Genetic Structure and Gene Flow in Red Foxes (Vulpes vulpes) in Scandinavia: Implications for the Potential Future Spread of Echinococcus multilocularis Tapeworm
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Considerations for Management Authorities
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clobert, J.; Danchin, E.; Dhondt, A.A.; Nichols, J. Dispersal; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Sutherland, G.D.; Harestad, A.S.; Price, K.; Lertzman, K.P. Scaling of natal dispersal distances in terrestrial birds and mammals. Conserv. Ecol. 2000, 4, 1. [Google Scholar] [CrossRef]
- Allen, S.H.; Sargeant, A.B. Dispersal patterns of red foxes relative to population density. J. Wildl. Manag. 1993, 57, 526–533. [Google Scholar] [CrossRef]
- Lindström, E.R.; Andrén, H.; Angelstam, P.; Cederlund, G.; Hörnfeldt, B.; Jäderberg, L.; Lemnell, P.A.; Martinsson, B.; Sköld, K.; Swenson, J.E. Disease reveals the predator: Sarcoptic mange, red fox predation, and prey populations. Ecology 1994, 75, 1042–1049. [Google Scholar] [CrossRef]
- Nathan, R.; Perry, G.; Cronin, J.T.; Strand, A.E.; Cain, M.L. Methods for estimating long-distance dispersal. Oikos 2003, 103, 261–273. [Google Scholar] [CrossRef]
- Whitmee, S.; Orme, C.D.L. Predicting dispersal distance in mammals: A trait-based approach. J. Anim. Ecol. 2013, 82, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Norén, K.; Statham, M.J.; Ågren, E.O.; Isomursu, M.; Flagstad, Ø.; Eide, N.E.; Berg, T.B.G.; Bech-Sanderhoff, L.; Sacks, B.N. Genetic footprints reveal geographic patterns of expansion in Fennoscandian red foxes. Glob. Chang. Biol. 2015, 21, 3299–3312. [Google Scholar] [CrossRef]
- Storm, G.; Andrews, R.D.; Phillips, R.L.; Bishop, R.A.; Siniff, D.B.; Tester, J.R. Morphology, reproduction, dispersal, and mortality of midwestern red fox populations. Wildl. Monogr. 1976, 49, 3–82. [Google Scholar]
- Colson, K.; Smith, J.D.; Hundertmark, K.J. St. Matthew Island colonized through multiple long-distance red fox (Vulpes vulpes) dispersal events. Can. J. Zool. 2017, 95, 607–609. [Google Scholar] [CrossRef]
- Walton, Z.; Samelius, G.; Odden, M.; Willebrand, T. Long-distance dispersal in red foxes Vulpes vulpes revealed by GPS tracking. Eur. J. Wildl. Res. 2018, 64, 64. [Google Scholar] [CrossRef]
- Larivière, S.; Pasitschniak-Arts, M. Vulpes vulpes. Mamm. Species 1996, 537, 1–11. [Google Scholar] [CrossRef]
- Lowe, W.H. What drives long-distance dispersal? A test of theoretical predictions. Ecology 2009, 90, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Walton, Z.; Samelius, G.; Odden, M.; Willebrand, T. Variation in home range size of red foxes Vulpes vulpes along a gradient of productivity and human landscape alteration. PLoS ONE 2017, 12, e0175291. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, H.G. The Red Fox; BT Batsford: London, UK, 1980. [Google Scholar]
- Whiteside, H.M.; Dawson, D.A.; Soulsbury, C.D.; Harris, S. Mother knows best: Dominant females determine offspring dispersal in red foxes (Vulpes vulpes). PLoS ONE 2011, 6, e22145. [Google Scholar] [CrossRef] [PubMed]
- Englund, J. Population dynamics of the red fox (Vulpes vulpes L., 1758) in Sweden. In The Red Fox; Springer: Cham, Switzerland, 1980; pp. 107–121. [Google Scholar]
- Zimen, E. Long Range Movements of the Red Fox, Vulpes vulpes L.; Acta Zoologica Fennica: Helsinki, Finland, 1984. [Google Scholar]
- Trewhella, W.J.; Harris, S.; McAllister, F.E. Dispersal distance, home-range size and population density in the red fox (Vulpes vulpes): A quantitative analysis. J. Appl. Ecol. 1988, 25, 423–434. [Google Scholar] [CrossRef]
- Bresson-Hadni, S.; Piarroux, R.; Bartholomot, B.; Miguet, J.-P.; Mantion, G.; Vuitton, D.-A. Echinococcose alvéolaire. EMC Hépato Gastroentérologie 2005, 2, 86–104. [Google Scholar] [CrossRef]
