Microstructure and Tribological Properties of Self-Lubricating FeS Coating Prepared by Chemical Bath Deposition Coating Technique
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
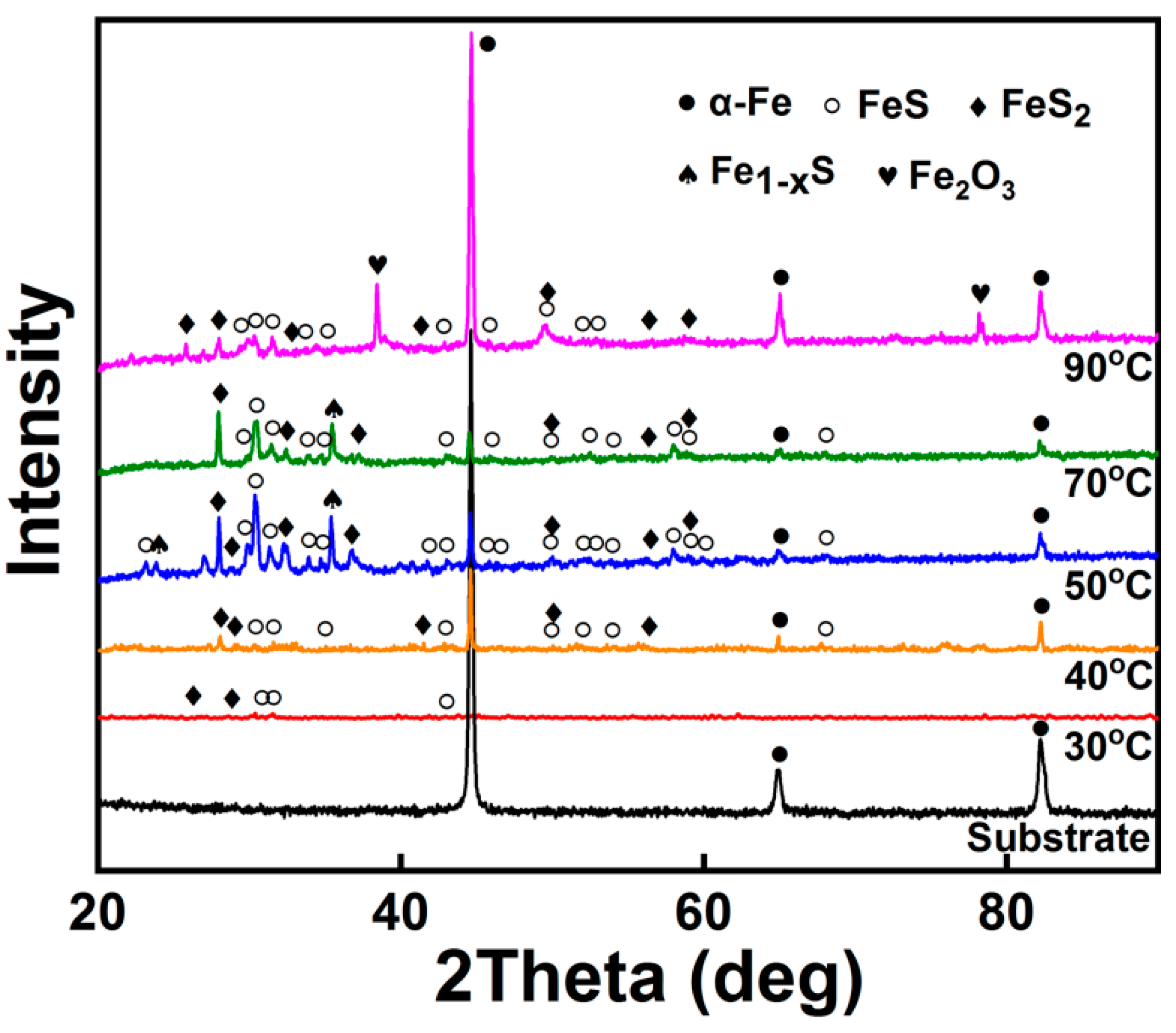
3.1. Phase Composition
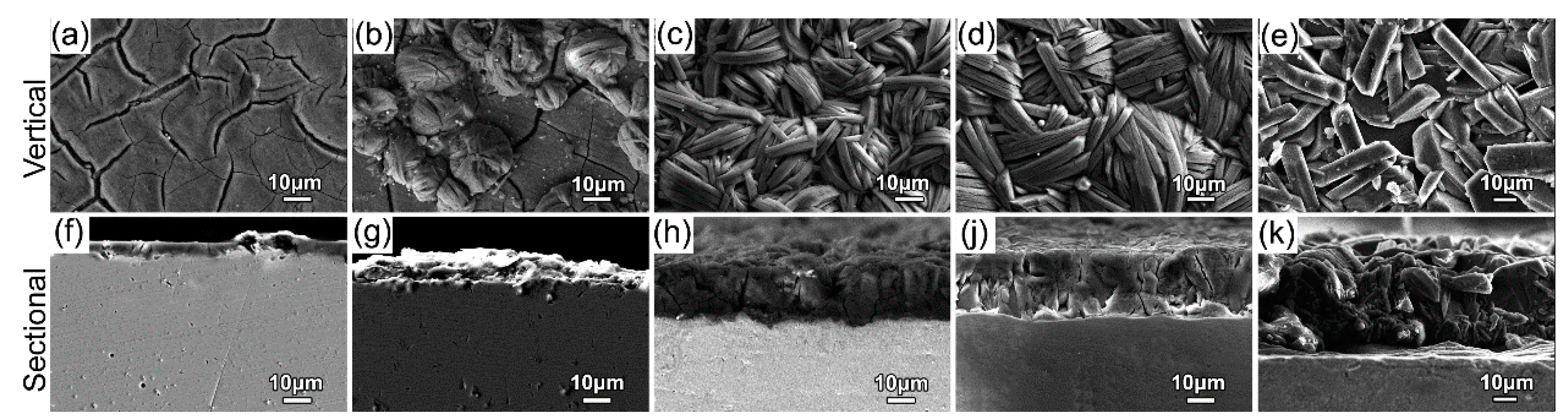
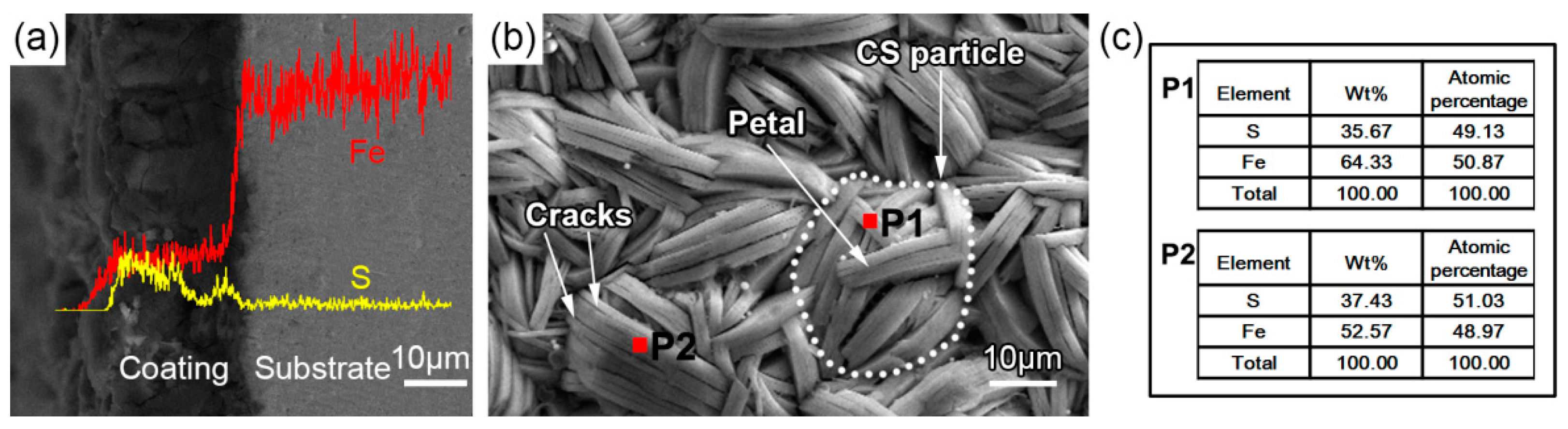
