The Wettability and Numerical Model of Different Silicon Microstructural Surfaces
Abstract
:1. Introduction
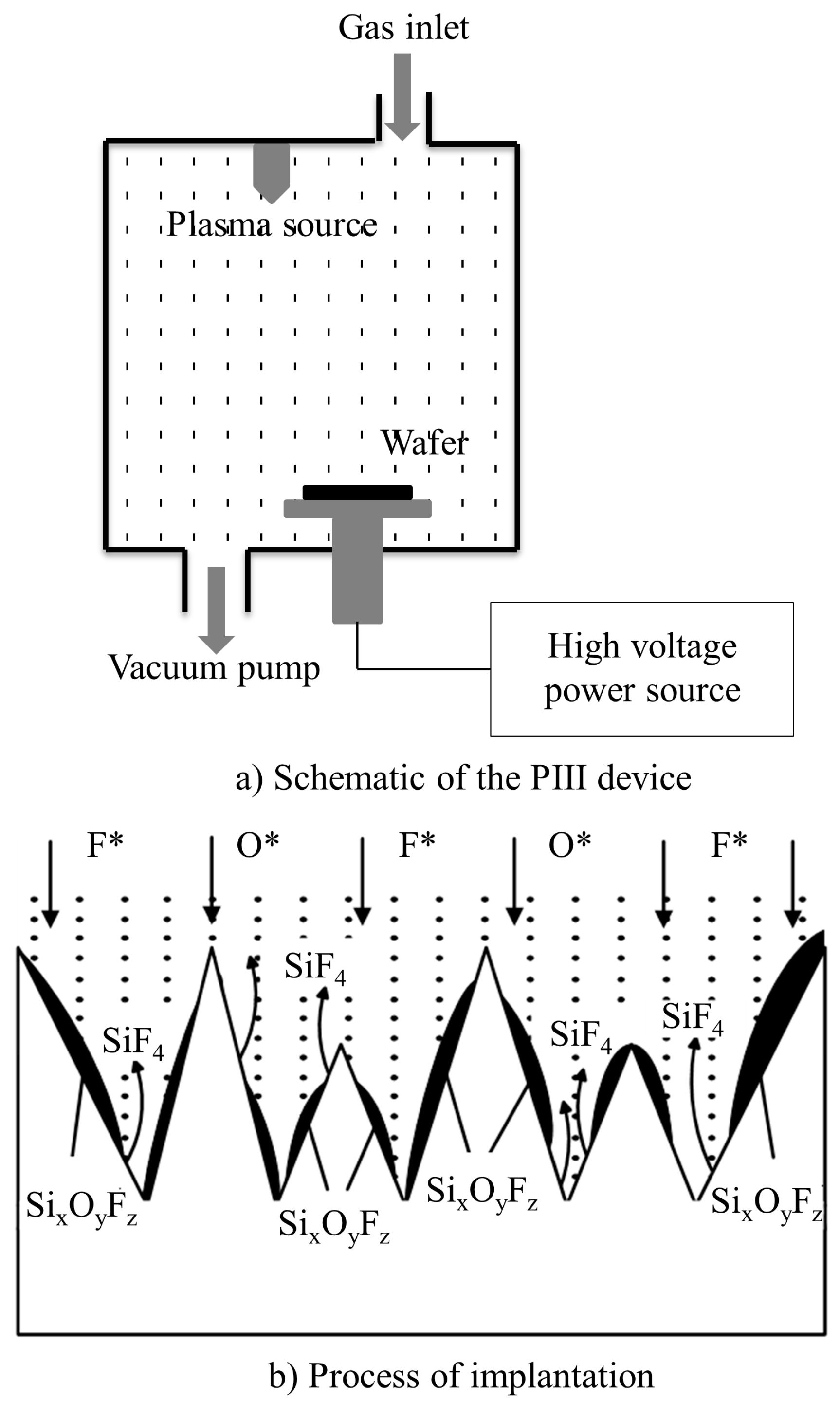
2. Surface Properties of Silicon Prepared by PIII Technology
2.1. Preparation and Characterization of Microstructural Silicon Surfaces
2.2. Static Contact Angle of the Sample Surfaces
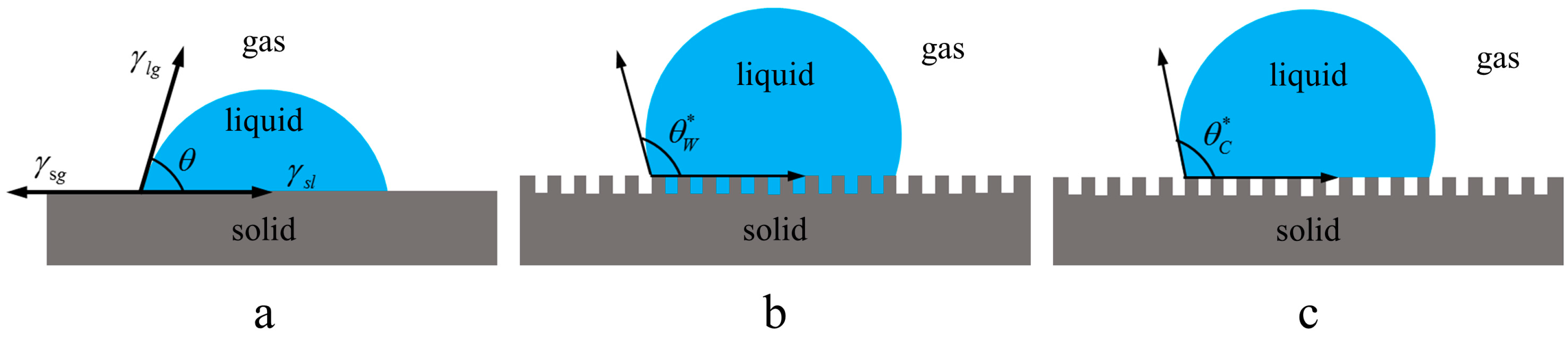
2.3. Theoretical Value of Static Contact Angle