- Vuitton, D.A.; Zhang, S.L.; Yang, Y.; Godot, V.; Beurton, I.; Mantion, G.; Bresson-Hadni, S. Survival strategy of Echinococcus multilocularis in the human host. Parasitol. Int. 2006, 55, S51–S55. [Google Scholar] [CrossRef] [PubMed]
- Vuitton, D.; Zhou, H.; Bresson-Hadni, S.; Wang, Q.; Piarroux, M.; Raoul, F.; Giraudoux, P. Epidemiology of alveolar echinococcosis with particular reference to China and Europe. Parasitology 2003, 127, S87–S107. [Google Scholar] [CrossRef]
- Craig, P. Echinococcus multilocularis. Curr. Opin. Infect. Dis. 2003, 16, 437–444. [Google Scholar] [CrossRef]
- Osterman, E.L.; Juremalm, M.; Christensson, D.; Widgren, S.; Hallgren, G.; Ågren, E.; Uhlhorn, H.; Lindberg, A.; Cedersmyg, M.; Wahlström, H. First detection of Echinococcus multilocularis in Sweden, February to March 2011. Eurosurveillance 2011, 16, 696–705. [Google Scholar]
- Miller, A.L.; Olsson, G.E.; Walburg, M.R.; Sollenberg, S.; Skarin, M.; Ley, C.; Wahlström, H.; Höglund, J. First identification of Echinococcus multilocularis in rodent intermediate hosts in Sweden. Int. J. Parasitol. Parasites Wildl. 2016, 5, 56–63. [Google Scholar] [CrossRef]
- Wahlström, H.; Lindberg, A.; Lindh, J.; Wallensten, A.; Lindqvist, R.; Plym-Forshell, L.; Lind, E.O.; Ågren, E.; Widgren, S.; Carlsson, U. Investigations and actions taken during 2011 due to the first finding of Echinococcus multilocularis in Sweden. Eurosurveillance 2012, 17, 20215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Madslien, K.; Albin-Amiot, C.; Jonsson, M.E.; Clausen, T.; Henriksen, K.; Hamnes, I.S.; Urdahl, A.M.; Heier, B.T.; Enemark, H.L. The Surveillance Programme for Echinococcus multilocularis in Red Foxes (Vulpes Vulpes) in Norway 2016; Norwegian Veterinary Institute: Oslo, Norway, 2017; p. 7. [Google Scholar]
- Miller, A.L.; Olsson, G.E.; Sollenberg, S.; Skarin, M.; Wahlström, H.; Höglund, J. Support for targeted sampling of red fox (Vulpes vulpes) feces in Sweden: A method to improve the probability of finding Echinococcus multilocularis. Parasites Vectors 2016, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Holmes, N.; Dickens, H.; Parker, H.; Binns, M.; Mellersh, C.; Sampson, J. Eighteen canine microsatellites. Anim. Genet. 1995, 26, 132a–133. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, M.; Winterø, A. Variation of short tandem repeats within and between species belonging to the Canidae family. Mamm. Genome 1995, 6, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Breen, M.; Jouquand, S.; Renier, C.; Mellersh, C.S.; Hitte, C.; Holmes, N.G.; Chéron, A.; Suter, N.; Vignaux, F.; Bristow, A.E. Chromosome-specific single-locus FISH probes allow anchorage of an 1800-marker integrated radiation-hybrid/linkage map of the domestic dog genome to all chromosomes. Genome Res. 2001, 11, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Wandeler, P.; Funk, S. Short microsatellite DNA markers for the red fox (Vulpes vulpes). Mol. Ecol. Notes 2006, 6, 98–100. [Google Scholar] [CrossRef]
- Molecular Ecology Resources Primer Development Consortium; An, J.; Bechet, A.; Berggren, Å.; Brown, S.K.; Bruford, M.W.; Cai, Q.; Cassel-Lundhagen, A.; Cezilly, F.; Chen, S.-L.; et al. Permanent genetic resources added to molecular ecology resources database 1 October 2009–30 November 2009. Mol. Ecol. Resour. 2010, 10, 404–408. [Google Scholar] [CrossRef]
- Shen, Z.; Qu, W.; Wang, W.; Lu, Y.; Wu, Y.; Li, Z.; Hang, X.; Wang, X.; Zhao, D.; Zhang, C. MPprimer: A program for reliable multiplex PCR primer design. BMC Bioinform. 2010, 11, 143. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT, a Program to Estimate and Test Gene Diversities and Fixation Indices, Version 2.9.3. 2001. Available online: http://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 8 November 2019).
