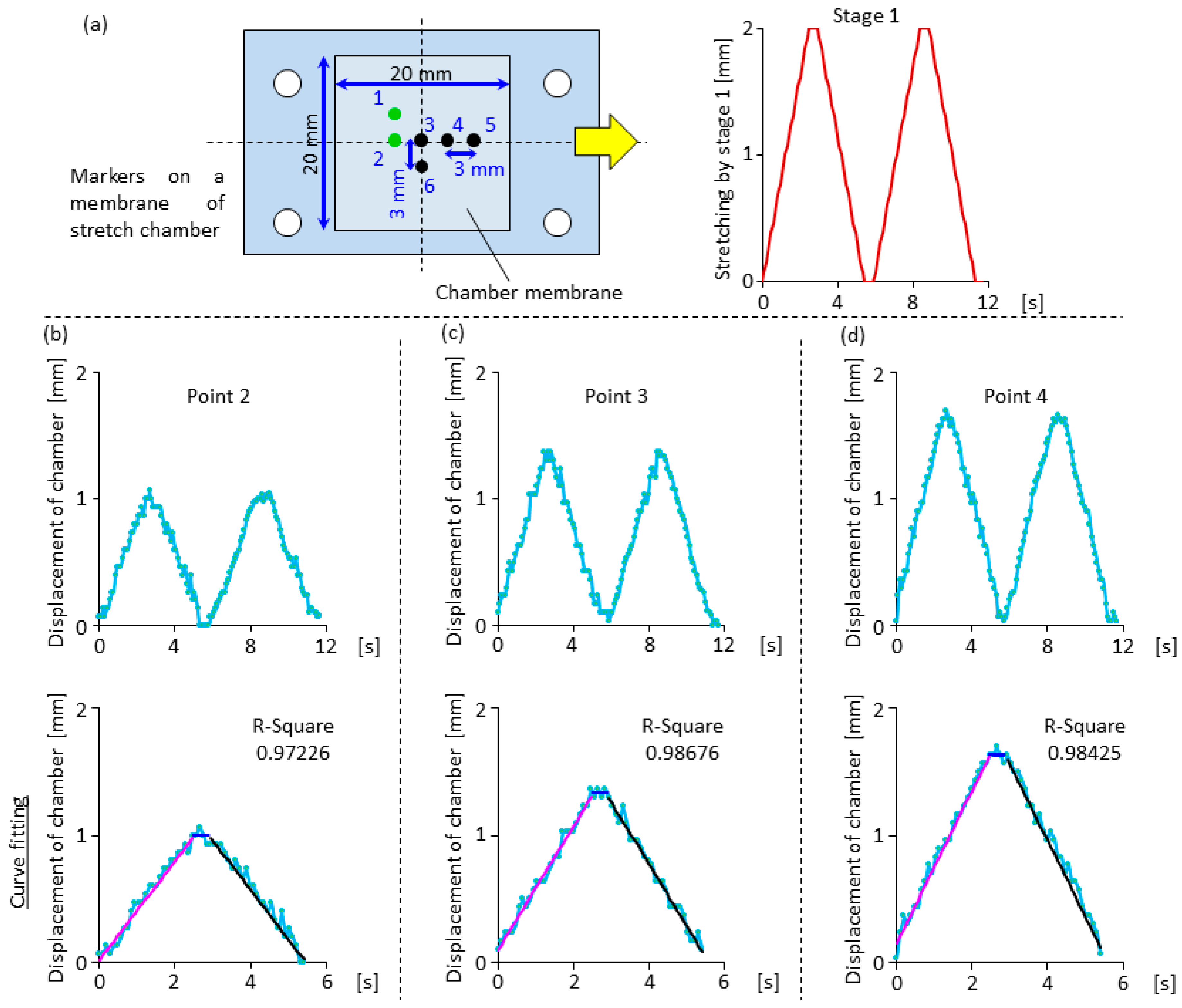
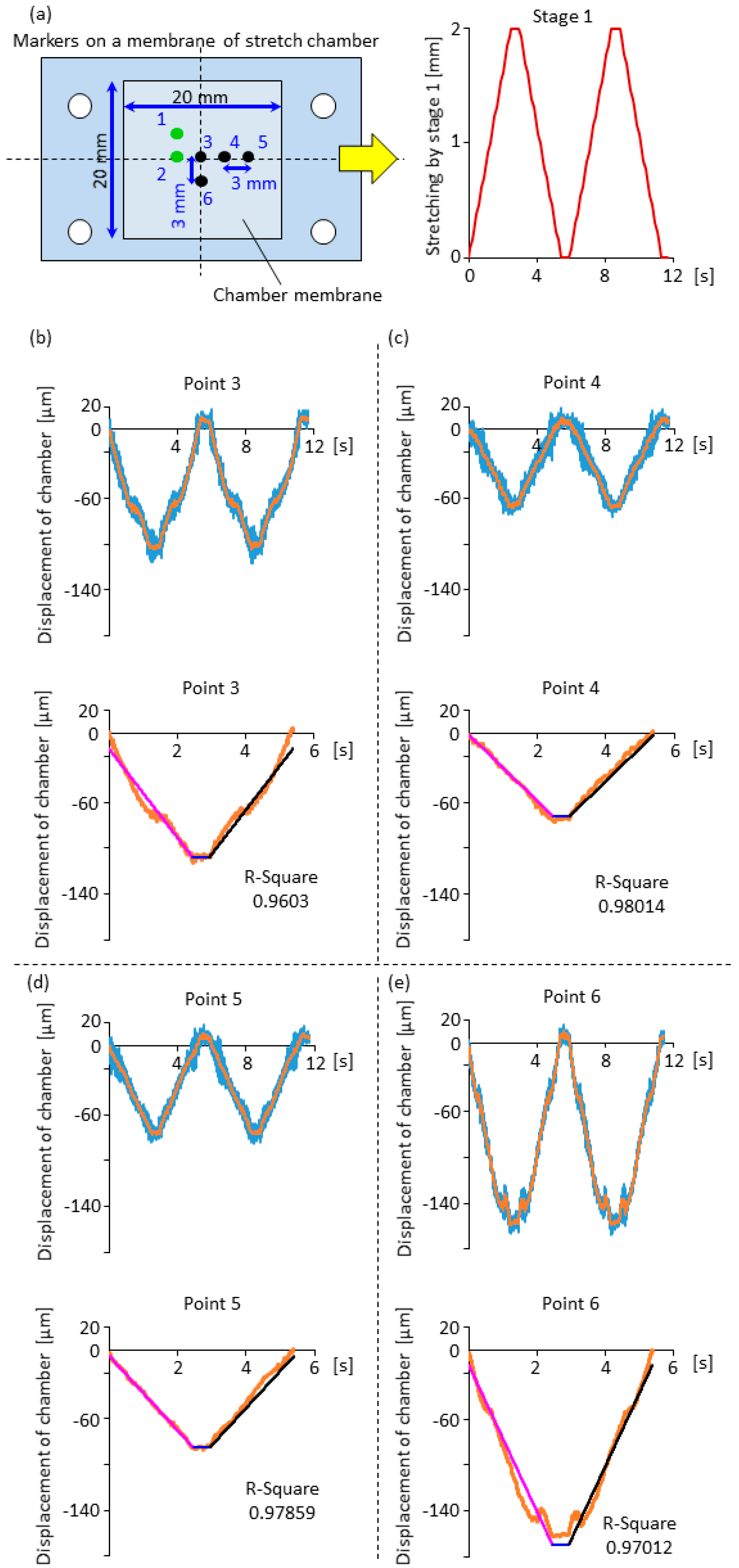
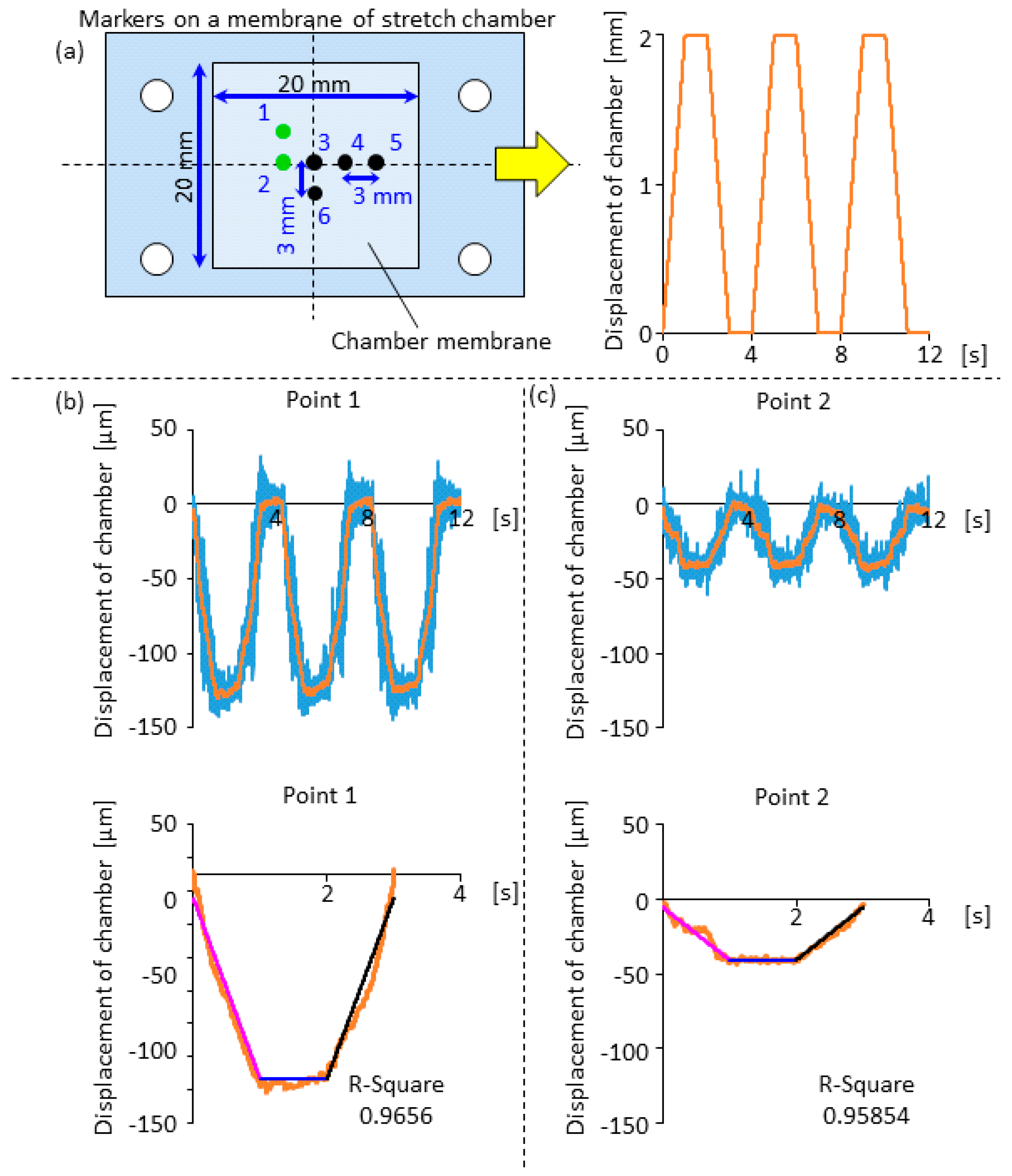
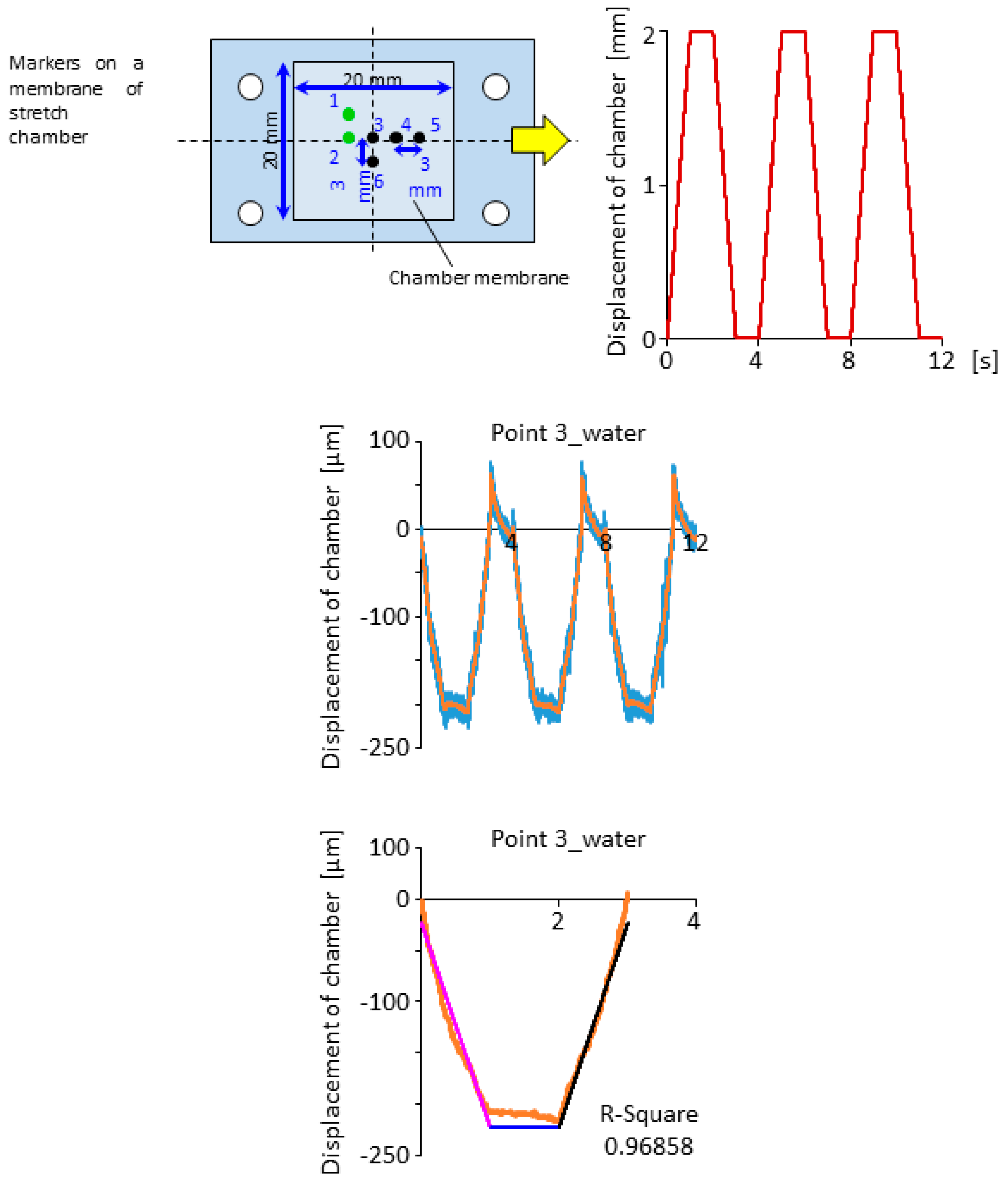
2.3. Displacement Characteristics in the Horizontal and Vertical Direction for the Design of Compensation Waveforms
- if(xd < M)
- y = A×xd + B;
- else if(xd >= M && N >= xd)
- y = K;
- elsey = D×xd + E
3. Results
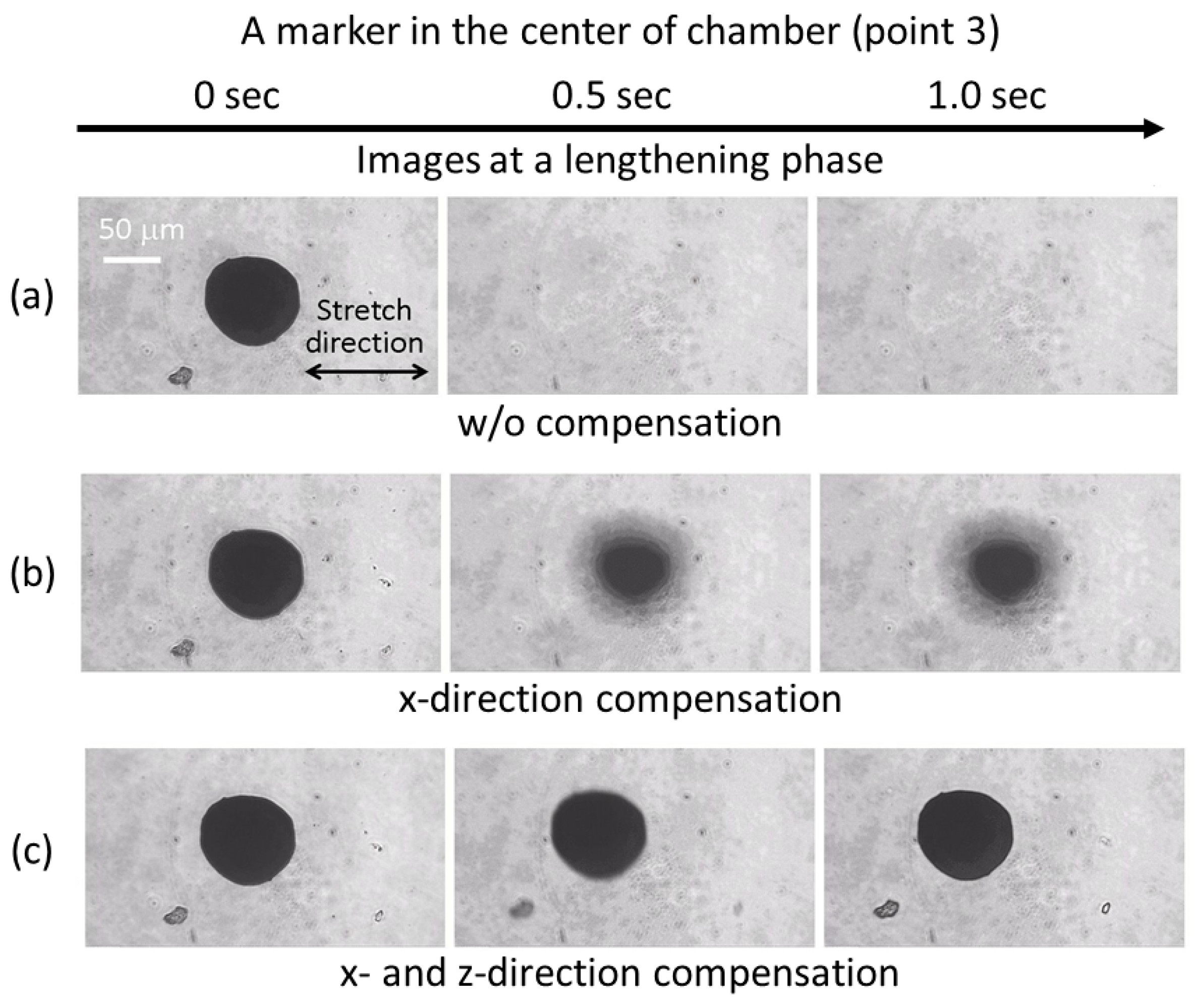
3.1. Effectiveness of the Compensation Waveforms
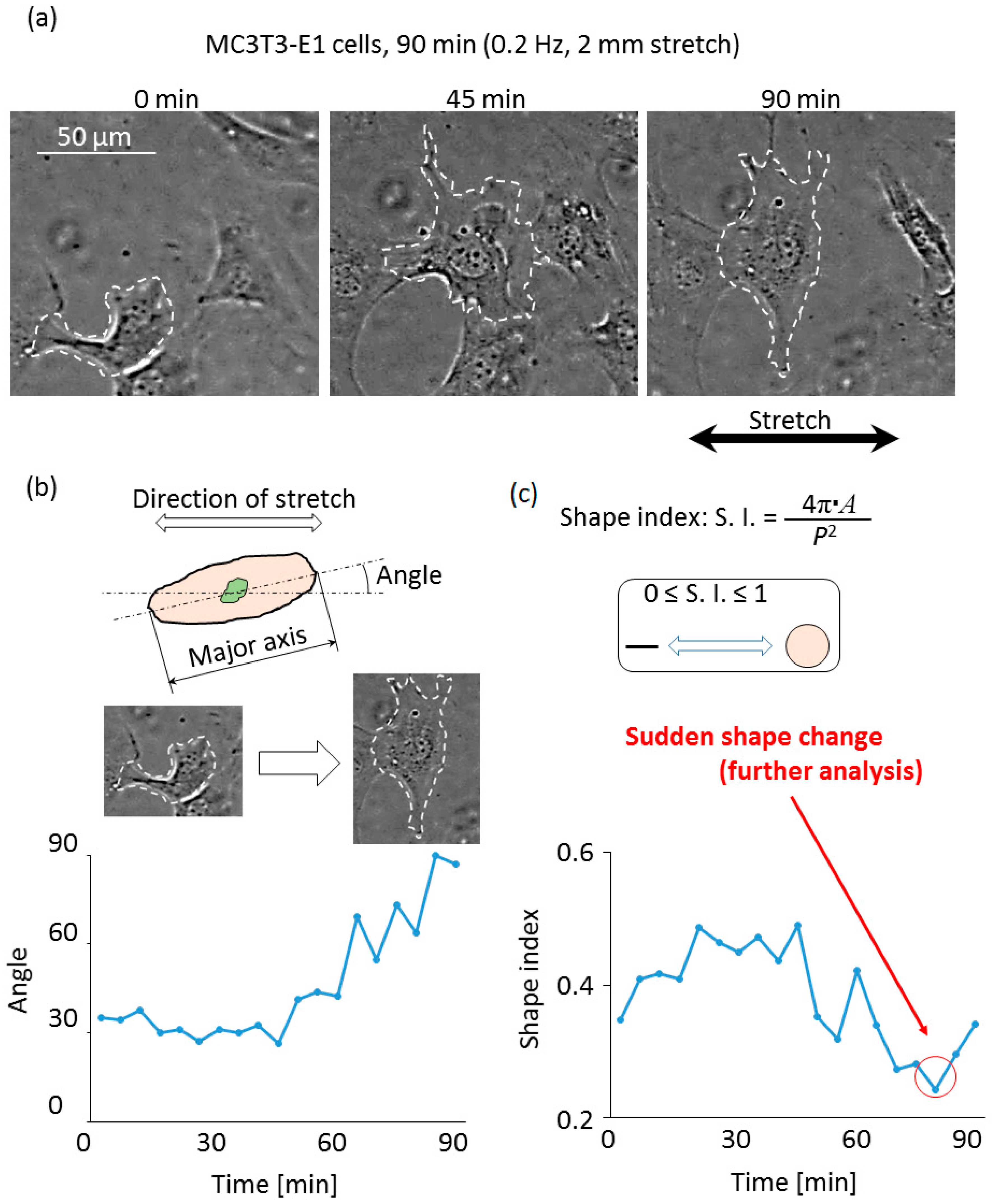
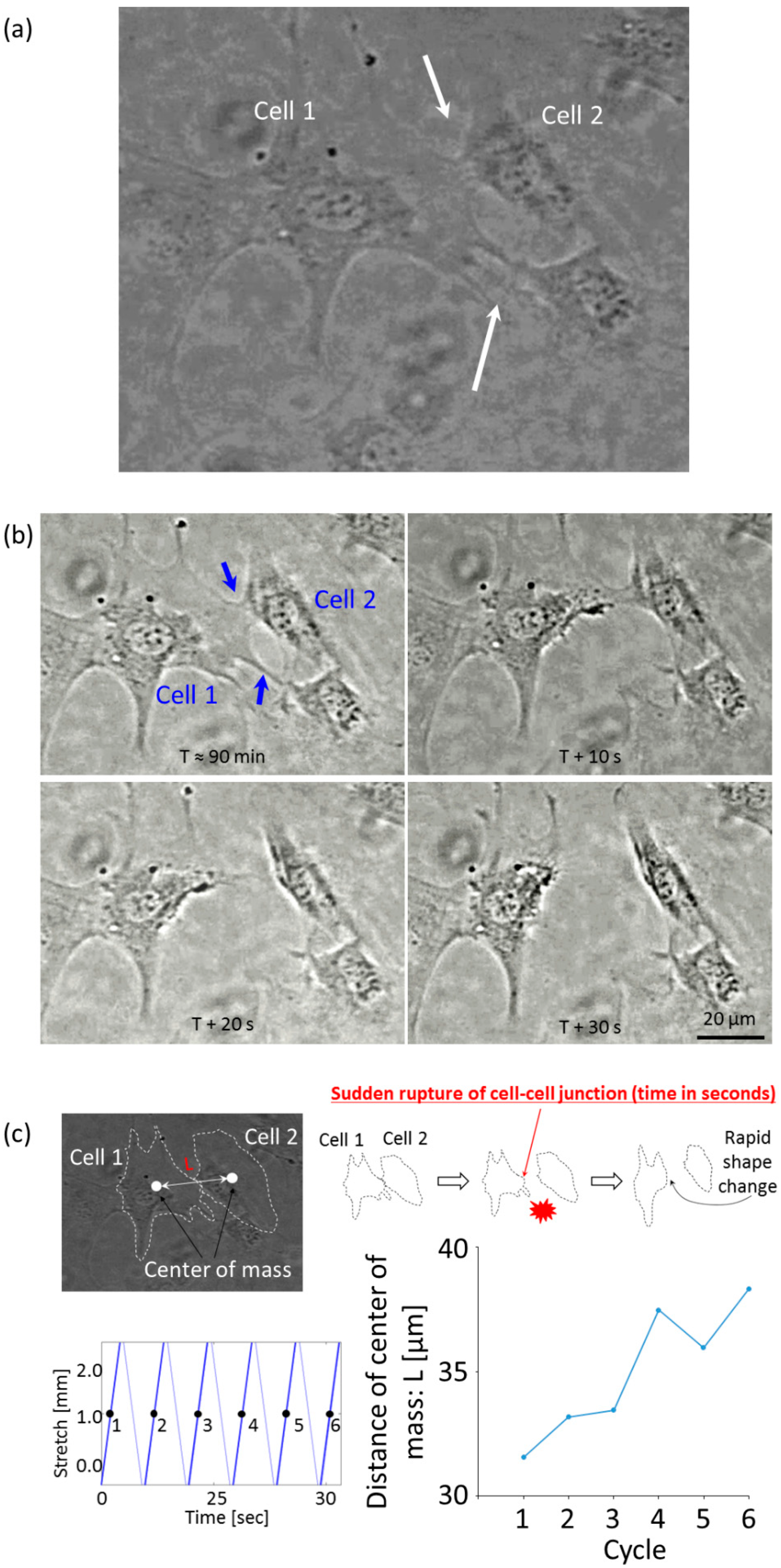
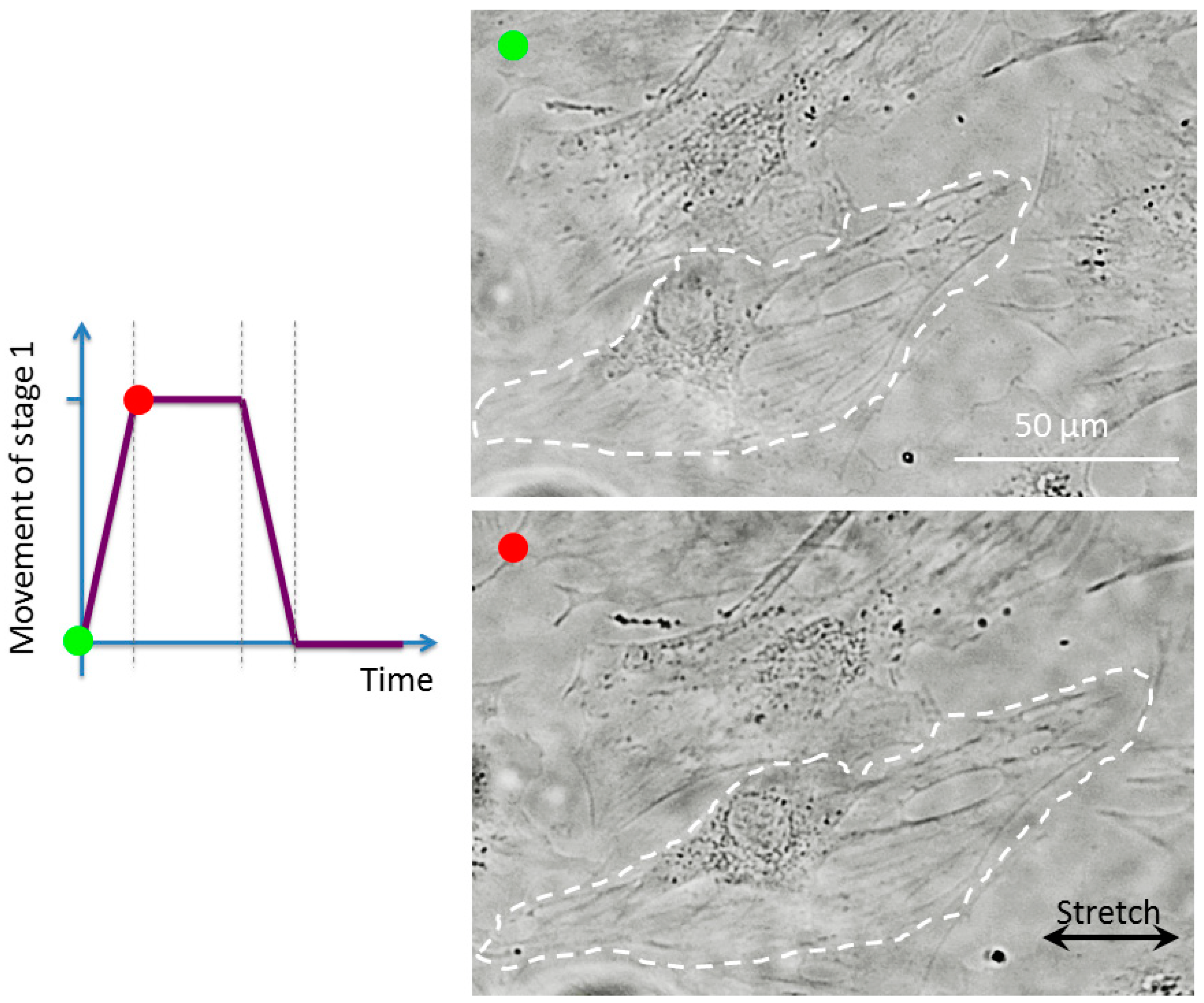
3.2. Cell Observation with a High Time Resolution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nerem, R.M.; Cornhill, J.F. Hemodynamics and atherogenesis. Atherosclerosis 1980, 36, 151–157. [Google Scholar] [CrossRef]
- Katanosaka, Y.; Bao, J.-H.; Komatsu, T.; Suemori, T.; Yamada, A.; Mohri, S.; Naruse, K. Analysis of cyclic-stretching responses using cell-adhesion-patterned cells. J. Biotechnol. 2008, 133, 82–89. [Google Scholar] [CrossRef]
- Kataoka, N.; Ujita, S.; Kimura, K.; Sato, M. The morphological responses of cultured bovine aortic endothelial cells to fluid-imposed shear stress under sparse and colony conditions. JSME Int. J. Ser. C 1998, 41, 76–82. [Google Scholar] [CrossRef]
- Naruse, K.; Yamada, T.; Sai, X.; Hamaguchi, M.; Sokabe, M. Pp125fak is required for stretch dependent morphological response of endothelial cells. Oncogene 1998, 17, 455–463. [Google Scholar] [CrossRef]
- Ngu, H.; Feng, Y.; Lu, L.; Oswald, S.J.; Longmore, G.D.; Yin, F.C.P. Effect of focal adhesion proteins on endothelial cell adhesion, motility and orientation response to cyclic strain. Ann. Biomed. Eng. 2010, 38, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Ballermann, B.J.; Dardik, A.; Eng, E.; Liu, A. Shear stress and the endothelium. Kidney Int. 1998, 54, S100–S108. [Google Scholar] [CrossRef] [Green Version]
