Characterization and Application of Agave salmiana Cuticle as Bio-membrane in Low-temperature Electrolyzer and Fuel Cells
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
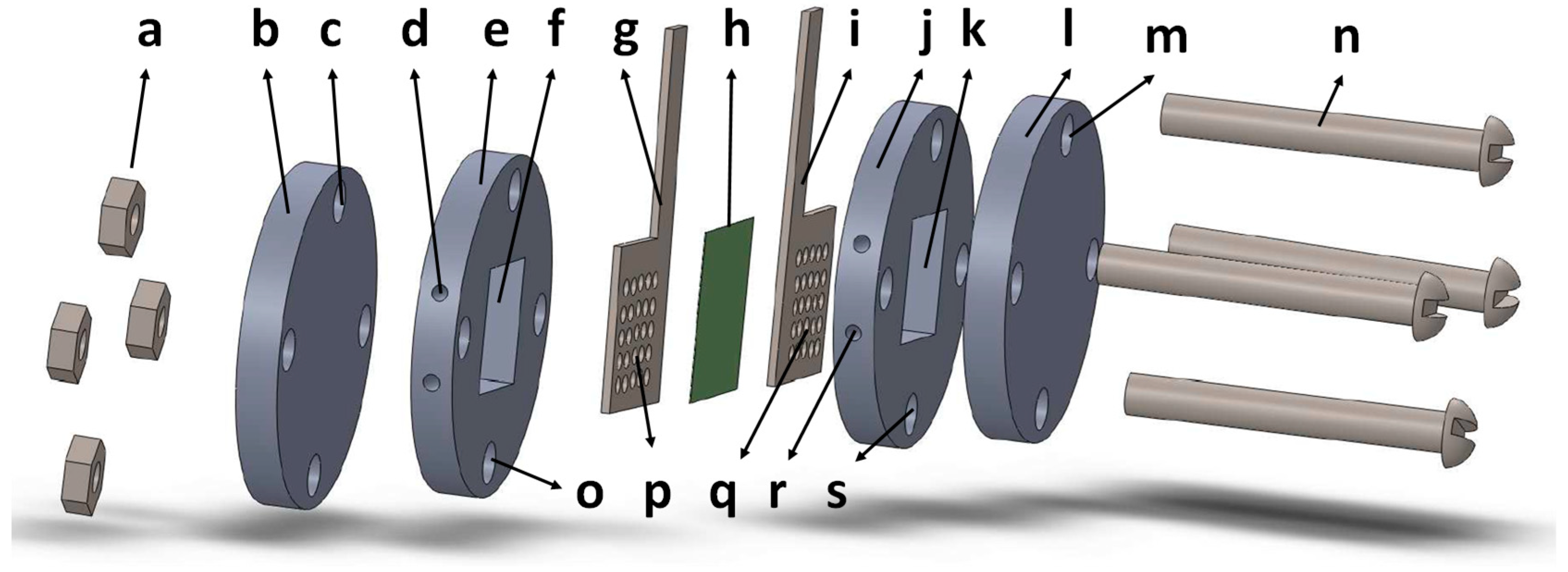
2.2. Apparatus
2.3. Bio-Membrane Treatment and Activation
- Individual immersion (24 h) of the bio-membrane in 50 ml of: An acid solution (HCl 0.1 M), an alkaline solution (NaOH 0.1 M) and a neutral medium (deionized H2O).
- Clean-up treatment of the abaxial face with a cotton ball soaked in organic solvents (chloroform, ethyl ether, hexane and methanol).
- Polymeric coating of the bio-membrane by immersion in a Nafion (5 wt%) ethanol:water (1:2 v:v) solution during 60 minutes with agitation.
- Scraping of the cuticle abaxial face to remove any wax from its surface.
- Thermal treatment by immersing the bio-membrane into boiling water for 4 h.