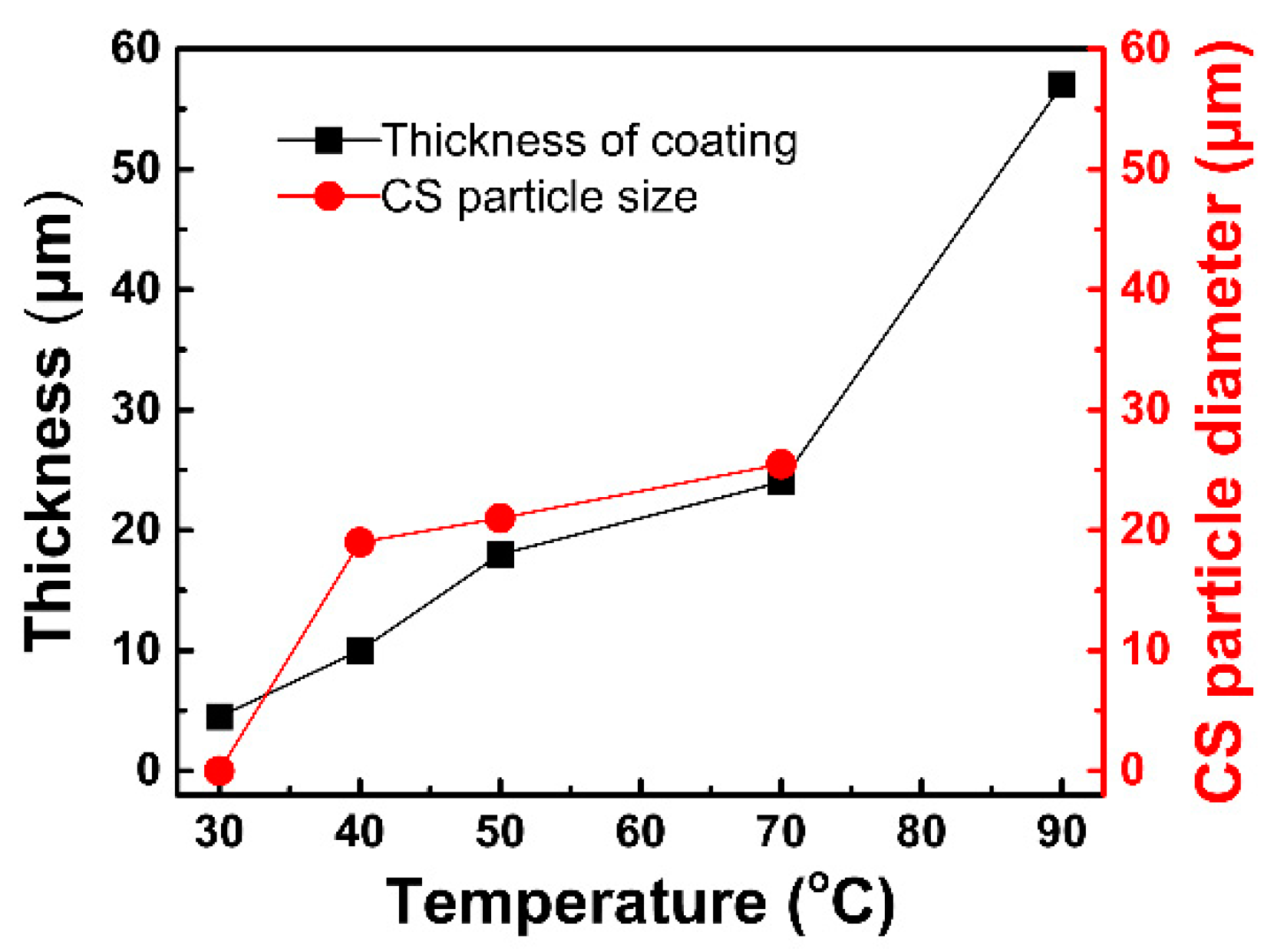
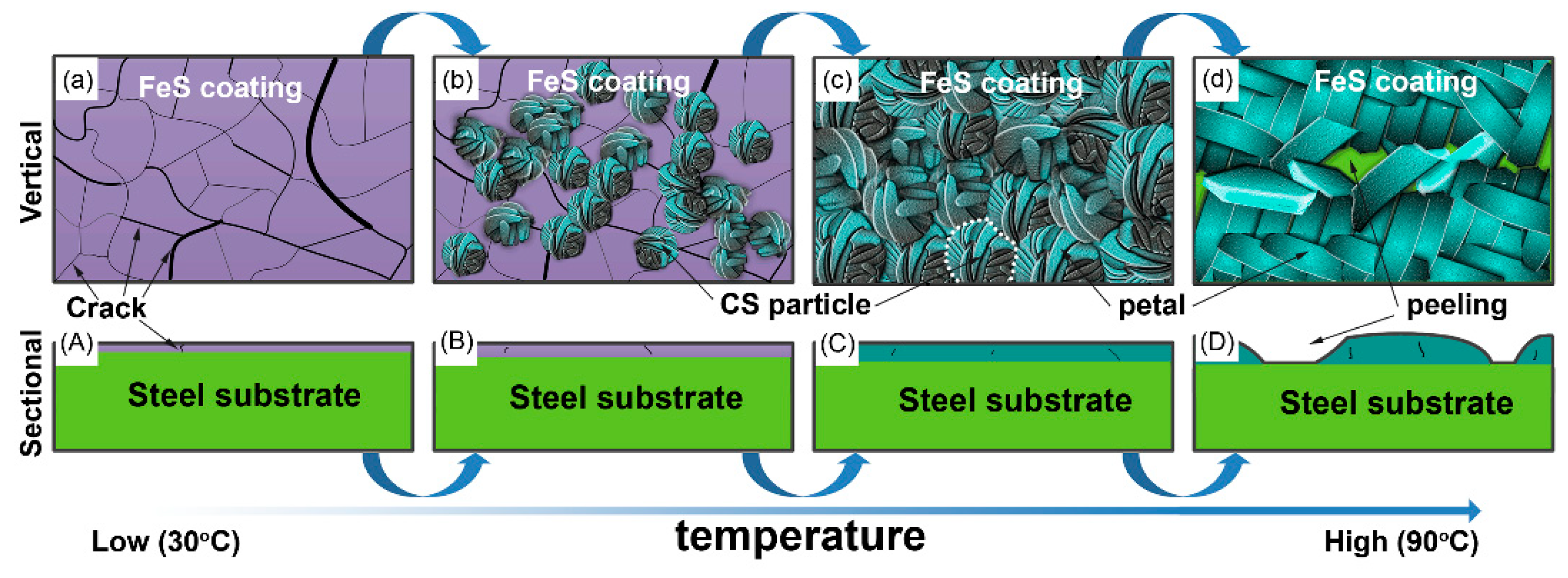
3.2. Microstructure Evolution
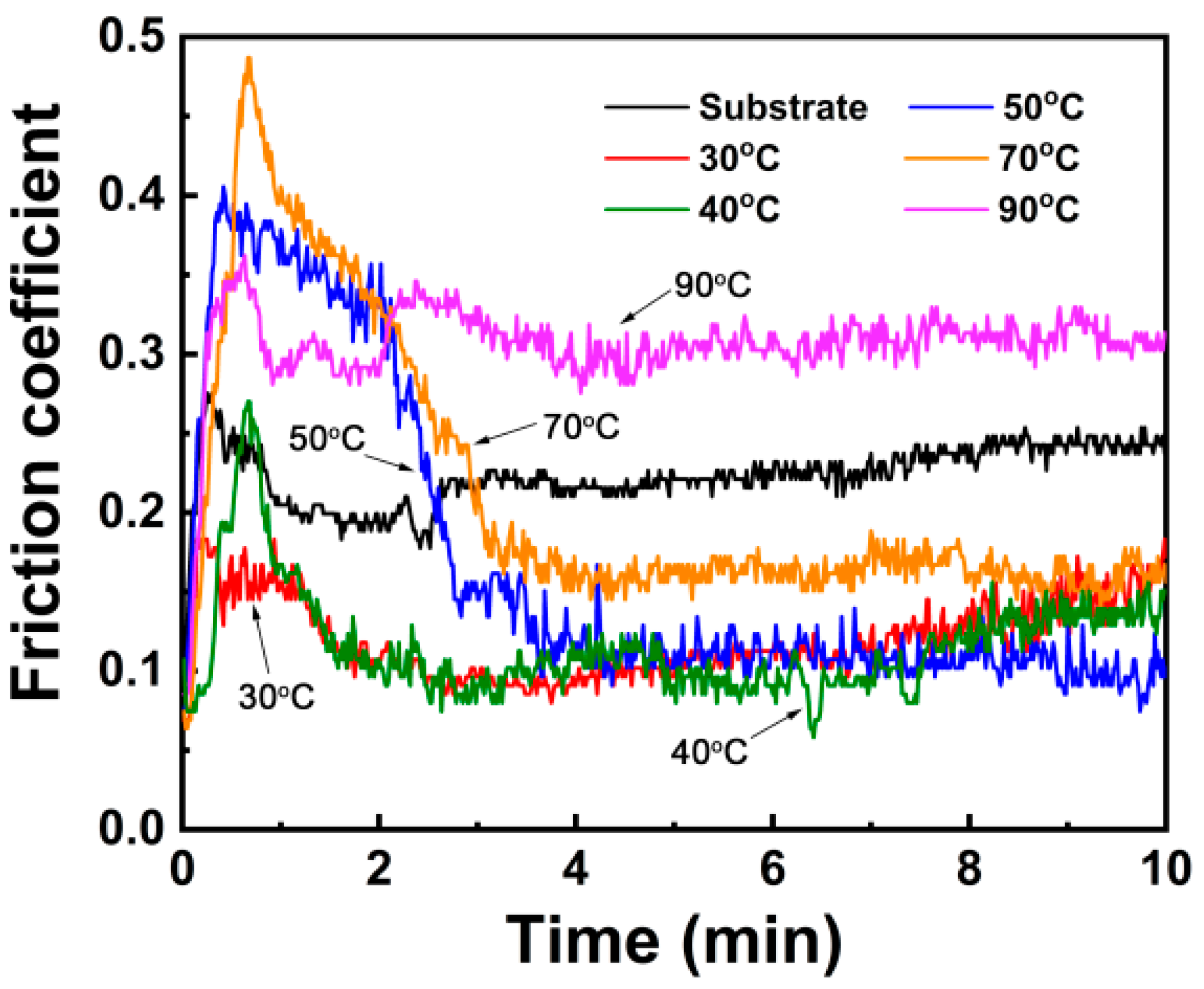
3.3. Friction and Wear Behavior
4. Conclusions
- The optimum CBD temperature is between 50 and 70 °C. Because the coating is mainly composed of the soft self-lubricating active ingredients FexSy (FeS, FeS2, and Fe1−xS) in this temperature range. When the temperature is lower than this temperature range, the FexSy content/thickness in the coating does not reach a peak, and above this temperature range oxidation occurs in the coating.
- The FeS coating has a chrysanthemum-shaped (CS) morphology composed of the FeS crystals. The CS particles nucleate at about 30 °C, rapidly grow and reach a peak thickness at about 50–70 °C, and finally disappear and are replaced by disordered thick petals at 90 °C. Additionally, in CBD treatment at high temperature (90 °C), the FeS coating peels off in a partial area because of the rapid evaporation of moisture from the chemical solution bath.
- The wear resistance of the steel was improved obviously after CBD treatment, because of which the treated coatings can provide better lubrication and improve the wear resistance. The steel treated at a medium temperature (about 50 °C in this study) has a moderate coating thickness and a coating density, corresponding to the best wear resistance.