- Jufri, N.F.; Mohamedali, A.; Avolio, A.; Baker, M.S. Mechanical stretch: Physiological and pathological implications for human vascular endothelial cells. Vasc. Cell 2015, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Woolf, N.; Rowles, P.M.; Pepper, J. Morphology of the endothelium over atherosclerotic plaques in human coronary arteries. Br. Heart J. 1988, 60, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Sokabe, M.; Naruse, K.; Sai, S.; Yamada, T.; Kawakami, K.; Inoue, M.; Murase, K.; Miyazu, M. Mechanotransduction and Intracellular Signaling Mechanisms of Stretch-Induced Remodeling in Endothelial Cells. Heart Vessels 1997, (Suppl. 12), 191–193. [Google Scholar]
- Childs, P.G.; Boyle, C.A.; Pemberton, G.D.; Nikukar, H.; Curtis, A.S.G.; Henriquez, F.L.; Dalby, M.J.; Reid, S. Use of nanoscale mechanical stimulation for control and manipulation of cell behaviour. Acta Biomater. 2016, 34, 159–168. [Google Scholar] [CrossRef]
- Marturano-Kruik, A.; Villasante, A.; Yaeger, K.; Ambati, S.R.; Chramiec, A.; Raimondi, M.T.; Vunjak-Novakovic, G. Biomechanical regulation of drug sensitivity in an engineered model of human tumor. Biomaterials 2017, 150, 150–161. [Google Scholar] [CrossRef]
- Sumpio, B.E.; Banes, A.J.; Link, G.W.; Iba, T. Modulation of endothelial cell phenotype by cyclic stretch: Inhibition of collagen production. J. Surg. Res. 1990, 48, 415–420. [Google Scholar] [CrossRef]
- Huang, W.; Sakamoto, N.; Hanamura, K.; Miyazawa, R.; Sato, M. Role of intercellular junctions in redistribution of focal adhesions and orientation of vascular endothelial cells exposed to cyclic stretching. Cell. Mol. Bioeng. 2011, 4, 368. [Google Scholar] [CrossRef]