3. Results
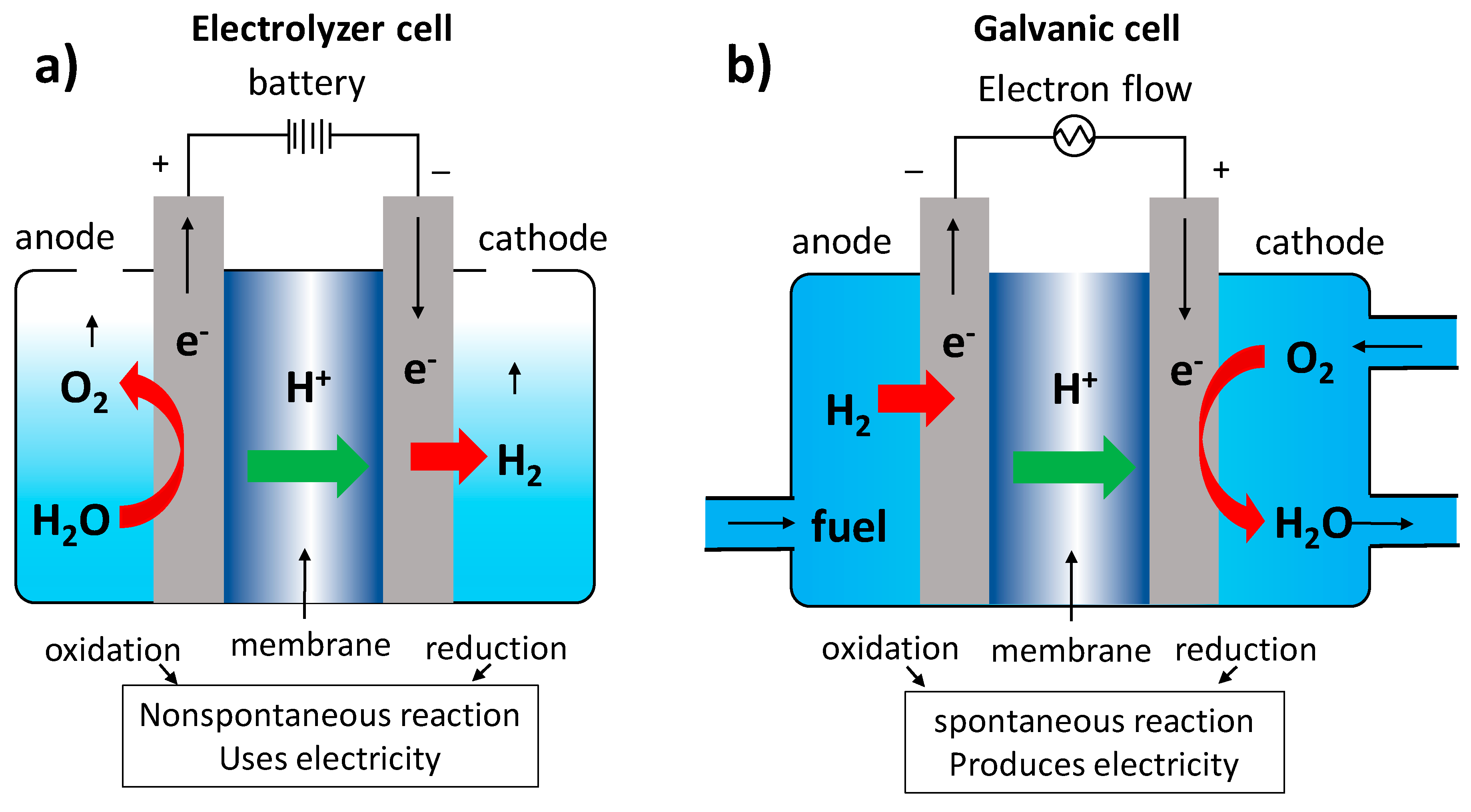
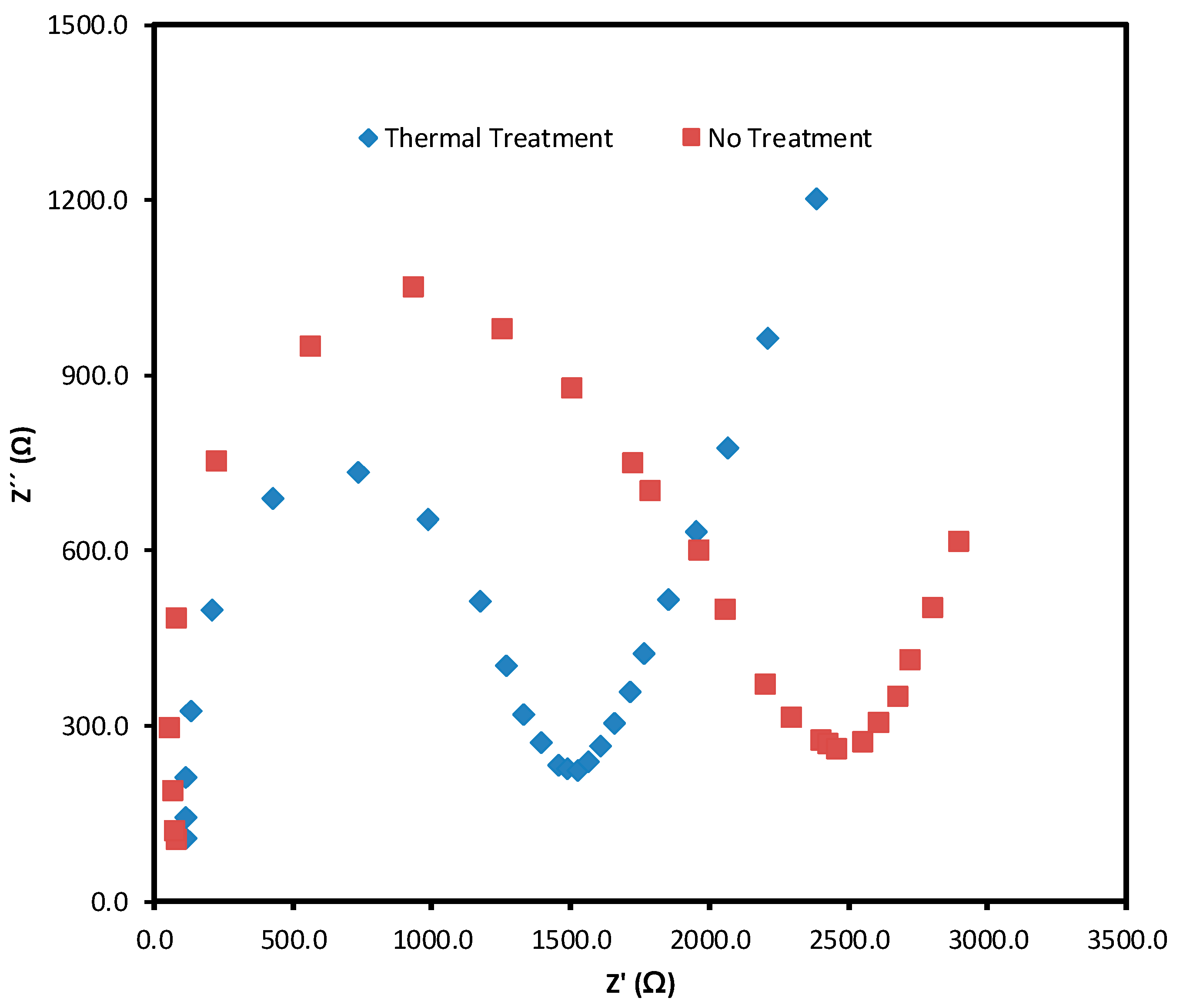
3.1. Biomembrane Characterization in a Coupled Electrolyzer Cell
3.2. Bio-Membrane Characterization Coupled Fuel Cell
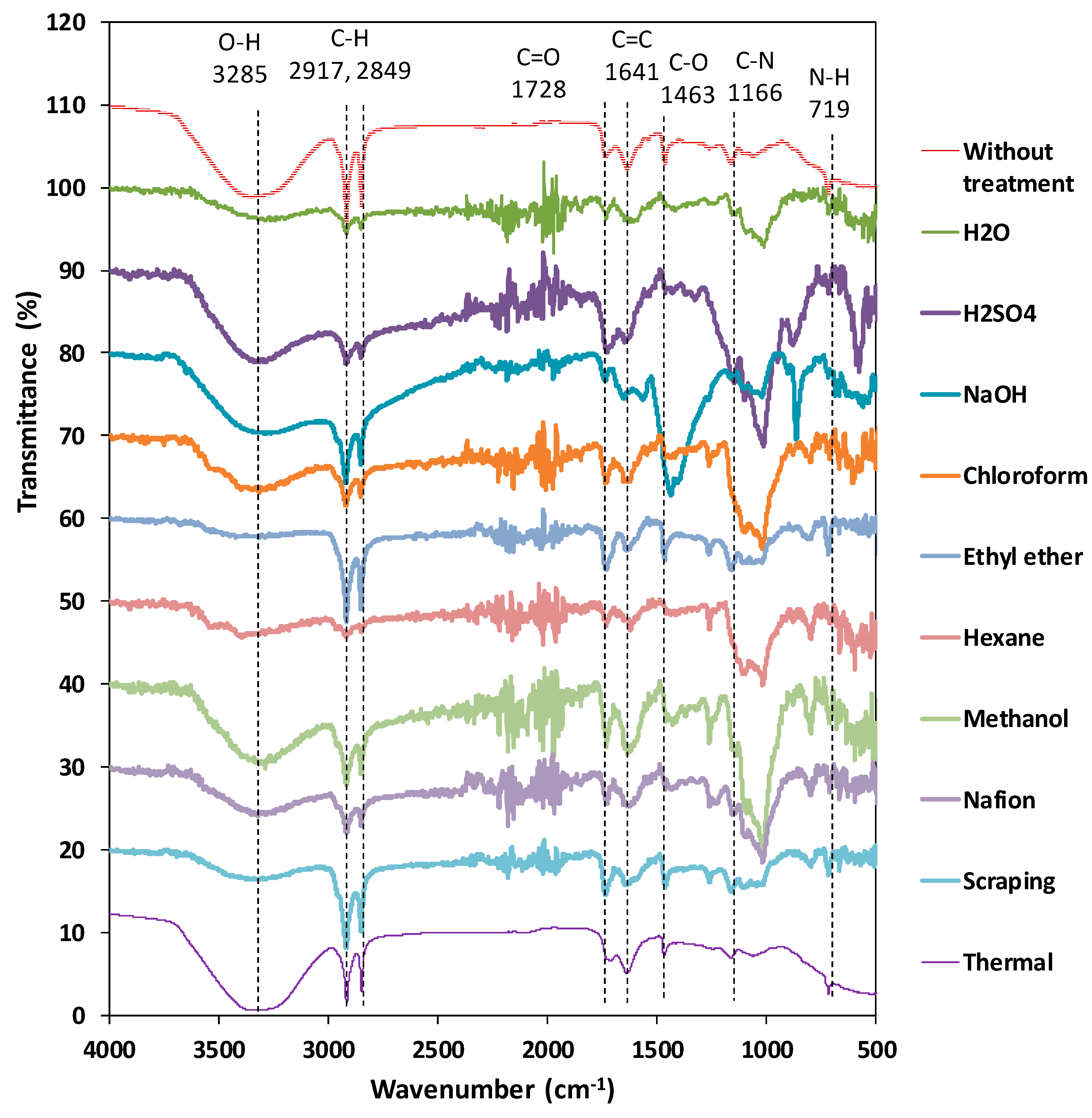
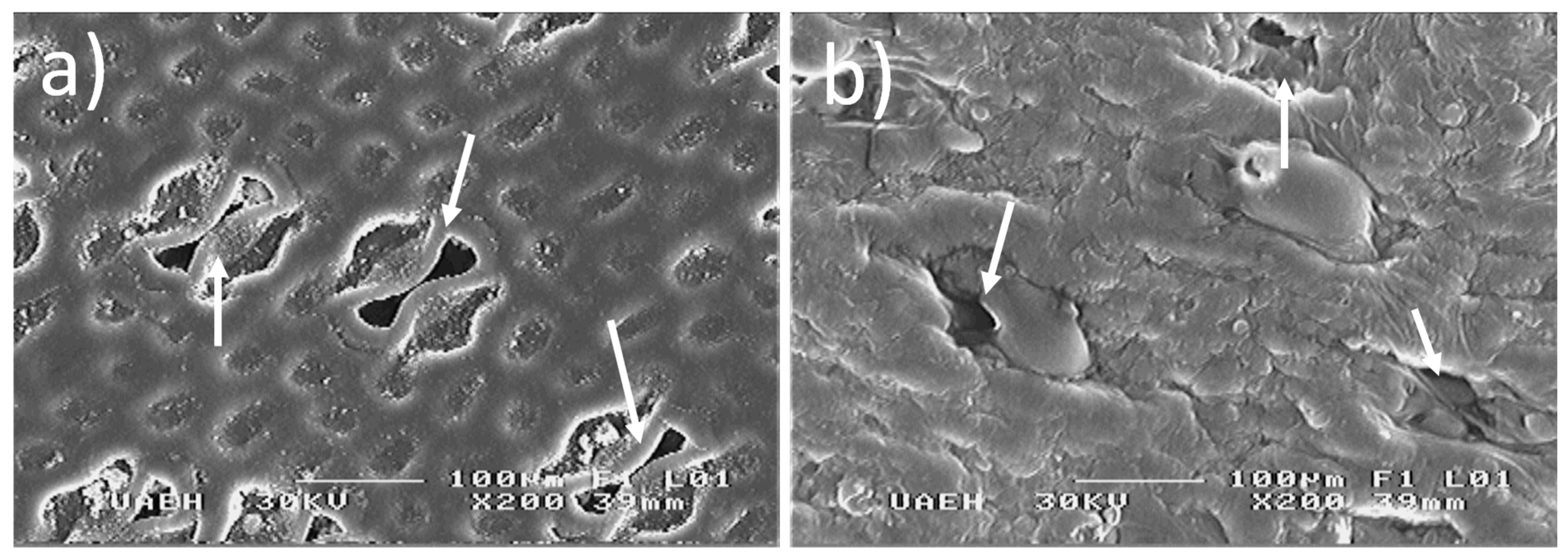
3.3. Structural Characteristics of the Bio-Membrane with Thermal Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Day, J.; Senthilarasu, S.; Mallick, T.K. Improving spectral modification for applications in solar cells: A Review. Renew. Energy 2019, 132, 186–205. [Google Scholar] [CrossRef]
- Siniscalchi-Minna, S.; Bianchi, F.D.; De-Prada-Gil, M.; Ocampo-Martinez, C. A wind farm control strategy for power reserve maximization. Renew. Energy 2019, 131, 37–44. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Hemer, M.A.; Manasseh, R.; McInnes, K.L.; Penesis, I.; Pitman, T. Perspectives on a way forward for ocean renewable energy in Australia. Renew. Energy 2018, 127, 733–745. [Google Scholar] [CrossRef]
- Eriksson, E.L.V.; Gray, E.M.A. Optimization and integration of hybrid renewable energy hydrogen fuel cell energy systems—A critical review. Appl. Energy 2017, 202, 348–364. [Google Scholar] [CrossRef]
- Hames, Y.; Kaya, K.; Baltacioglu, E.; Turksoy, A. Analysis of the control strategies for fuel saving in the hydrogen fuel cell vehicles. Int. J. Hydrogen Energy 2018, 43, 10810–10821. [Google Scholar] [CrossRef]