- Raymond, M.; Rousset, F. GENEPOP (version 1.2): Population genetics software for exact tests and ecumenicism. J. Hered. 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Guo, S.W.; Thompson, E.A. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics 1992, 48, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Pike, N. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol. Evol. 2011, 2, 278–282. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Package ‘factoextra’. Extr. Vis. Results Multivar. Data Anal. 2017, 76. [Google Scholar]
- R Foundation for Statistical Computing. R-Core-Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Guillot, G.; Santos, F.; Estoup, A. Analysing georeferenced population genetics data with Geneland: A new algorithm to deal with null alleles and a friendly graphical user interface. Bioinformatics 2008, 24, 1406–1407. [Google Scholar] [CrossRef]
- Coulon, A.; Guillot, G.; Cosson, J.F.; Angibault, J.; Aulagnier, S.; Cargnelutti, B.; Galan, M.; Hewison, A. Genetic structure is influenced by landscape features: Empirical evidence from a roe deer population. Mol. Ecol. 2006, 15, 1669–1679. [Google Scholar] [CrossRef]
- Guillot, G.; Mortier, F.; Estoup, A. GENELAND: A computer package for landscape genetics. Mol. Ecol. Resour. 2005, 5, 712–715. [Google Scholar] [CrossRef]
- Beerli, P.; Felsenstein, J. Maximum-likelihood estimation of migration rates and effective population numbers in two populations using a coalescent approach. Genetics 1999, 152, 763–773. [Google Scholar] [PubMed]
- Beerli, P.; Felsenstein, J. Maximum likelihood estimation of a migration matrix and effective population sizes in n subpopulations by using a coalescent approach. Proc. Natl. Acad. Sci. USA 2001, 98, 4563–4568. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.A.; Rannala, B. Bayesian inference of recent migration rates using multilocus genotypes. Genetics 2003, 163, 1177–1191. [Google Scholar] [PubMed]
- Teacher, A.G.; Thomas, J.A.; Barnes, I. Modern and ancient red fox (Vulpes vulpes) in Europe show an unusual lack of geographical and temporal structuring, and differing responses within the carnivores to historical climatic change. BMC Evol. Biol. 2011, 11, 214. [Google Scholar] [CrossRef]
- Edwards, C.J.; Soulsbury, C.D.; Statham, M.J.; Ho, S.Y.; Wall, D.; Dolf, G.; Iossa, G.; Baker, P.J.; Harris, S.; Sacks, B.N. Temporal genetic variation of the red fox, Vulpes vulpes, across western Europe and the British Isles. Quat. Sci. Rev. 2012, 57, 95–104. [Google Scholar] [CrossRef]
- Frati, F.; Hartl, G.; Lovari, S.; Delibes, M.; Markov, G. Quaternary radiation and genetic structure of the red fox Vulpes vulpes in the Mediterranean Basin, as revealed by allozymes and mitochondrial DNA. J. Zool. 1998, 245, 43–51. [Google Scholar] [CrossRef]
- Cohen, T.M.; King, R.; Dolev, A.; Boldo, A.; Lichter-Peled, A.; Bar-Gal, G.K. Genetic characterization of populations of the golden jackal and the red fox in Israel. Conserv. Genet. 2013, 14, 55–63. [Google Scholar] [CrossRef]
- Wandeler, P.; Funk, S.M.; Largiader, C.; Gloor, S.; Breitenmoser, U. The city-fox phenomenon: Genetic consequences of a recent colonization of urban habitat. Mol. Ecol. 2003, 12, 647–656. [Google Scholar] [CrossRef]
- Gachot-Neveu, H.; Lefevre, P.; Roeder, J.-J.; Henry, C.; Poulle, M.-L. Genetic detection of sex-biased and age-biased dispersal in a population of wild carnivore, the red fox, Vulpes vulpes. Zool. Sci. 2009, 26, 145–153. [Google Scholar] [CrossRef]