- Ursekar, C.P.; Teo, S.-K.; Hirata, H.; Harada, I.; Chiam, K.-H.; Sawada, Y. Design and construction of an equibiaxial cell stretching system that is improved for biochemical analysis. PLoS ONE 2014, 9, e90665. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Tan, X.; Novitski, R.; Muqaddam, M.; List, P.; Williamson, L.; Fu, J.; Liu, A.P. Uniaxial cell stretching device for live-cell imaging of mechanosensitive cellular functions. Rev. Sci. Instrum. 2013, 84, 114304. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Sakamoto, N.; Miyazawa, R.; Sato, M. Role of paxillin in the early phase of orientation of the vascular endothelial cells exposed to cyclic stretching. Biochem. Biophys. Res. Commun. 2012, 418, 708–713. [Google Scholar] [CrossRef]
- Chen, K.; Vigliotti, A.; Bacca, M.; McMeeking, R.M.; Deshpande, V.S.; Holmes, J.W. Role of boundary conditions in determining cell alignment in response to stretch. Proc. Natl. Acad. Sci. USA 2018, 115, 986. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Bosetti, M.; Gatti, S.; Cannas, M. Dynamic fibroblast cultures: Response to mechanical stretching. Cell Adhes. Migr. 2007, 1, 124–128. [Google Scholar] [CrossRef]
- Kamble, H.; Barton, M.J.; Jun, M.; Park, S.; Nguyen, N.-T. Cell stretching devices as research tools: Engineering and biological considerations. Lab Chip 2016, 16, 3193–3203. [Google Scholar] [CrossRef]
- Bayas, M.V.; Leung, A.; Evans, E.; Leckband, D. Lifetime measurements reveal kinetic differences between homophilic cadherin bonds. Biophys. J. 2006, 90, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Diez, S.; Gerisch, G.; Anderson, K.; Müller-Taubenberger, A.; Bretschneider, T. Subsecond