- Abdin, Z.; Webb, C.J.; Gray, E.M.A. Modelling and simulation of an alkaline electrolyser cell. Energy 2017, 138, 316–331. [Google Scholar] [CrossRef]
- Ito, H.; Maeda, T.; Nakano, A.; Takenaka, H. Properties of Nafion membranes under PEM water electrolysis conditions. Int. J. Hydrogen Energy 2011, 36, 10527–10540. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Özgür, T.; Yakaryılmaz, A.C. A review: Exergy analysis of PEM and PEM fuel cell based CHP systems. Int. J. Hydrogen Energy 2018, 43, 17993–18000. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Basri, S.; Shyuan, L.K.; Masdar, M.S.; Nordin, D. Enhanced Proton Conductivity and Methanol Permeability Reduction via Sodium Alginate Electrolyte-Sulfonated Graphene Oxide Bio-membrane. Nanoscale Res. Lett. 2018, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Choi, S.-Y.; Jung, K.-H.; Kim, T.-H.; Rhee, H.-W. Sulfonated Poly (etheretherketone) Based Nanocomposite Membranes Containing POSS-SA for Polymer Electrolyte Membrane Fuel Cells (PEMFC). J. Memb. Sci. 2018, 566, 69–76. [Google Scholar] [CrossRef]
- Odgaard, M. The Use of NafionP as Electrolyte in Fuel Cells. In Fluorinated Materials for Energy Conversion; Elsevier: Amsterdam, The Netherlands, 2005; pp. 439–468. [Google Scholar] [CrossRef]
- Ercelik, M.; Ozden, A.; Devrim, Y.; Colpan, C.O. Investigation of Nafion based composite membranes on the performance of DMFCs. Int. J. Hydrogen Energy 2017, 42, 2658–2668. [Google Scholar] [CrossRef]