- Mullins, J.; McDevitt, A.D.; Kowalczyk, R.; Ruczyńska, I.; Górny, M.; Wójcik, J.M. The influence of habitat structure on genetic differentiation in red fox populations in north-eastern Poland. Acta Theriol. 2014, 59, 367–376. [Google Scholar] [CrossRef]
- Garroway, C.J.; Bowman, J.; Holloway, G.L.; Malcolm, J.R.; Wilson, P.J. The genetic signature of rapid range expansion by flying squirrels in response to contemporary climate warming. Glob. Chang. Biol. 2011, 17, 1760–1769. [Google Scholar] [CrossRef]
- Hersteinsson, P.; Macdonald, D.W. Interspecific competition and the geographical distribution of red and arctic foxes Vulpes vulpes and Alopex lagopus. Oikos 1992, 64, 505–515. [Google Scholar] [CrossRef]
- Lynch, M. Evolution and extinction in response to environmental change. In Biotic Interactions and Global Change; Kareiva, P.M., Kingsolver, J.G., Huey, R.B., Eds.; Sinauer: Sunderland, MA, USA, 1993; pp. 234–250. [Google Scholar]
- Elmhagen, B.; Kindberg, J.; Hellström, P.; Angerbjörn, A. A boreal invasion in response to climate change? Range shifts and community effects in the borderland between forest and tundra. Ambio 2015, 44, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Elmhagen, B.; Berteaux, D.; Burgess, R.M.; Ehrich, D.; Gallant, D.; Henttonen, H.; Ims, R.A.; Killengreen, S.T.; Niemimaa, J.; Norén, K. Homage to Hersteinsson and Macdonald: Climate warming and resource subsidies cause red fox range expansion and Arctic fox decline. Polar Res. 2017, 36, 3. [Google Scholar] [CrossRef]
- Smedshaug, C.A.; Selås, V.; Lund, S.E.; Sonerud, G.A. The effect of a natural reduction of red fox Vulpes vulpes on small game hunting bags in Norway. Wildl. Biol. 1999, 5, 157–167. [Google Scholar] [CrossRef]
- Angelstam, P.; Lindström, E.; Widén, P. Role of predation in short-term population fluctuations of some birds and mammals in Fennoscandia. Oecologia 1984, 62, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Storm, G. Dispersal and mortality of red foxes. J. Wildl. Manag. 1972, 36, 237–248. [Google Scholar]
- Gosselink, T.E.; Piccolo, K.A.; Van Deelen, T.R.; Warner, R.E.; Mankin, P.C. Natal dispersal and philopatry of red foxes in urban and agricultural areas of Illinois. J. Wildl. Manag. 2010, 74, 1204–1217. [Google Scholar] [CrossRef]
- Lambin, X. Dispersal, intraspecific competition, kin competition and kin facilitation: A review of the empirical evidence. In Dispersal; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Davidson, R.K.; Romig, T.; Jenkins, E.; Tryland, M.; Robertson, L.J. The impact of globalisation on the distribution of Echinococcus multilocularis. Trends Parasitol. 2012, 28, 239–247. [Google Scholar] [CrossRef]
- Torgerson, P.R.; Schweiger, A.; Deplazes, P.; Pohar, M.; Reichen, J.; Ammann, R.W.; Tarr, P.E.; Halkik, N.; Müllhaupt, B. Alveolar echinococcosis: From a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J. Hepatol. 2008, 49, 72–77. [Google Scholar] [CrossRef]
- Malkamäki, S.; Näreaho, A.; Oksanen, A.; Sukura, A. Berries as a potential transmission vehicle for taeniid eggs. Parasitol. Int. 2019, 70, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Regjeringen. Krav om Parasittbehandling av Alle Hunder og Katter Som Innføres fra Sverige. Available online: https://www.regjeringen.no/no/dokumentarkiv/stoltenberg-ii/lmd/Nyheter-og-pressemeldinger/nyheter/2011/krav-om-parasittbehandling-alle-hunder-o/id635397/ (accessed on 2 October 2019).