3. Numerical Methods and Setup
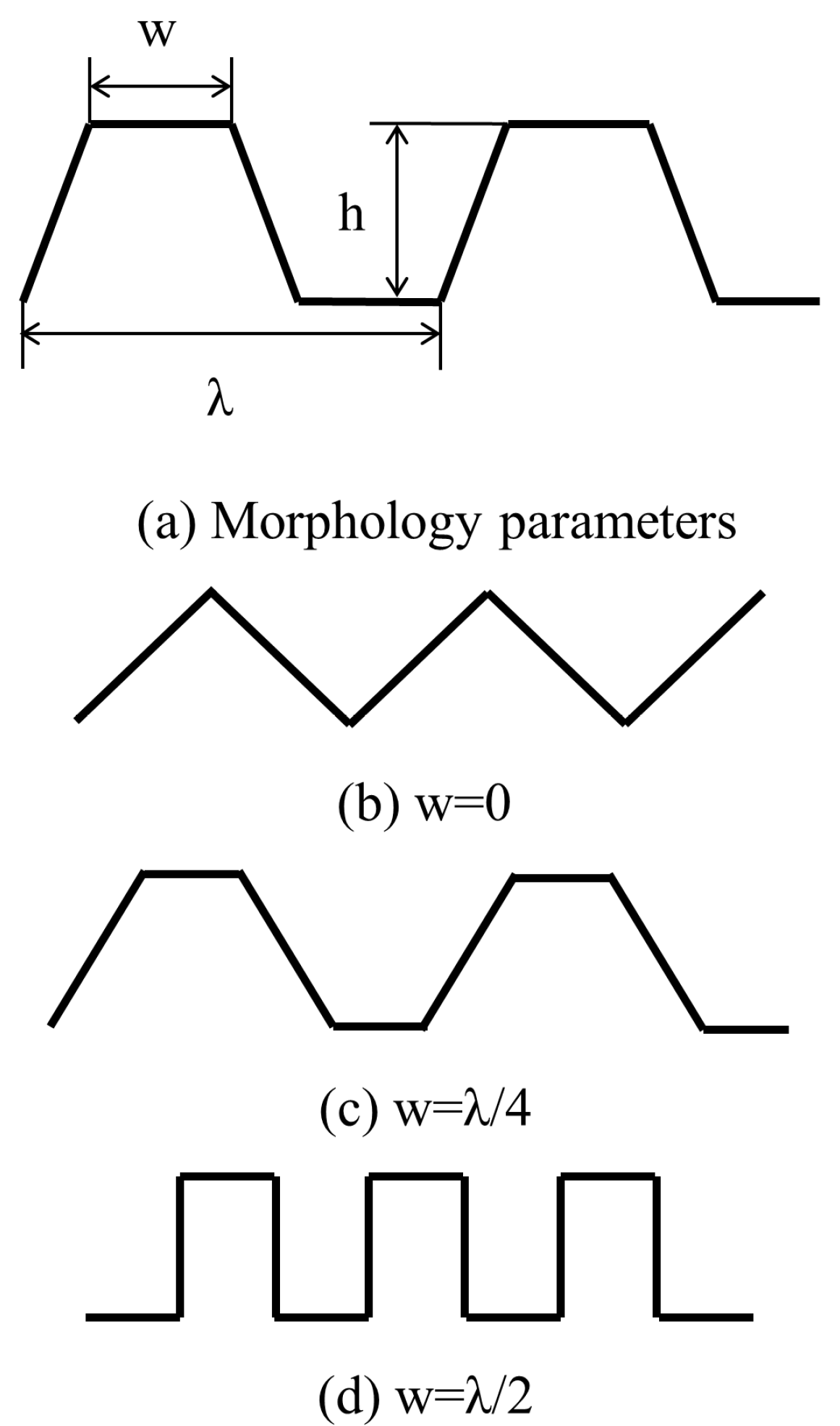
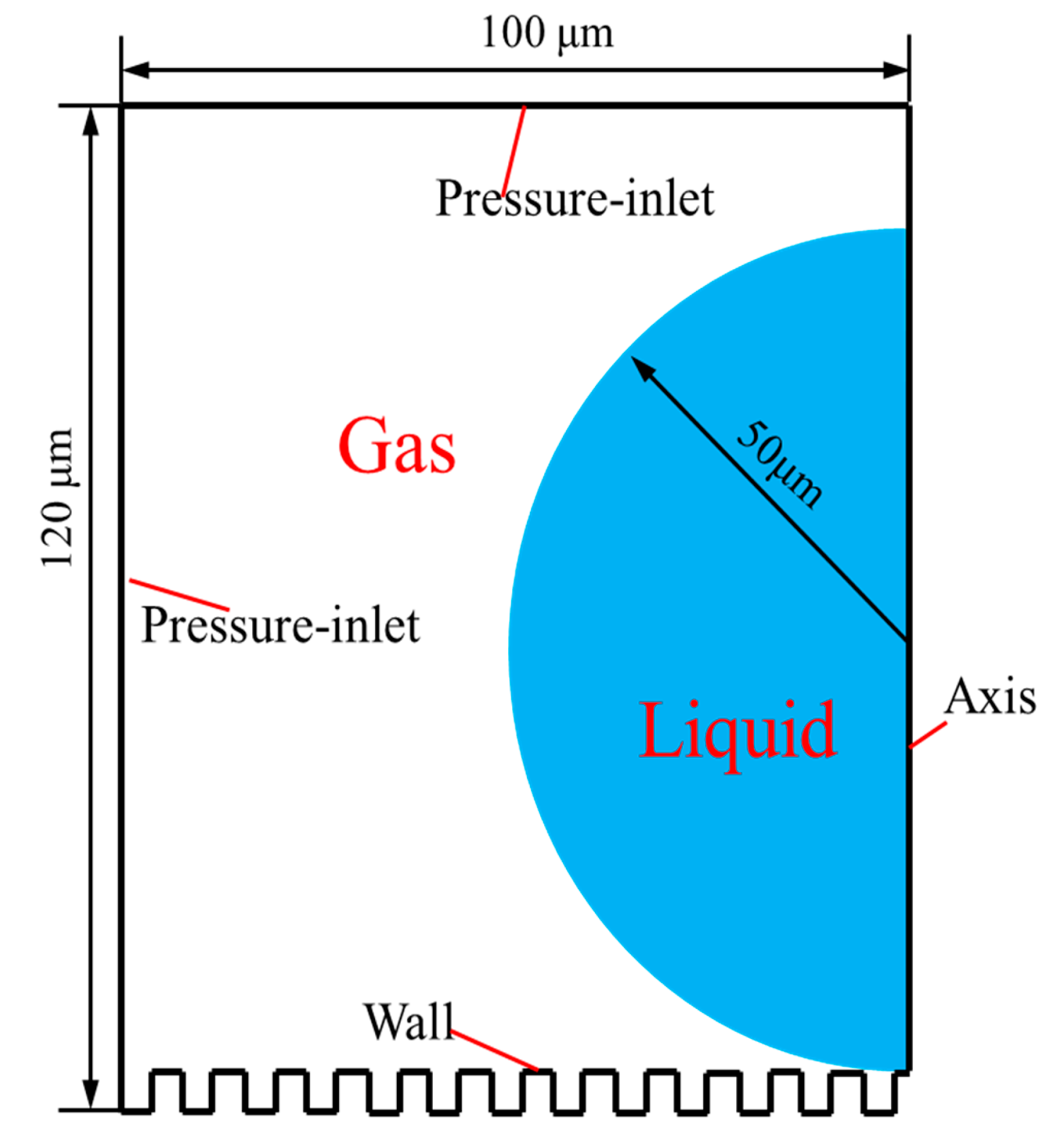
3.1. Establishment of the Parameterized Geometric Model
3.2. Geometric Model
3.3. VOF Model
3.4. Initial Conditions and Boundary Conditions
- The water droplet was circle in shape and located above the upper wall of the parameterized microstructure. The water droplet position was shown in Figure 9.