- Basile, A.; Iulianelli, A. Chapter 3: Alternative Sulfonated Polymers to Nafion for PEM Fuel Cell. In Fuel Cell Research Trends; Vasquez, L.O., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2007; pp. 135–160. ISBN 1-60021-669-2. [Google Scholar]
- Li, P.; Wu, W.; Liu, J.; Shi, B.; Du, Y.; Li, Y.; Wang, J. Investigating the nanostructures and proton transfer properties of Nafion-GO hybrid membranes. J. Memb. Sci. 2018, 555, 327–336. [Google Scholar] [CrossRef]
- Alshorman, A.A. Characteristic Study of Bio-Membrane PEM Fuel Cell for Performance Upgrading. Procedia Comput. Sci. 2016, 83, 839–846. [Google Scholar] [CrossRef]
- Higashihara, T.; Matsumoto, K.; Ueda, M. Sulfonated aromatic hydrocarbon polymers as proton exchange membranes for fuel cells. Polymer 2009, 50, 5341–5357. [Google Scholar] [CrossRef]
- Gomadam, P.M.; Weidner, J.W. Analysis of electrochemical impedance spectroscopy in proton exchange membrane fuel cells. Int. J. Energy Res. 2005, 29, 1133–1151. [Google Scholar] [CrossRef]
- Siracusano, S.; Trocino, S.; Briguglio, N.; Baglio, V.; Aricò, A.S. Electrochemical Impedance Spectroscopy as a Diagnostic Tool in Polymer Electrolyte Membrane Electrolysis. Materials 2018, 11, 1368. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Niya, S.M.; Hoorfar, M. Study of proton exchange membrane fuel cells using electrochemical impedance spectroscopy technique—A review. J. Power Sources 2013, 240, 281–293. [Google Scholar] [CrossRef]