Author Contributions
Funding
Conflicts of Interest
References
- Chaubey, S.K.; Jain, N.K. State-of-art review of past research on manufacturing of meso and micro cylindrical gears. Precis. Eng. 2018, 51, 702–728. [Google Scholar] [CrossRef]
- Tobie, T.; Hippenstiel, F.; Mohrbacher, H. Optimizing gear performance by alloy modification of carburizing steels. Metals 2017, 7, 415. [Google Scholar] [CrossRef]
- Dengo, C.; Meneghetti, G.; Dabalà, M. Experimental analysis of bending fatigue strength of plain and notched case-hardened gear steels. Int. J. Fatigue 2015, 80, 145–161. [Google Scholar] [CrossRef]
- Hu, J.; Ma, C.; Xu, H.; Guo, N.; Hou, T. Development of a composite technique for preconditioning of 41Cr4 steel used as gear material: Examination of its microstructural characteristics and properties. Sci. Technol. Nucl. Install. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, J.; Li, H.; Yang, X.; Xu, H.; Jin, Y.; Ma, C.; Dong, Q.; Guo, N. Effect of annealing treatment on microstructure and properties of Cr-Coatings deposited on AISI 5140 Steel by Brush-Plating. Coatings 2018, 8, 193. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, X.; Gu, F.; Wang, T.; Ball, A. Gear wear process monitoring using a sideband estimator based on modulation signal bispectrum. Appl. Sci. 2017, 7, 274. [Google Scholar] [CrossRef]
- Li-na, Z.; Cheng-biao, W.; Hai-dou, W.; Bin-shi, X.; Da-ming, Z.; Jia-jun, L.; Guo-lu, L. Microstructure and tribological properties of WS2/MoS2 multilayer films. Appl. Surf. Sci. 2012, 258, 1944–1948. [Google Scholar] [CrossRef]
- Peng, C.; Xiao, Y.; Wang, Y.; Guo, W. Effect of laser shock peening on bending fatigue performance of AISI 9310 steel spur gear. Opt. Laser Technol. 2017, 94, 15–24. [Google Scholar] [CrossRef]
- Ma, G.Z.; Xu, B.S.; Wang, H.D.; Li, G.L.; Zhang, S. The Low-temperature ion sulfurizing technology and its applications. Phys. Procedia 2013, 50, 131–138. [Google Scholar] [CrossRef]
- Tao, Q.; Wang, J.; Fu, L.; Chen, Z.; Shen, C.; Zhang, D.; Sun, Z. Ultrahigh hardness of carbon steel surface realized by novel solid carburizing with rapid diffusion of carbon nanostructures. J. Mater. Sci. Technol. 2017, 33, 1210–1218. [Google Scholar] [CrossRef]
- Pertek, A.; Kulka, M. Two-step treatment carburizing followed by boriding on medium-carbon steel. Surf. Coat. Technol. 2003, 173, 309–314. [Google Scholar] [CrossRef]
- Basu, A.; Majumdar, J.D.; Alphonsa, J.; Mukherjee, S.; Manna, I. Corrosion resistance improvement of high carbon low alloy steel by plasma nitriding. Mater. Lett. 2008, 62, 3117–3120. [Google Scholar] [CrossRef]
- Aizawa, T.; Morita, H.; Wasa, K. Low-Temperature plasma nitriding of Mini-/Micro-Tools and parts by Table-Top system. Appl. Sci. 2019, 9, 1667. [Google Scholar] [CrossRef]
- Kulka, M.; Pertek, A. Characterization of complex (B + C + N) diffusion layers formed on chromium and nickel-based low-carbon steel. Appl. Surf. Sci. 2003, 218, 114–123. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Fan, L.; Dong, L.; Deng, J.; Li, D.; Xue, W. Investigation of anodic plasma electrolytic carbonitriding on medium carbon steel. Surf. Coat. Technol. 2017, 313, 288–293. [Google Scholar] [CrossRef]
- Xu, H.; Hu, J.; Ma, C.; Chai, L.; Guo, N. Influence of electron beam irradiation on surface roughness of commercially AISI 5140 steel. Mater. Trans. 2017, 58, 1519–1523. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Yang, X.; Li, H.; Xu, H.; Ma, C.; Dong, Q.; Guo, N.; Yao, Z. Effect of pack-chromizing temperature on microstructure and performance of AISI 5140 steel with Cr-coatings. Surf. Coat. Technol. 2018, 344, 656–663. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, J.; Yang, Y.; Yang, X.; Li, H.; Guo, N. Microstructures and wear resistance of Boron-Chromium Duplex-Alloyed coatings prepared by a Two-Step pack cementation process. Coatings 2019, 9, 529. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Jiang, J.; Yang, X.; Xu, H.; Li, H.; Guo, N. Effect of heating treatment on the microstructure and properties of Cr–Mo Duplex-Alloyed coating prepared by double glow plasma surface alloying. Coatings 2019, 9, 336. [Google Scholar] [CrossRef]