- The microstructure channels were filled with air, without water.
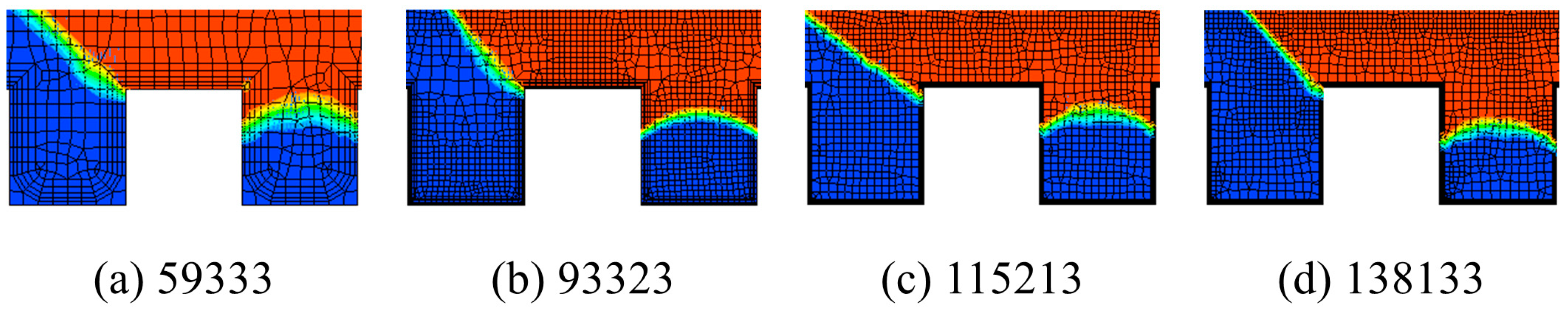
3.5. Grid Sensitivity Analysis and Model Verification
4. Analysis of the Numerical Results
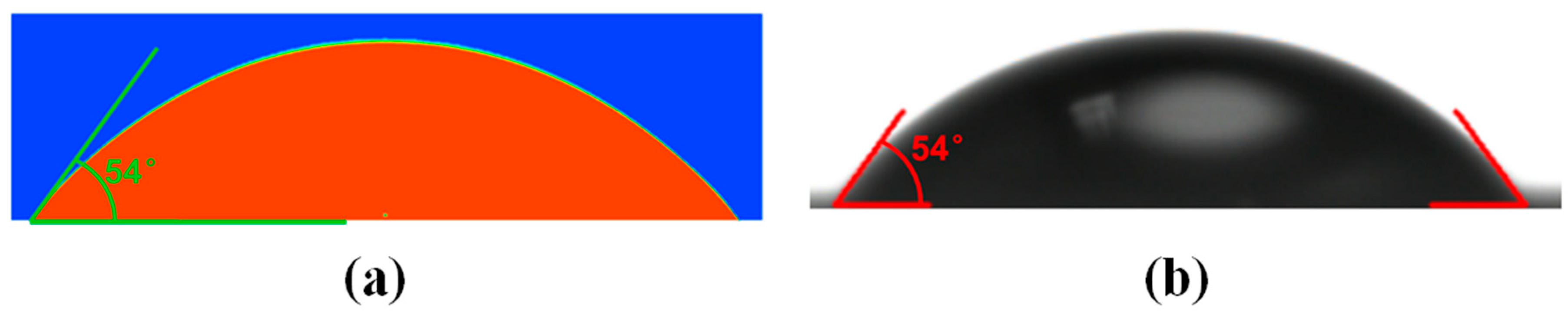
4.1. Static Contact Angle of the Smooth Surface
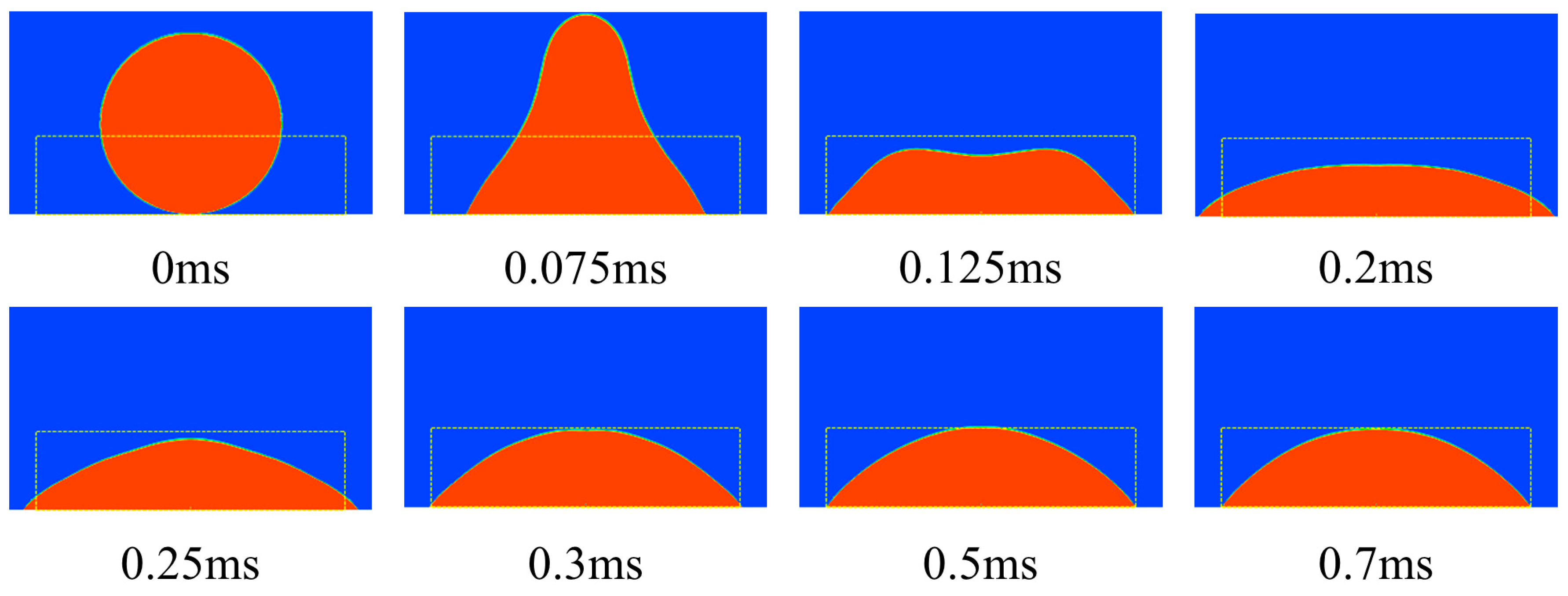
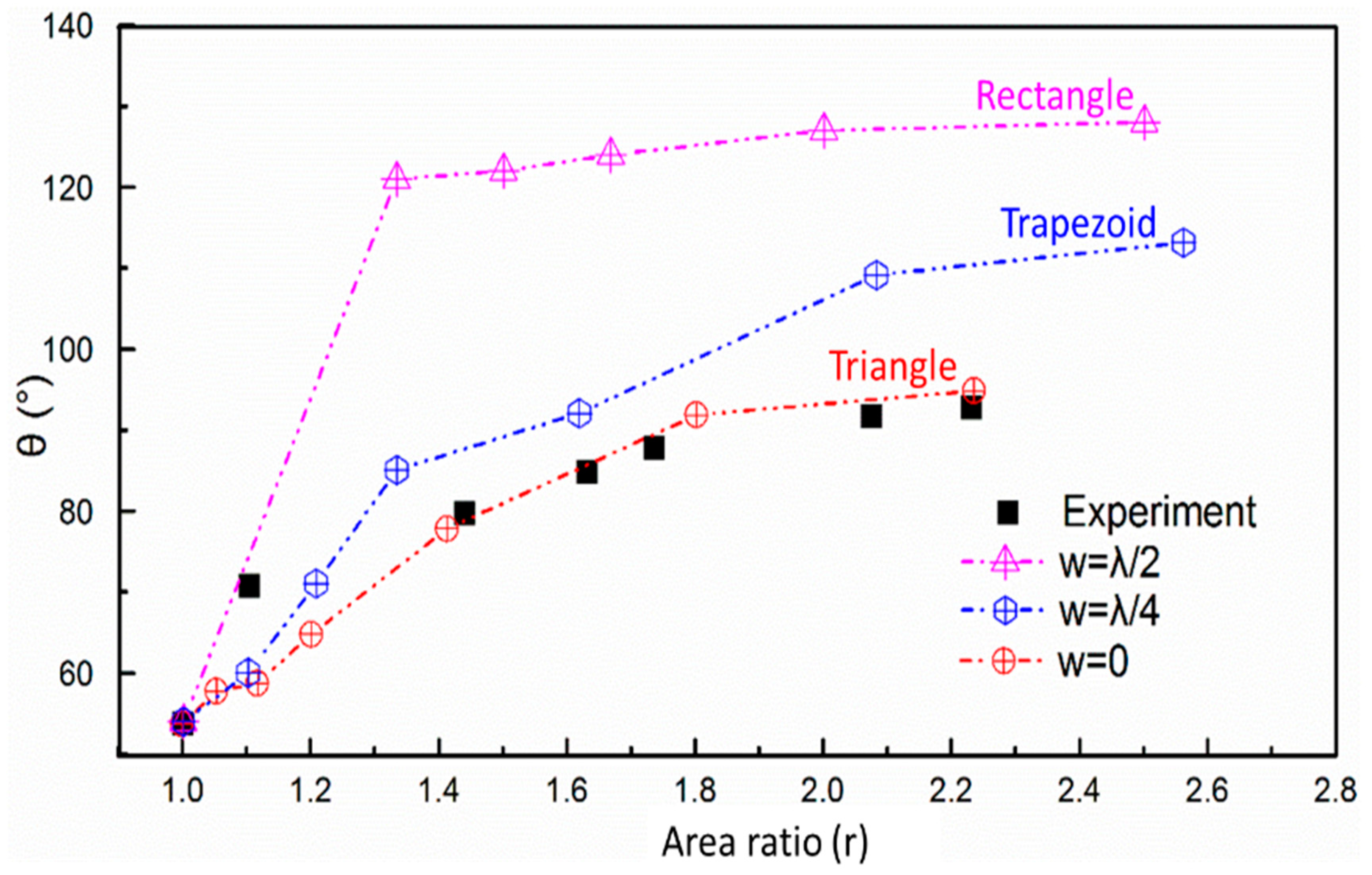
4.2. Static Contact Angle of the Microstructural Surface
4.3. The Simplified Waveform of the Microstructural Surface
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| r | ratio of the true surface area to the projected |
| fs | percent of liquid–solid contact area |
| Ra | surface roughness, μm |
| F | fluid volume fraction |
| h | height of the structure, μm |
| λ | width of the structure, μm |
| w | width of the morphology, μm |
| S | source term |
| V | scalar speed |
| vector speed | |
| A | area |
| σ | surface-tension coefficient |
| Greek letters | |
| contact angle depending on the liquid surface and vapor | |
| surface tension at solid–vapor interface | |
| surface tension at solid–liquid interface | |
| surface tension at liquid–vapor interface | |
| apparent contact angle | |
| Wenzel’s liquid–solid contact angle | |
| Cassie–Baxter’s liquid–solid contact angle | |
| Subscripts, superscripts, and acronyms | |
| PIII | plasma immersion ion implantation |
| VOF | volume of fluid |
| SEM | scanning electron microscopy |
| AFM | atomic force microscopy |
| C | Cassie and Baxter state |
| W | Wenzel state |
| l | liquid |
| s | solid |
| g | gas |
| a | air |
| w | water |
References