- Zhiani, M.; Majidi, S.; Silva, V.B.; Gharibi, H. Comparison of the performance and EIS (electrochemical impedance spectroscopy) response of an activated PEMFC (proton exchange membrane fuel cell) under low and high thermal and pressure stresses. Energy 2016, 97, 560–567. [Google Scholar] [CrossRef]
- Monja-Mio, K.M.; Barredo Pool, F.; Herrera Herrera, G.; Esqueda Valle, M.; Robert, M.L. Development of the stomatal complex and leaf surface of Agave angustifolia Haw. ‘Bacanora’ plantlets during the in vitro to ex vitro transition process. Sci. Hortic. 2015, 189, 32–40. [Google Scholar] [CrossRef]
- Owen, A.; Griffiths, H. Marginal land bioethanol yield potential of four crassulacean acid metabolism candidates (Agave fourcroydes, Agave salmiana, Agave tequilana and Opuntia ficus-indica) in Australia. Glob. Chang. Biol. Bioenergy 2014, 6, 687–703. [Google Scholar] [CrossRef]
- González-López, J.R.; Ramos-Lara, J.F.; Zaldivar-Cadena, A.; Chávez-Guerrero, I. Small addition effect of agave biomass ashes in cement mortars. Fuel Process. Technol. 2015, 133, 35–42. [Google Scholar] [CrossRef]
- Peña-Reyes, V.L.; Marin-Bustamante, M.Q.; Manzo-Robledo, A.; Chanona-Pérez, J.J.; Cásarez-Santiago, R.G.; Suarez-Najera, E. Effect of crosslinking of alginate/pva and chitosan/pva, reinforced with cellulose nanoparticles obtained from agave Atrovirens karw. Procedia Eng. 2017, 200, 434–439. [Google Scholar] [CrossRef]
- Tafolla-Arellano, J.C.; González-León, A.; Tiznado-Hernández, M.E.; Zacarías García, L.; Báez-Sañudo, R. Composition, physiology and biosynthesis of plant cuticle. Rev. Fitotec. Mex. 2013, 36, 3–12. [Google Scholar]