reorganization of the actin network in cell motility and chemotaxis. Proc. Natl. Acad. Sci. USA 2005, 102, 7601–7606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, G.; Huang, A.H.; Cai, Y.; Tanase, M.; Sheetz, M.P. Rigidity sensing at the leading edge through alphavbeta3 integrins and rptpalpha. Biophys. J. 2006, 90, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Pierres, A.; Prakasam, A.; Touchard, D.; Benoliel, A.-M.; Bongrand, P.; Leckband, D. Dissecting subsecond cadherin bound states reveals an efficient way for cells to achieve ultrafast probing of their environment. FEBS Lett. 2007, 581, 1841–1846. [Google Scholar] [CrossRef]
- Panorchan, P.; Thompson, M.S.; Davis, K.J.; Tseng, Y.; Konstantopoulos, K.; Wirtz, D. Single-molecule analysis of cadherin-mediated cell-cell adhesion. J. Cell Sci. 2006, 119, 66. [Google Scholar] [CrossRef] [PubMed]
- Kurazumi, H.; Kubo, M.; Ohshima, M.; Yamamoto, Y.; Takemoto, Y.; Suzuki, R.; Ikenaga, S.; Mikamo, A.; Udo, K.; Hamano, K.; et al. The effects of mechanical stress on the growth, differentiation, and paracrine factor production of cardiac stem cells. PLoS ONE 2011, 6, e28890. [Google Scholar] [CrossRef]
- Suzuki, M.; Naruse, K.; Asano, Y.; Okamoto, T.; Nishikimi, N.; Sakurai, T.; Nimura, Y.; Sokabe, M. Up-regulation of integrin β3expression by cyclic stretch in human umbilical endothelial cells. Biochem. Biophys. Res. Commun. 1997, 239, 372–376. [Google Scholar] [CrossRef]
- Na, S.; Trache, A.; Trzeciakowski, J.; Sun, Z.; Meininger, G.A.; Humphrey, J.D. Time-dependent changes in smooth muscle cell stiffness and focal adhesion area in response to cyclic equibiaxial stretch. Ann. Biomed. Eng. 2008, 36, 369–380. [Google Scholar] [CrossRef]