- Attinger, D.; Frankiewicz, C.; Betz, A.R.; Schutzius, T.M.; Ganguly, R.; Das, A.; Kim, C.J.; Megaridis, C.M. Surface engineering for phase change heat transfer: A review. MRS Energy Sustain. 2014, 1. [Google Scholar] [CrossRef]
- Quan, Y.Y.; Zhang, L.Z.; Qi, R.H.; Cai, R.R. Self-cleaning of surfaces: The role of surface wettability and dust types. Sci. Rep. 2016, 6, 38239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Yang, J.; Yang, E.H. Self-cleaning engineered cementitious composites. Cem. Concr. Compos. 2015, 64, 74–83. [Google Scholar] [CrossRef]
- Li, W.; Kang, Z. Fabrication of corrosion resistant superhydrophobic surface with self-cleaning property on magnesium alloy and its mechanical stability. Surf. Coat. Technol. 2014, 253, 205–213. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Gao, R.; Wang, J.; Yang, W.; Liu, L. One-step method for the fabrication of superhydrophobic surface on magnesium alloy and its corrosion protection, antifouling performance. Corros. Sci. 2014, 80, 177–183. [Google Scholar] [CrossRef]
- Lyu, S.; Dang, C.N.; Kim, D.; Hwang, W.; Yoon, B. Experimental drag reduction study of super-hydrophobic surface with dual-scale structures. Appl. Surf. Sci. 2013, 286, 206–211. [Google Scholar] [CrossRef]
- Kang, B.J.; Chang, K.L.; Oh, J.H. All-inkjet-printed electrical components and circuit fabrication on a plastic substrate. Microelectron. Eng. 2012, 97, 251–254. [Google Scholar] [CrossRef]
- Ogihara, H.; Xie, J.; Saji, T. Factors determining wettability of superhydrophobic paper prepared by spraying nanoparticle suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 35–41. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Qu, M.; Feng, J.; He, J.; Yao, Y.; Liu, S. Facile fabrication of superhydrophobic fabric with mechanical durable property. Nanosci. Nanotechnol. Lett. 2017, 9, 592–595. [Google Scholar] [CrossRef]