- Domínguez, E.; Cuarteto, J.; Heredia, A. An overview on plant cuticle biomechanics. Plant Sci. 2011, 181, 77–84. [Google Scholar] [CrossRef]
- Villena, J.F.; Dominguez, E.; Heredia, A. Monitoring Biopolymers Present in Plant Cuticles by FT-IR Spectroscopy. J. Plant Physiol. 2000, 156, 419–422. [Google Scholar] [CrossRef]
- Van Winkle, L.J.; Bussolati, O.; Gazzola, G.; McGiven, J.; Mackenzie, B.; Saier, M.H.; Taylor, P.M.; Rennie, M.J.; Low, S.Y. Chapter 1—Importance of Biomembrane Transport, Biomembrane Transport, 1st ed.; Academic Press: Cambridge, MA, USA, 1999; pp. 1–11. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, H.B.; Lee, Y.M.; Lee, R.D. Importance of Proton Conductivity Measurement in Polymer Electrolyte Membrane for Fuel Cell Application. Ind. Eng. Chem. Res. 2005, 44, 7617–7626. [Google Scholar] [CrossRef]
- Martínez Salvador, M.; Rubio Arias, H.; Ortega Rubio, A. Population Structure of Maguey (Agave salmiana ssp. crassispina) in Southeast Zacatecas, Mexico. Arid Land Res. Manag. 2005, 19, 101–109. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N. Membranes for direct ethanol fuel cells: An overview. Appl. Energy 2016, 163, 334–342. [Google Scholar] [CrossRef]
- Bayer, T.; Selyanchyn, R.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. Spray-painted graphene oxide membrane fuel cells. J. Memb. Sci. 2017, 541, 347–357. [Google Scholar] [CrossRef]