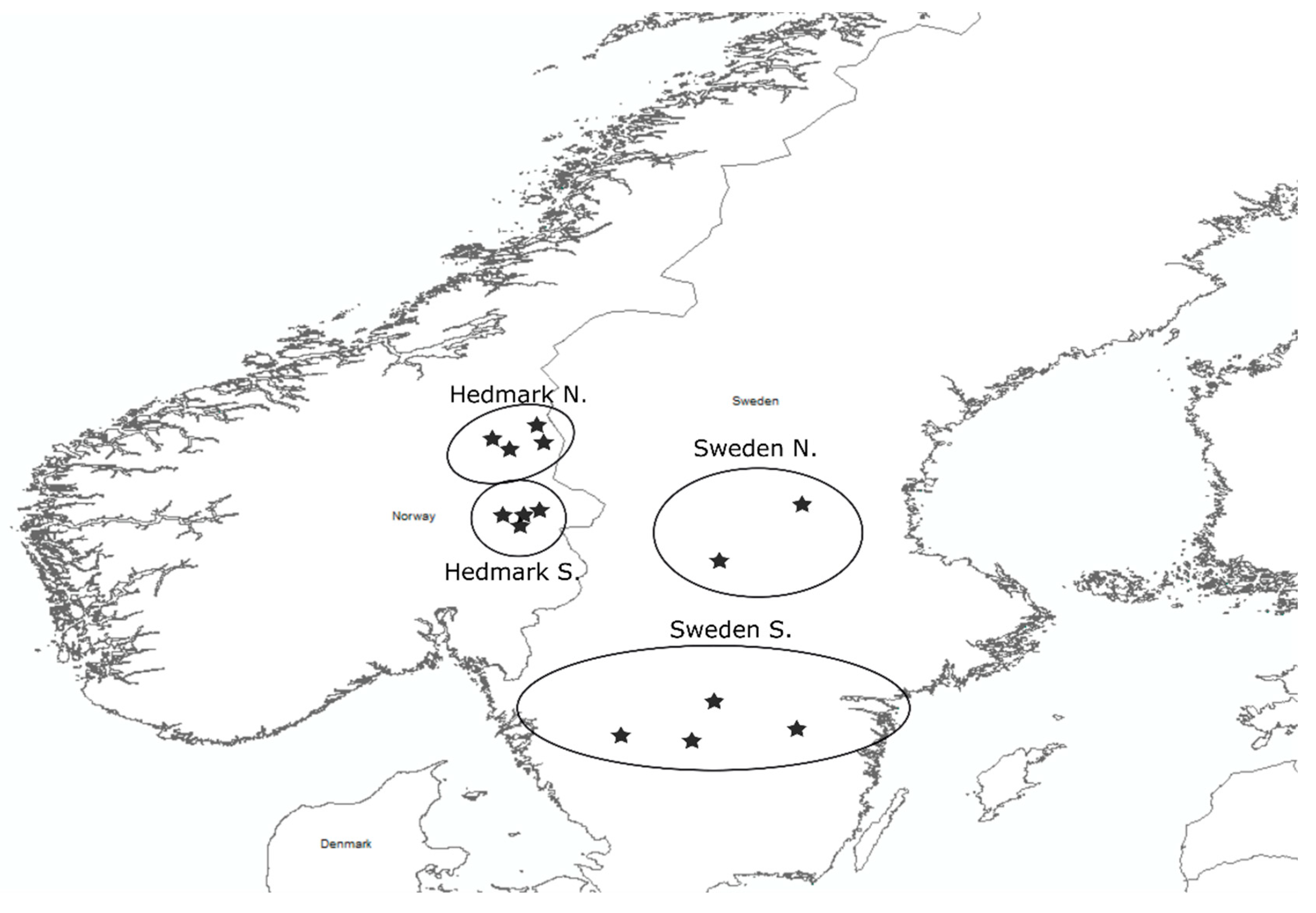
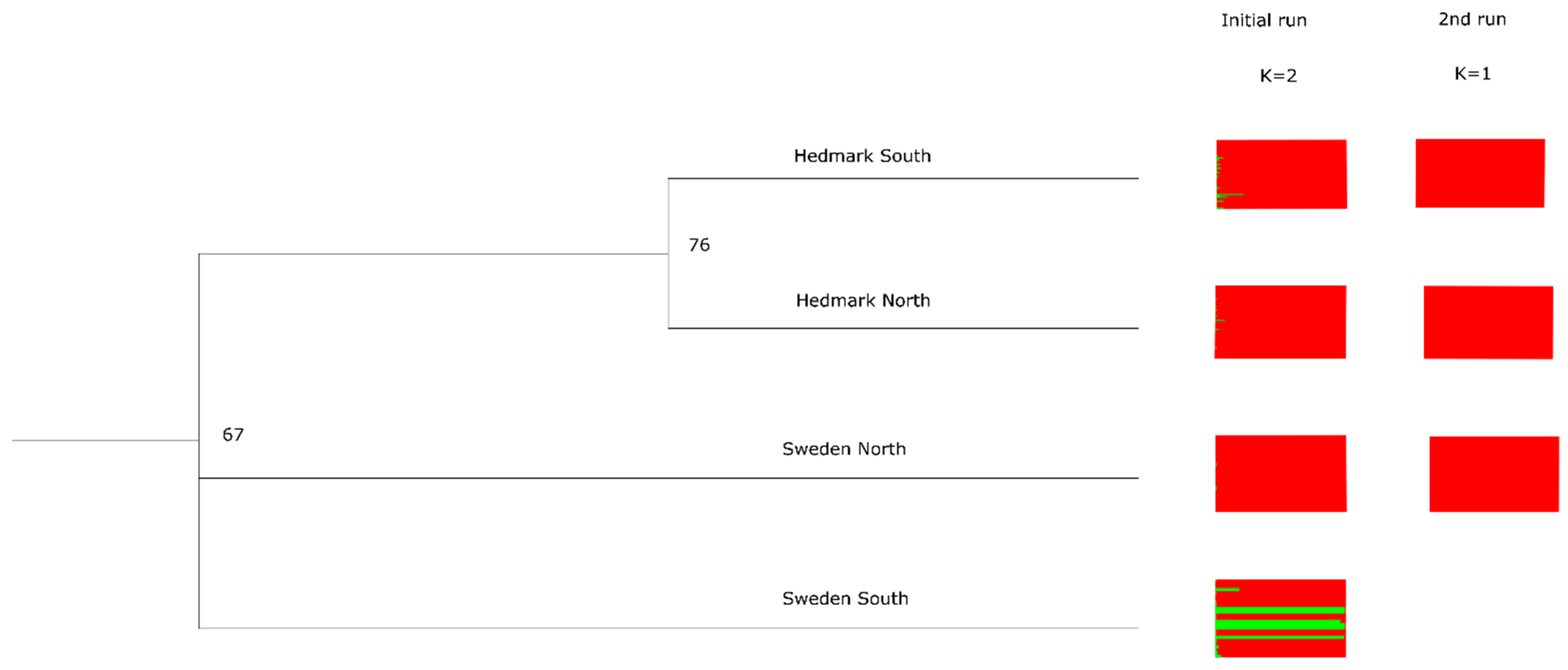
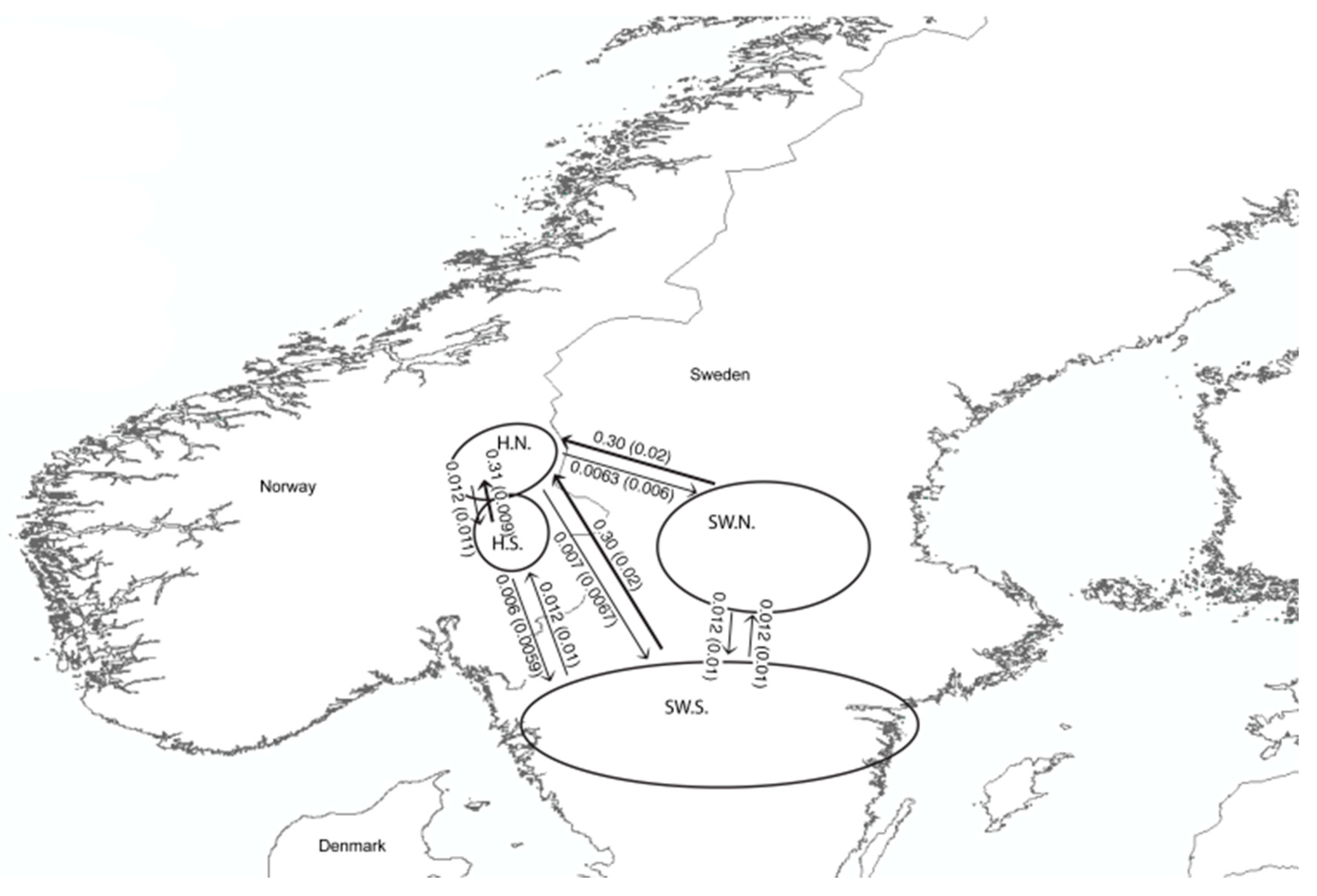
| Study Area | Country | Total N | N Males | N Females | GPS Fox | Shot Fox |
|---|---|---|---|---|---|---|
| Sweden N. | Sweden | 24 | 13 | 11 | 20 | 3 |
| Sweden S. 1 | Sweden | 14 | 3 | 11 | 13 | 1 |
| Sweden S. 2 | Sweden | 6 | 4 | 2 | 0 | 6 |
| Sweden S. 3 | Sweden | 5 | 3 | 2 | 0 | 5 |
| Hedmark N. 1 | Norway | 9 | 3 | 6 | 0 | 9 |
| Hedmark S. 1 | Norway | 8 | 3 | 5 | 0 | 8 |
| Hedmark S. 2 | Norway | 25 | 10 | 15 | 0 | 25 |
| Hedmark S. 3 | Norway | 19 | 9 | 10 | 0 | 19 |
| Hedmark N. 2 | Norway | 23 | 14 | 9 | 0 | 23 |
| Hedmark N. 3 | Norway | 17 | 9 | 8 | 0 | 17 |
| Fst | Hedmark N. | Sweden N. | Sweden S. |