- Wang, Z.; Elimelech, M.; Lin, S. Environmental applications of interfacial materials with special wettability. Environ. Sci. Technol. 2016, 50, 2132. [Google Scholar] [CrossRef] [PubMed]
- Betz, A.R.; Jenkins, J.; Kim, C.J.; Attinger, D. Boiling heat transfer on superhydrophilic, superhydrophobic, and;superbiphilic surfaces. Int. J. Heat Mass Transf. 2013, 57, 733–741. [Google Scholar] [CrossRef]
- Qi, B.; Wei, J.; Li, Z.; Xu, H. A fractal dropwise condensation heat transfer model including the effects of contact angle and drop size distribution. Int. J. Heat Mass Transf. 2015, 83, 259–272. [Google Scholar] [CrossRef]
- Chau, T.T.; Bruckard, W.J.; Koh, P.T.L.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv Colloid Interface Sci 2009, 150, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Akyildiz, H.I.; Padbury, R.P.; Parsons, G.N.; Jur, J.S. Temperature and exposure dependence of hybrid organic-inorganic layer formation by sequential vapor infiltration into polymer fibers. Langmuir 2012, 28, 15697–15704. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.S.; Chiang, Y.H.; Li, M.H.; Guo, T.F.; Chen, P. Research update: Hybrid organic-inorganic perovskite (hoip) thin films and solar cells by vapor phase reaction. APL Mater. 2016, 4, 1035. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, X.; Bai, Y.; Padture, N.P.; Huang, J. Progress in tandem solar cells based on hybrid organic-inorganic perovskites. Adv. Energy Mater. 2017, 7, 1602400. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, H.; Zhou, Z. Superhydrophobic surfaces created by a one-step solution-immersion process and their drag-reduction effect on water. RSC Adv. 2015, 5, 18909–18914. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, H.; Wang, Z.; Liu, Y. Superhydrophobic aluminum alloy surface: Fabrication, structure, and corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 319–325. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C. Analysis of anti-condensation mechanism on superhydrophobic anodic aluminum oxide surface. Appl. Therm. Eng. 2013, 58, 664–669. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Shagieva, F.M.; Domantovsky, A.G.; Boinovich, L.B. Nanosecond laser micro- and nanotexturing for the design of a superhydrophobic coating robust against long-term contact with water, cavitation, and abrasion. Appl. Surf. Sci. 2015, 332, 513–517. [Google Scholar] [CrossRef]
- Xin, B.; Hao, J. Reversibly switchable wettability. Chem. Soc. Rev. 2010, 39, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Lv, C.; Zhang, X. Freezing of sessile water droplets on surfaces with various roughness and wettability. Appl. Phys. Lett. 2014, 104, 117. [Google Scholar] [CrossRef]
- Song, D.; Song, B.; Hu, H.; Du, X.; Ma, Z. Contact angle and impinging process of droplets on partially grooved hydrophobic surfaces. Appl. Therm. Eng. 2015, 85, 356–364. [Google Scholar] [CrossRef]
- Das, S.; Kumar, D.S.; Bhaumik, S. Experimental study of nucleate pool boiling heat transfer of water on silicon oxide nanoparticle coated copper heating surface. Appl. Therm. Eng. 2016, 96, 555–567. [Google Scholar] [CrossRef]