- Deshmukh, A.P.; Simpson, A.J.; Hadad, C.M.; Hatcher, P.G. Insights into the structure of cutin and cutan from Agave americana leaf cuticle using HRMAS NMR spectroscopy. Org. Geochem. 2005, 36, 1072–1085. [Google Scholar] [CrossRef]
- Matic, M. The chemistry of plant cuticles: A study of cutin from Agave americana L. Biochem. J. 1956, 63, 168–176. [Google Scholar] [CrossRef] [PubMed]
- North, G.B.; Nobel, P.S. Changes in hydraulic conductivity and anatomy caused by drying and rewetting roots of agave deserti (Agavaceae). Am. J. Bot. 1991, 78, 906–915. [Google Scholar] [CrossRef]
- Liu, J.G.; Zhao, T.S.; Chen, R.; Wong, C.W. The effect of methanol concentration on the performance of a passive DMFC. Electrochem. Commun. 2005, 7, 288–294. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Potential (V) | σ mS cm−2 | Half-life (h) | |
|---|---|---|---|---|
| Without treatment | 9.3 | 1.39 ± 0.01 | 0.05 | |
| Immersion | H2O | 3.9 | 0.03 ± 0.01 | 0.06 |
| NaOH 0.1 M | 3.8 | 0.02 ± 0.03 | 0.08 | |
| H2SO4 0.1 M | 3.6 | 0.02 ± 0.06 | 0.08 | |
| Clean-up | chloroform | 4.4 | 0.11 ± 0.03 | 0.05 |
| ethyl ether | 3.5 | 4.26 ± 0.02 | 0.16 | |
| hexane | 3 | 6.25 ± 0.03 | 0.06 | |
| methanol | 3 | 0.07 ± 0.01 | 0.25 | |
| Polymeric coating | Nafion | 2.7 | 0.02 ± 0.01 | 1.85 |
| Scraping | 3.4 | 5.13 ± 0.02 | 0.65 | |
| Thermal | 2 | 10.00 ± 0.03 | 336 | |
| E (volts) | i (mA) | Conductivity mS cm−2 | |
|---|---|---|---|
| Hydrogen fuel cell with Nafion membrane | 0.81 ± 0.01 | 1.00 ± 0.05 | 4.29 |