- Wei, D.; Li, F.; Li, S.; Chen, X.; Ding, F.; Zhang, P.; Wang, Z. A new plasma surface alloying to improve the wear resistance of the metallic card clothing. Appl. Sci. 2019, 9, 1849. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, Z.; Dong, Q.; Yu, H.; Song, B.; Chai, L.; Liu, C.; Yao, Z.; Daymond, M.R. Strengthening and toughening austenitic steel by introducing gradient martensite via cyclic forward/reverse torsion. Mater. Des. 2018, 143, 150–159. [Google Scholar] [CrossRef]
- Hai-dou, W.; Da-ming, Z.; Kun-lin, W.; Jia-jun, L. Comparison of the tribological properties of an ion sulfurized coating and a plasma sprayed FeS coating. Mater. Sci. Eng. 2003, 357, 321–327. [Google Scholar] [CrossRef]
- Wakuda, M.; Yamauchi, Y.; Kanzaki, S.; Yasuda, Y. Effect of surface texturing on friction reduction between ceramic and steel materials under lubricated sliding contact. Wear 2003, 254, 356–363. [Google Scholar] [CrossRef]
- Dai, W.; Kheireddin, B.; Gao, H.; Liang, H. Roles of nanoparticles in oil lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar] [CrossRef]
- Zhang, G.; Wetzel, B.; Wang, Q. Tribological behavior of PEEK-based materials under mixed and boundary lubrication conditions. Tribol. Int. 2015, 88, 153–161. [Google Scholar] [CrossRef]
- Ronen, A.; Etsion, I.; Kligerman, Y. Friction-Reducing Surface-Texturing in reciprocating automotive components. Tribol. Trans. 2001, 44, 359–366. [Google Scholar] [CrossRef]
- Wei, S.; Shang, H.; Liao, C.; Huang, J.; Shi, B. Tribology performance of surface texturing plunger. Biomimetics 2019, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Lin, J.; Ma, L. Study on lubrication performance of journal bearing with multiple texture distributions. Appl. Sci. 2018, 8, 244. [Google Scholar] [CrossRef]
- Bijani, D.; Deladi, E.L.; Akchurin, A.; de Rooij, M.B.; Schipper, D.J. The influence of surface texturing on the frictional behaviour of parallel sliding lubricated surfaces under conditions of mixed lubrication. Lubricants 2018, 6, 91. [Google Scholar] [CrossRef]
- Kang, J.J.; Wang, C.B.; Wang, H.D.; Xu, B.S.; Liu, J.J.; Li, G.L. Research on the tribological property of synthetic multilayer MoS2/FeS film under dry condition. Adv. Mater. Res. 2011, 217–218, 1117–1122. [Google Scholar] [CrossRef]
- Kang, J.J.; Wang, C.B.; Wang, H.D.; Xu, B.S.; Liu, J.J.; Li, G.L. Preparation and characterization of FeS film by low temperature ion sulfurizing technique. Adv. Mater. Res. 2011, 217–218, 1113–1116. [Google Scholar] [CrossRef]
- Wang, H.D.; Xu, B.S.; Liu, J.J.; Zhuang, D.M. Investigation on friction and wear behaviors of FeS films on L6 steel surface. Appl. Surf. Sci. 2005, 252, 1084–1091. [Google Scholar] [CrossRef]
- Zang, Y.; Hu, C.H.; Qiao, Y.L. Anti-Friction mechanism of FeS layer as solid lubrication cmposite coating croduced by Low-Temperature ion sulfurization. Adv. Mater. Res. 2011, 314–316, 1383–1386. [Google Scholar] [CrossRef]
- Lee, I.; Park, I. Solid lubrication coating of FeS layer on the surface of SKD 61 steel produced by plasma sulfnitriding. Surf. Coat. Technol. 2006, 200, 3540–3543. [Google Scholar] [CrossRef]
- Liu, Y.D.; Wang, C.B.; Yuan, J.J.; Liu, J.J. The effect of FeS solid lubricant on the tribological properties of bearing steel under grease lubrication. Tribol. Trans. 2010, 53, 667–677. [Google Scholar] [CrossRef]