- Yang, L.X.; Chao, Y.M.; Jia, L.; Li, C.B. Wettability and boiling heat transfer study of black silicon surface produced using the plasma immersion ion implantation method. Appl. Therm. Eng. 2016, 99, 253–261. [Google Scholar] [CrossRef]
- Bell, M.S.; Azar, S.; Fichthorn, K.A.; Ali, B. Effects of hierarchical surface roughness on droplet contact angle. Langmuir 2015, 31, 6752–6762. [Google Scholar] [CrossRef]
- Hashim, J.; Looney, L.; Hashmi, M.S.J. The wettability of sic particles by molten aluminium alloy. J. Mater. Process. Tech. 2001, 119, 324–328. [Google Scholar] [CrossRef]
- Liu, J. The Research of Plasma Immersion Ion Implantation System and Its Application; Lanzhou University: Lanzhou, China, 2012. [Google Scholar]
- Xia, Y.; Liu, B.; Liu, J.; Shen, Z.; Li, C. A novel method to produce black silicon for solar cells. Sol. Energy 2011, 85, 1574–1578. [Google Scholar] [CrossRef]
- Rahman, M.A.; Jacobi, A.M. Experimental investigation of wetting anisotropy on microgrooved brass surfaces. Procedia Eng. 2014, 90, 611–617. [Google Scholar] [CrossRef]
- Rahman, M.A.; Jacobi, A.M. Experimental study on frosting/defrosting characteristics of microgrooved metal surfaces. Int. J. Refrig. 2015, 50, 44–56. [Google Scholar] [CrossRef]
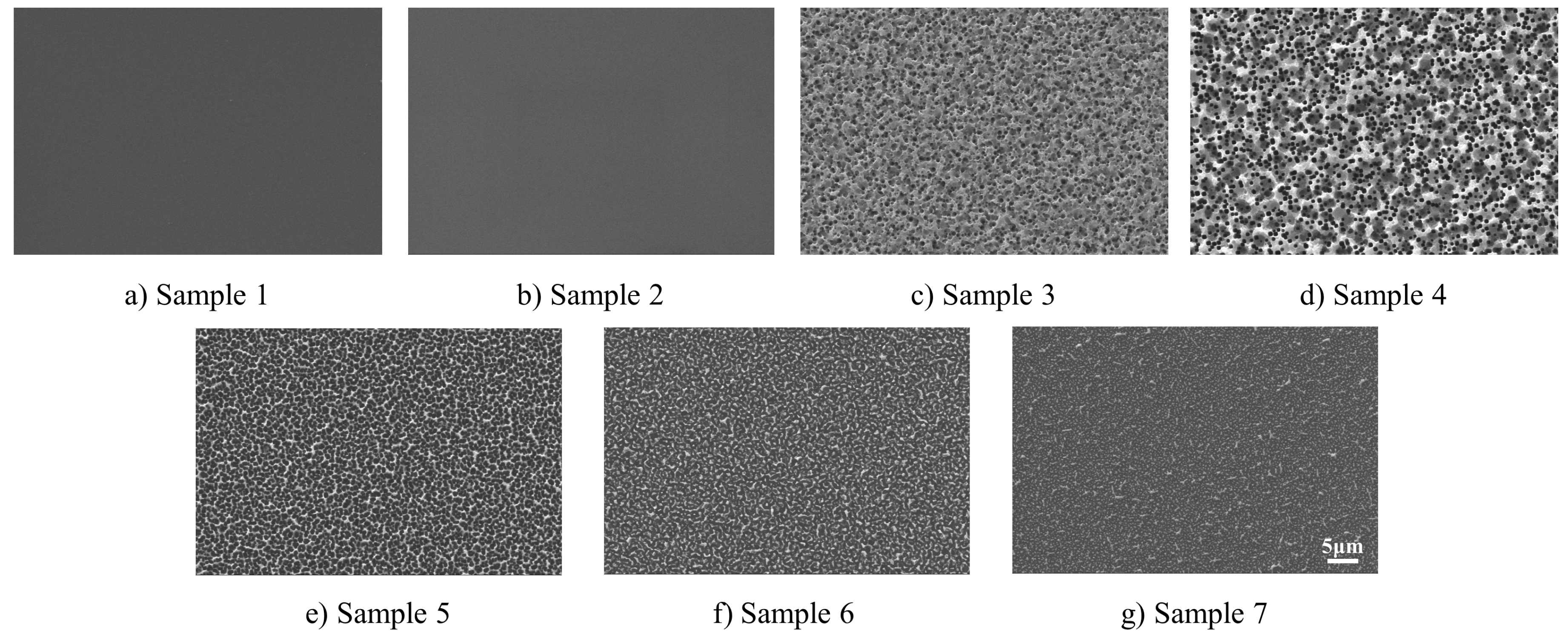
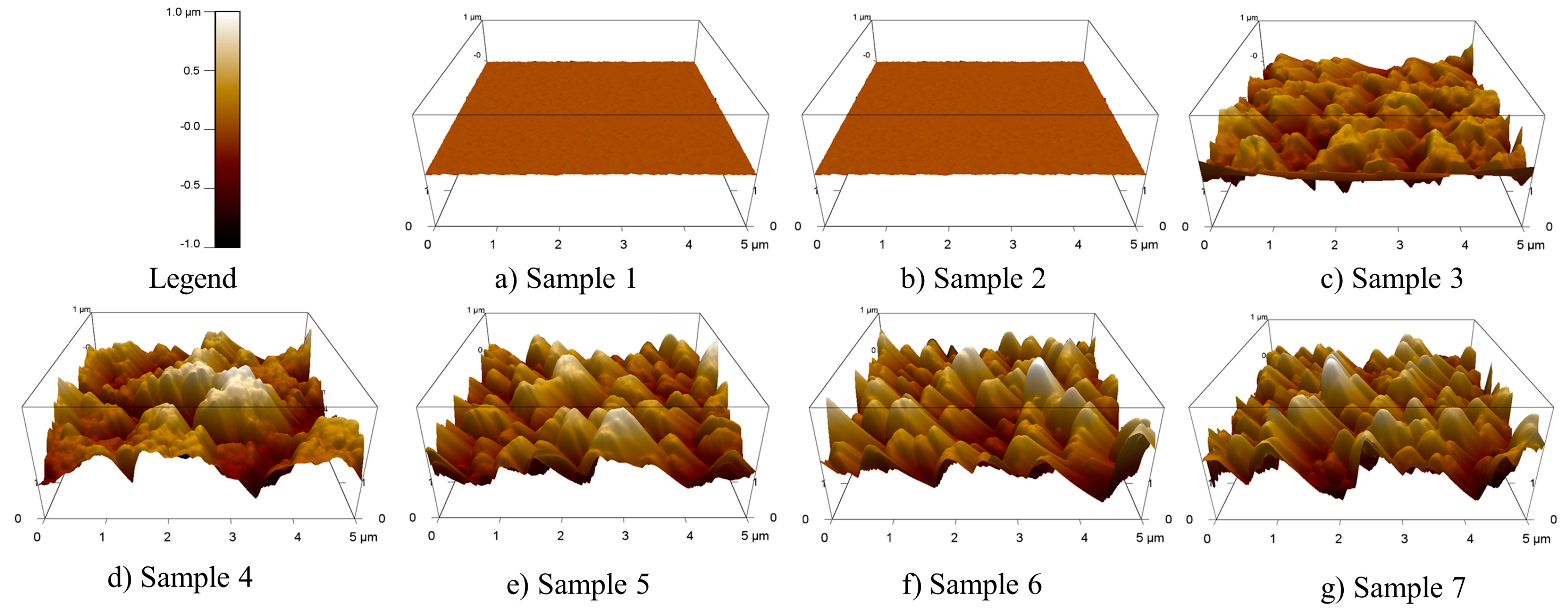
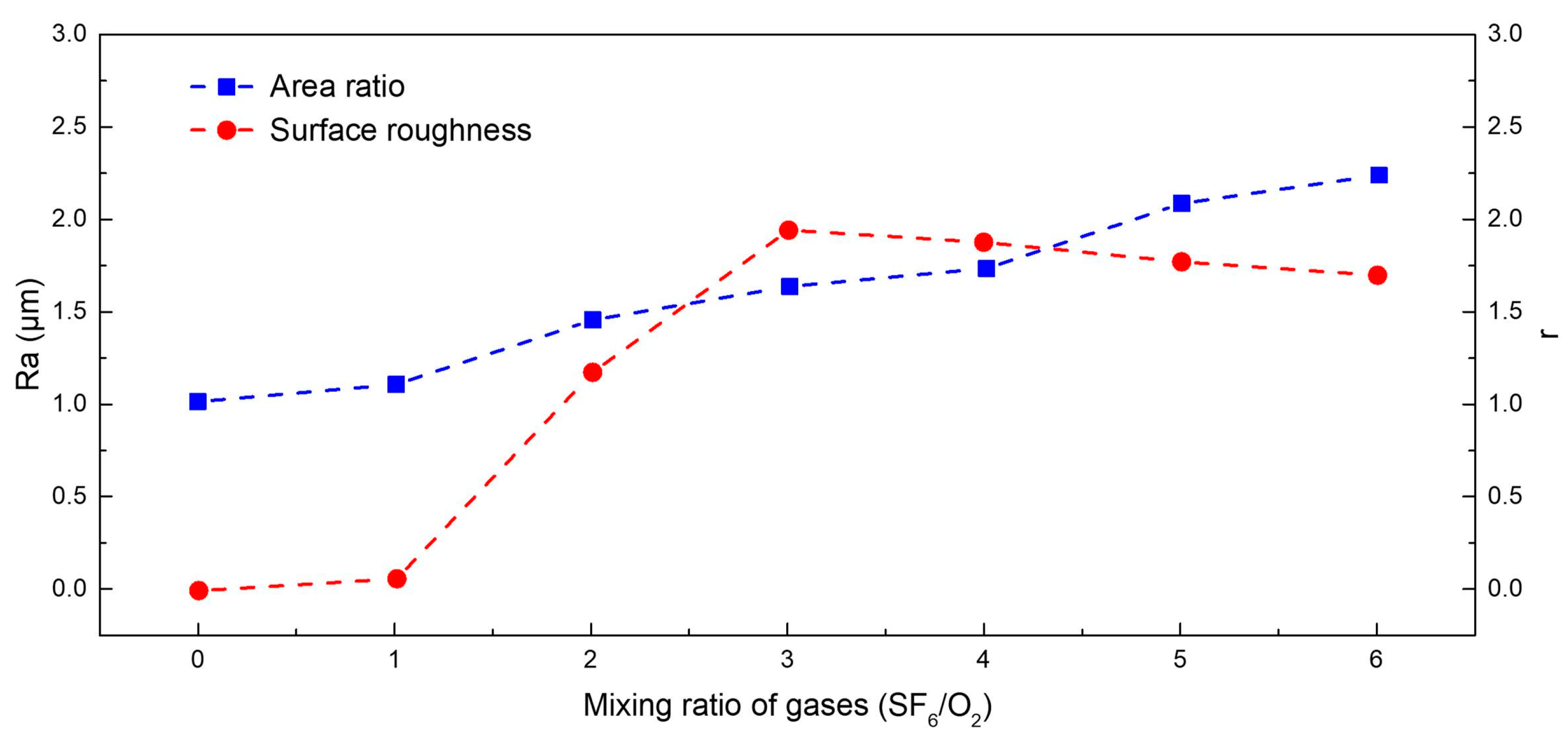
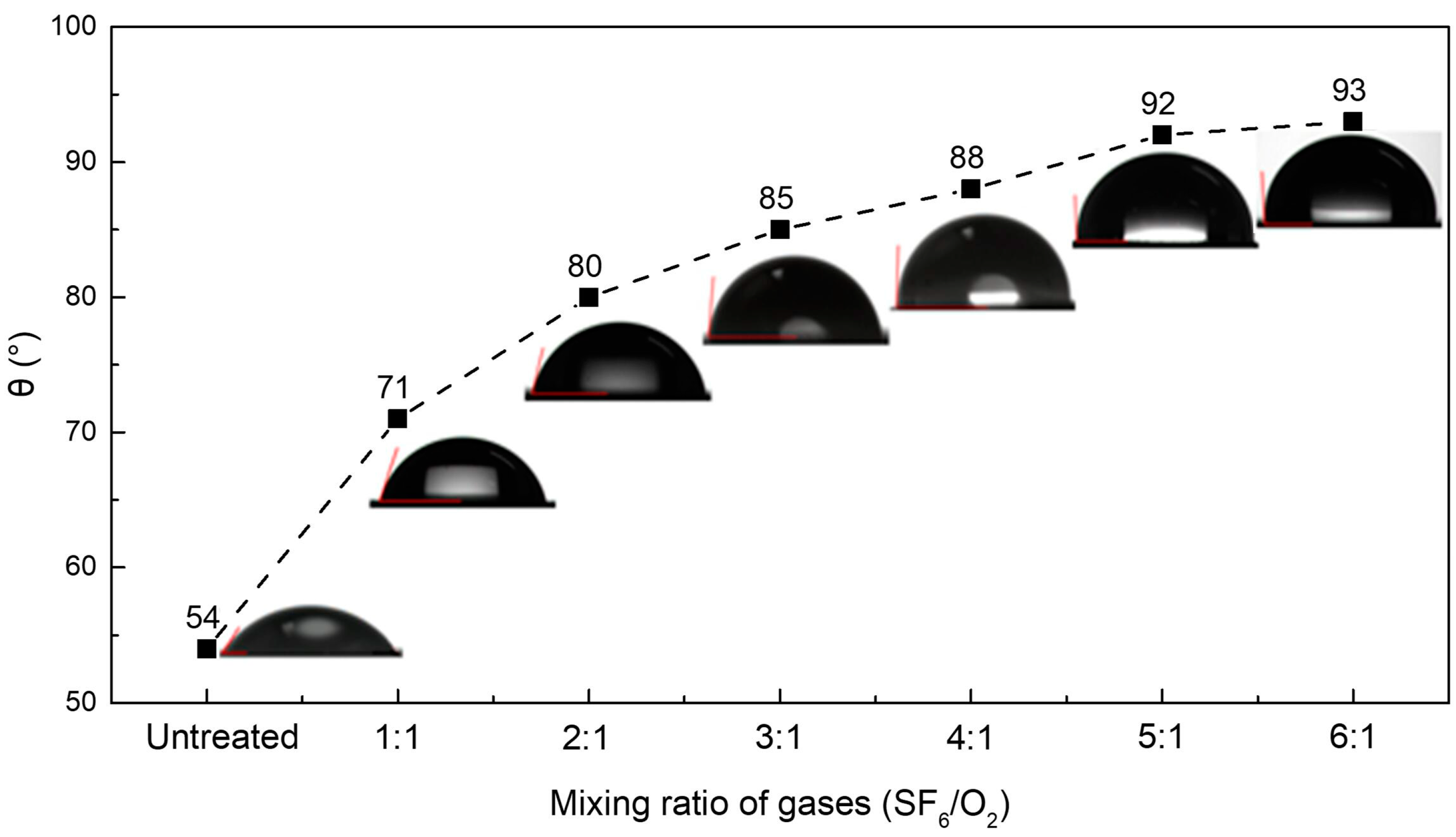
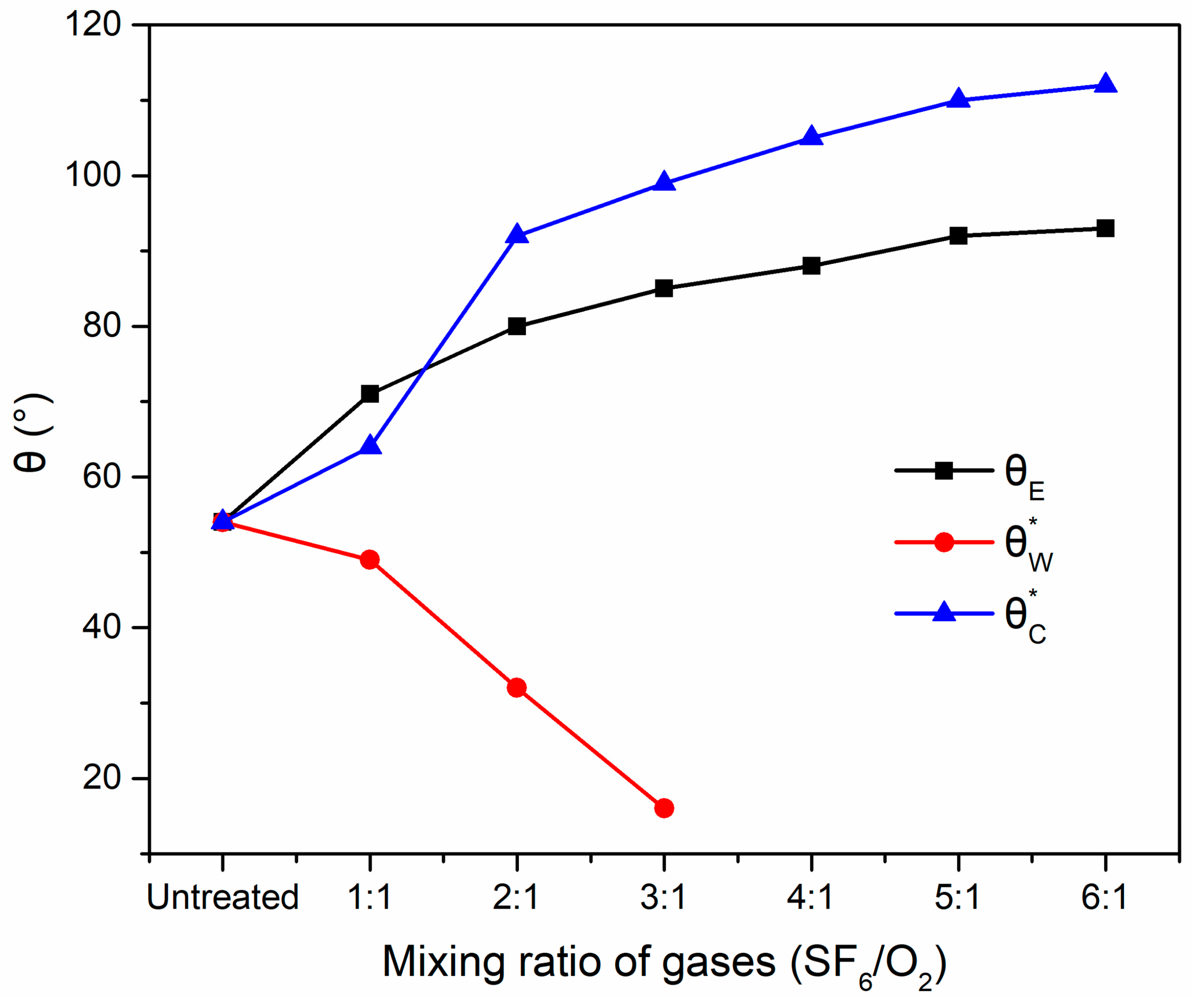
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Mixing ratio of the gases (SF6/O2) | Untreated | 1:1 | 2:1 | 3:1 | 4:1 | 5:1 | 6:1 |