- Goldyn, A.M.; Rioja, B.A.; Spatz, J.P.; Ballestrem, C.; Kemkemer, R. Force-induced cell polarisation is linked to rhoa-driven microtubule-independent focal-adhesion sliding. J. Cell Sci. 2009, 122, 3644–3651. [Google Scholar] [CrossRef]
- Naruse, K.; Sai, X.; Yokoyama, N.; Sokabe, M. Uni-axial cyclic stretch induces c-src activation and translocation in human endothelial cells via sa channel activation. FEBS Lett. 1998, 441, 111–115. [Google Scholar] [CrossRef]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265. [Google Scholar] [CrossRef]
- Davies, P.F.; Tripathi, S.C. Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ. Res. 1993, 72, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Thoumine, O.; Ott, A. Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. J. Cell Sci. 1997, 110, 2109. [Google Scholar] [PubMed]
- Bongrand, P. Physical Basis of Cell-Cell Adhesion; CRC Press Inc: Boca Raton, FL, USA, 2018; p. 149. [Google Scholar]
- Gavara, N.; Roca-Cusachs, P.; Sunyer, R.; Farré, R.; Navajas, D. Mapping cell-matrix stresses during stretch reveals inelastic reorganization of the cytoskeleton. Biophys. J. 2008, 95, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Melcrová, A.; Pokorna, S.; Pullanchery, S.; Kohagen, M.; Jurkiewicz, P.; Hof, M.; Jungwirth, P.; Cremer, P.S.; Cwiklik, L. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016, 6, 38035. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, U.R.; Leidy, C.; Westh, P.; Peters, G.H. The effect of calcium on the properties of charged phospholipid bilayers. Biochim. Biophys. Acta 2006, 1758, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Xie, Y.; Yuan, B.; Xu, J.; Gong, P.; Jiang, X. A stretching device for imaging real-time molecular dynamics of live cells adhering to elastic membranes on inverted microscopes during the entire process of the stretch. Integr. Biol. 2010, 2, 288–293. [Google Scholar] [CrossRef]