| Hydrogen fuel cell with thermal bio-membrane | 0.70 ± 0.03 | 0.09 ± 0.01 | 1.06 |
| Methanol fuel cell with Nafion membrane | 0.46 ± 0.02 | 0.30 ± 0.02 | 1.11 |
| Methanol fuel cell with thermal bio-membrane | 0.50 ± 0.01 | 0.50 ± 0.02 | 7.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arredondo, C.H.; Aguilar-Lira, G.; Perez-Silva, I.; Rodriguez, J.A.; Islas, G.; Hernandez, P. Characterization and Application of Agave salmiana Cuticle as Bio-membrane in Low-temperature Electrolyzer and Fuel Cells. Appl. Sci. 2019, 9, 4461. https://doi.org/10.3390/app9204461
Arredondo CH, Aguilar-Lira G, Perez-Silva I, Rodriguez JA, Islas G, Hernandez P. Characterization and Application of Agave salmiana Cuticle as Bio-membrane in Low-temperature Electrolyzer and Fuel Cells. Applied Sciences. 2019; 9(20):4461. https://doi.org/10.3390/app9204461
Chicago/Turabian StyleArredondo, Cristhian H., Guadalupe Aguilar-Lira, Irma Perez-Silva, Jose Antonio Rodriguez, Gabriela Islas, and Prisciliano Hernandez. 2019. "Characterization and Application of Agave salmiana Cuticle as Bio-membrane in Low-temperature Electrolyzer and Fuel Cells" Applied Sciences 9, no. 20: 4461. https://doi.org/10.3390/app9204461
APA StyleArredondo, C. H., Aguilar-Lira, G., Perez-Silva, I., Rodriguez, J. A., Islas, G., & Hernandez, P. (2019). Characterization and Application of Agave salmiana Cuticle as Bio-membrane in Low-temperature Electrolyzer and Fuel Cells. Applied Sciences, 9(20), 4461. https://doi.org/10.3390/app9204461