| Mixing Ratio of the Gases (SF6/O2) | 0 | 1:1 | 2:1 | 3:1 | 4:1 | 5:1 | 6:1 |
|---|---|---|---|---|---|---|---|
| 1 | 1.104 | 1.440 | 1.632 | 1.736 | 2.076 | 2.232 | |
| 54 | 49 | 32 | 16 | – | – | – | |
| 1 | 0.903 | 0.604 | 0.536 | 0.470 | 0.417 | 0.393 | |
| 54 | 64 | 92 | 99 | 105 | 110 | 112 |
| Only air in the control unit | |
| Water and air in the control unit | |
| Only water in the control unit |
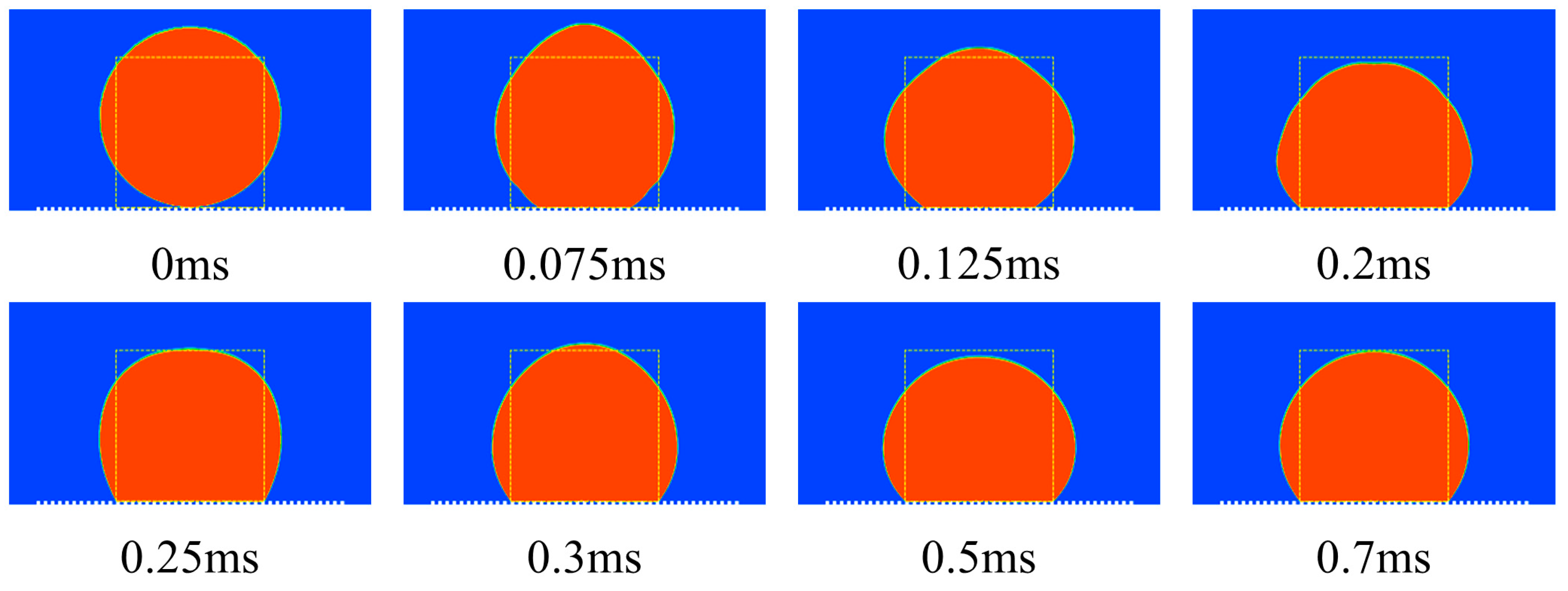
| 0 ms | The initial state |
| 0–0.075 ms | The droplet rose to its maximum height |
| 0.075–0.2 ms | The droplet spread to maximum diameter |
| 0.2–0.3 ms | The droplet shrank to stable state |
| 0.3–0.7 ms | The stable state |
| 0 ms | The initial state |
| 0–0.075 ms | The droplet rose to its maximum height |
| 0.075–0.5 ms | The oscillating state |
| 0.5–0.7 ms | The stable state |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Yang, R.; Li, C.; Yang, L. The Wettability and Numerical Model of Different Silicon Microstructural Surfaces. Appl. Sci. 2019, 9, 566. https://doi.org/10.3390/app9030566
Han S, Yang R, Li C, Yang L. The Wettability and Numerical Model of Different Silicon Microstructural Surfaces. Applied Sciences. 2019; 9(3):566. https://doi.org/10.3390/app9030566
Chicago/Turabian StyleHan, Shuang, Runhua Yang, Chaobo Li, and Lixin Yang. 2019. "The Wettability and Numerical Model of Different Silicon Microstructural Surfaces" Applied Sciences 9, no. 3: 566. https://doi.org/10.3390/app9030566
APA StyleHan, S., Yang, R., Li, C., & Yang, L. (2019). The Wettability and Numerical Model of Different Silicon Microstructural Surfaces. Applied Sciences, 9(3), 566. https://doi.org/10.3390/app9030566