- Peng, T.; Yan, Q.Z.; Zhang, Y.; Shi, X.J.; Ba, M.Y. Low-cost solid FeS lubricant as a possible alternative to MoS2 for producing Fe-based friction materials. Int. J. Miner. Metall. Mater. 2017, 24, 115–121. [Google Scholar] [CrossRef]
- Kang, J.J.; Wang, C.B.; Wang, H.D.; Xu, B.S.; Liu, J.J.; Li, G.L. Characterization and tribological behavior of FeS/ferroalloy composite coating under dry condition. Mater. Chem. Phys. 2011, 129, 625–630. [Google Scholar] [CrossRef]
- Kang, J.J.; Wang, C.B.; Wang, H.D.; Xu, B.S.; Liu, J.J.; Li, G.L. Characterization and tribological properties of composite 3Cr13/FeS layer. Surf. Coat. Technol. 2009, 203, 1927–1932. [Google Scholar] [CrossRef]
- Zhang, N.; Zhuang, D.M.; Liu, J.J. Tribological behaviors of steel surfaces treated with ion sulphurization duplex processes. Surf. Coat. Technol. 2009, 203, 3173–3177. [Google Scholar] [CrossRef]
- Wang, H.D.; Xu, B.S.; Liu, J.J.; Zhuang, D.M.; Wei, S.C.; Jin, G. The iron sulfide coatings prepared by plasma spraying and their friction-reduction performance. Surf. Coat. Technol. 2007, 201, 5286–5289. [Google Scholar] [CrossRef]
- Wang, H.D.; Xu, B.S.; Liu, J.J.; Zhuang, D.M. The friction–reduction model of the iron sulfide film prepared by plasma source ion sulfuration. Surf. Coat. Technol. 2007, 201, 5236–5239. [Google Scholar] [CrossRef]
- Chaki, S.H.; Deshpande, M.P.; Tailor, J.P. Characterization of CuS nanocrystalline thin films synthesized by chemical bath deposition and dip coating techniques. Thin Solid Films 2014, 550, 291–297. [Google Scholar] [CrossRef]
- Terasako, T.; Murakami, T.; Hyodou, A.; Shirakata, S. Structural and electrical properties of CuO films and n -ZnO/ p -CuO heterojunctions prepared by chemical bath deposition based technique. Sol. Energy Mater. Sol. Cells 2015, 132, 74–79. [Google Scholar] [CrossRef]
- Terasako, T.; Obara, S.; Sakaya, S.; Tanaka, M.; Fukuoka, R.; Yagi, M.; Nomoto, J.; Yamamoto, T. Morphology-controlled growth of ZnO nanorods by chemical bath deposition and seed layer dependence on their structural and optical properties. Thin Solid Films 2019, 669, 141–150. [Google Scholar] [CrossRef]
- Pi, P.; Hou, K.; Zhou, C.; Li, G.; Wen, X.; Xu, S.; Cheng, J.; Wang, S. Superhydrophobic Cu2S@Cu2O film on copper surface fabricated by a facile chemical bath deposition method and its application in oil-water separation. Appl. Surf. Sci. 2017, 396, 566–573. [Google Scholar] [CrossRef]
- Wang, H.; Xu, B.; Liu, J. Micro and Nano Sulfide Solid Lubrication; Springer: Beijing, China, 2012; pp. 61–114. [Google Scholar]
- Hu, C.H.; Huang, J.; Zhao, H.S.; Ma, S.N.; Qiao, Y.L. Tribological performances of FeS solid lubrication duplex layer under liquid paraffin oil with n-Al2O3 lubrication. Adv. Mater. Res. 2011, 291–294, 1526–1531. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; He, C.; Yang, X.; Li, H.; Xu, H.; Guo, N. Microstructure and Tribological Properties of Self-Lubricating FeS Coating Prepared by Chemical Bath Deposition Coating Technique. Appl. Sci. 2019, 9, 4422. https://doi.org/10.3390/app9204422
Hu J, He C, Yang X, Li H, Xu H, Guo N. Microstructure and Tribological Properties of Self-Lubricating FeS Coating Prepared by Chemical Bath Deposition Coating Technique. Applied Sciences. 2019; 9(20):4422. https://doi.org/10.3390/app9204422
Chicago/Turabian StyleHu, Jianjun, Chuan He, Xian Yang, Hui Li, Hongbin Xu, and Ning Guo. 2019. "Microstructure and Tribological Properties of Self-Lubricating FeS Coating Prepared by Chemical Bath Deposition Coating Technique" Applied Sciences 9, no. 20: 4422. https://doi.org/10.3390/app9204422
APA StyleHu, J., He, C., Yang, X., Li, H., Xu, H., & Guo, N. (2019). Microstructure and Tribological Properties of Self-Lubricating FeS Coating Prepared by Chemical Bath Deposition Coating Technique. Applied Sciences, 9(20), 4422. https://doi.org/10.3390/app9204422