- Huang, L.; Mathieu, P.S.; Helmke, B.P. A stretching device for high-resolution live-cell imaging. Ann. Biomed. Eng. 2010, 38, 1728–1740. [Google Scholar] [CrossRef]
- Huang, Y.; Nguyen, N.-T.; Lok, K.S.; Lee, P.P.F.; Su, M.; Wu, M.; Kocgozlu, L.; Ladoux, B. Multiarray cell stretching platform for high-magnification real-time imaging. Nanomedicine 2013, 8, 543–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minami, K.; Hayashi, T.; Sato, K.; Nakahara, T. Development of micro mechanical device having two-dimensional array of micro chambers for cell stretching. Biomed. Microdevices 2018, 20, 10. [Google Scholar] [CrossRef]
- Sato, K.; Kamada, S.; Minami, K. Development of microstretching device to evaluate cell membrane strain field around sensing point of mechanical stimuli. Int. J. Mech. Sci. 2010, 52, 251–256. [Google Scholar] [CrossRef]
- Huang, W.; Ahmad, B.; Kawahara, T. On-line tracking of living cell subjected to cyclic stretch. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3553–3556. [Google Scholar]
- Kato, T.; Ishiguro, N.; Iwata, H.; Kojima, T.; Ito, T.; Naruse, K. Up-regulation of cox2 expression by uni-axial cyclic stretch in human lung fibroblast cells. Biochem. Biophys. Res. Commun. 1998, 244, 615–619. [Google Scholar] [CrossRef]
- Inoh, H.; Ishiguro, N.; Sawazaki, S.-I.; Amma, H.; Miyazu, M.; Iwata, H.; Sokabe, M.; Naruse, K. Uni-axial cyclic stretch induces the activation of transcription factor nuclear factor κb in human fibroblast cells. FASEB J. 2002, 16, 405–407. [Google Scholar] [CrossRef]