|---|---|---|---|
| Hedmark S. | 0.208 | 0.008 | 0.008 |
| Hedmark N. | 0.008 | 0.008 | |
| Sweden N. | 0.008 |
| Hedmark S. | Hedmark N. | Sweden N. | Sweden S. | |
|---|---|---|---|---|
| Hedmark S. | 77.24 (20.09) | 77.24 (13.24) | 49.02 (16.98) | |
| Hedmark N. | 49.02 (54.98) | 38.35 (16.98) | 37.05 (17.62) | |
| Sweden N. | 52.84 (19.16) | 115.75 (21.58) | 49.82 (3.51) | |
| Sweden S. | 62.73 (18.6) | 32.58 (14.75) | 73.91 (19.42) |
| Hedmark S. | Hedmark N. | Sweden N. | Sweden S. | |
|---|---|---|---|---|
| Hedmark S. | 0.3139 (0.0108) | 0.0060 (0.0058) | 0.0060 (0.0059) | |
| Hedmark N. | 0.0113 (0.0092) | 0.0063 (0.0061) | 0.0070 (0.0067) | |
| Sweden N. | 0.0118 (0.0113) | 0.2979 (0.0189) | 0.0119 (0.0114) | |
| Sweden S. | 0.0116 (0.0113) | 0.2986 (0.0189) | 0.0116 (0.0111) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagenlund, M.; Linløkken, A.; Østbye, K.; Walton, Z.; Odden, M.; Samelius, G.; Willebrand, T.; Wilson, R. Genetic Structure and Gene Flow in Red Foxes (Vulpes vulpes) in Scandinavia: Implications for the Potential Future Spread of Echinococcus multilocularis Tapeworm. Appl. Sci. 2019, 9, 5289. https://doi.org/10.3390/app9245289
Hagenlund M, Linløkken A, Østbye K, Walton Z, Odden M, Samelius G, Willebrand T, Wilson R. Genetic Structure and Gene Flow in Red Foxes (Vulpes vulpes) in Scandinavia: Implications for the Potential Future Spread of Echinococcus multilocularis Tapeworm. Applied Sciences. 2019; 9(24):5289. https://doi.org/10.3390/app9245289
Chicago/Turabian StyleHagenlund, Mari, Arne Linløkken, Kjartan Østbye, Zea Walton, Morten Odden, Gustaf Samelius, Tomas Willebrand, and Robert Wilson. 2019. "Genetic Structure and Gene Flow in Red Foxes (Vulpes vulpes) in Scandinavia: Implications for the Potential Future Spread of Echinococcus multilocularis Tapeworm" Applied Sciences 9, no. 24: 5289. https://doi.org/10.3390/app9245289
APA StyleHagenlund, M., Linløkken, A., Østbye, K., Walton, Z., Odden, M., Samelius, G., Willebrand, T., & Wilson, R. (2019). Genetic Structure and Gene Flow in Red Foxes (Vulpes vulpes) in Scandinavia: Implications for the Potential Future Spread of Echinococcus multilocularis Tapeworm. Applied Sciences, 9(24), 5289. https://doi.org/10.3390/app9245289