- Hsu, H.-J.; Lee, C.-F.; Locke, A.; Vanderzyl, S.Q.; Kaunas, R. Stretch-induced stress fiber remodeling and the activations of jnk and erk depend on mechanical strain rate, but not fak. PLoS ONE 2010, 5, e12470. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Shalhoub, J.; Lim, C.S.; Gohel, M.S.; Davies, A.H. The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J. Vasc. Res. 2012, 49, 463–478. [Google Scholar] [CrossRef]
- Yu, S.; Duthaler, S.; Nelson, B.J. Autofocusing algorithm selection in computer microscopy. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, Edmonton, AB, Canada, 2–6 August 2005; pp. 70–76. [Google Scholar]
- Livne, A.; Bouchbinder, E.; Geiger, B. Cell reorientation under cyclic stretching. Nat. Commun. 2014, 5, 3938. [Google Scholar] [CrossRef] [PubMed]
- Barron, V.; Brougham, C.; Coghlan, K.; McLucas, E.; O’Mahoney, D.; Stenson-Cox, C.; McHugh, P.E. The effect of physiological cyclic stretch on the cell morphology, cell orientation and protein expression of endothelial cells. J. Mater. Sci. Mater. Med. 2007, 18, 1973–1981. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, T.; Hanamura, K.; Azuma, D.; Sakamoto, N.; Sato, M. Remodeling of endothelial cell nucleus exposed to three different mechanical stimuli. J. Biomech. Sci. Eng. 2008, 3, 63–74. [Google Scholar] [CrossRef]
- Jensen, E.C. Overview of live-cell imaging: Requirements and methods used. Anat. Rec. 2013, 296, 1–8. [Google Scholar] [CrossRef]
- Beier, H.T.; Ibey, B.L. Experimental comparison of the high-speed imaging performance of an EM-CCD and scmos camera in a dynamic live-cell imaging test case. PLoS ONE 2014, 9, e84614. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Kikuiri, T.; Hasegawa, T.; Matsuno, M.; Minamikawa, H.; Deyama, Y.; Suzuki, K. How much medium do you use for cell culture? Medium volume influences mineralization and osteoclastogenesis in vitro. Mol. Med. Rep. 2017, 16, 429–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubitscheck, U. Fluorescence Microscopy: From Principles to Biological Applications, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; p. 539. [Google Scholar]
- Ortiz de Zárate, J.M.; Sengers, J.V. Chapter 3—Fluctuations in fluids in thermodynamic equilibrium. In Hydrodynamic Fluctuations in Fluids and Fluid Mixtures; Ortiz de Zárate, J.M., Sengers, J.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 39–62. [Google Scholar]
- Stark, F.O.; Falender, J.R.; Wright, A.P. 9.3—Silicones. In Comprehensive Organometallic Chemistry; Wilkinson, G., Stone, F.G.A., Abel, E.W., Eds.; Pergamon Press: Oxford, UK, 1982; pp. 305–363. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Zhang, S.; Ahmad, B.; Kawahara, T. Three-Motorized-Stage Cyclic Stretching System for Cell Monitoring Based on Chamber Local Displacement Waveforms. Appl. Sci. 2019, 9, 1560. https://doi.org/10.3390/app9081560
Huang W, Zhang S, Ahmad B, Kawahara T. Three-Motorized-Stage Cyclic Stretching System for Cell Monitoring Based on Chamber Local Displacement Waveforms. Applied Sciences. 2019; 9(8):1560. https://doi.org/10.3390/app9081560
Chicago/Turabian StyleHuang, Wenjing, Sheng Zhang, Belal Ahmad, and Tomohiro Kawahara. 2019. "Three-Motorized-Stage Cyclic Stretching System for Cell Monitoring Based on Chamber Local Displacement Waveforms" Applied Sciences 9, no. 8: 1560. https://doi.org/10.3390/app9081560
APA StyleHuang, W., Zhang, S., Ahmad, B., & Kawahara, T. (2019). Three-Motorized-Stage Cyclic Stretching System for Cell Monitoring Based on Chamber Local Displacement Waveforms. Applied Sciences, 9(8), 1560. https://doi.org/10.3390/app9081560




