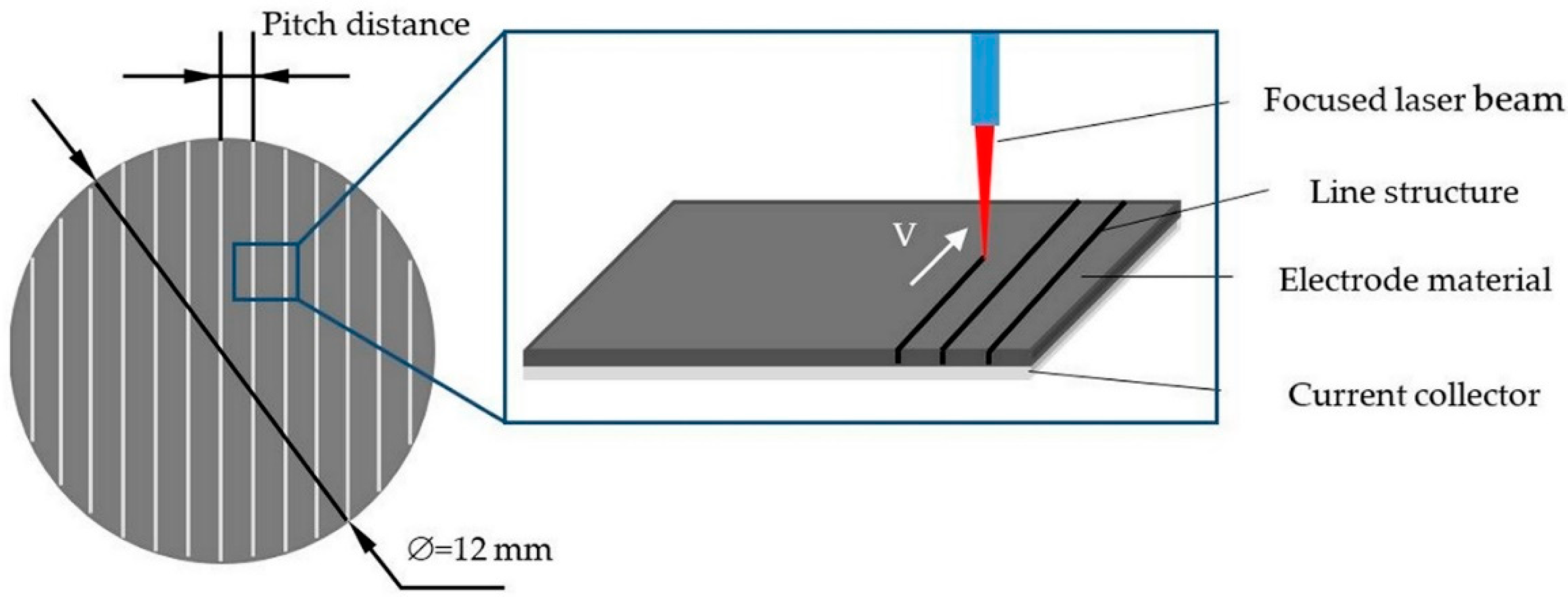
1. Introduction
Ever since the market’s introduction to them by Sony corporation in the early 1990s, rechargeable lithium-ion batteries (LIBs) have been a triumph in many applications and have changed the world tremendously, especially with the rapid development of 3C-markets (computer, communication, and consumer), electric power tools, and electric vehicles. Meanwhile, much effort is devoted to meet the demand for battery development and innovation, such demands include higher power density, higher energy density, rapid chargeability, and a longer cycle life, while lowering the costs simultaneously. Nowadays, more and more automobile industries are seeking to find an alternative and sustainable solution for mobility. Many have been committed to developing hybrid electric vehicles (HEVs) and battery electric vehicles (BEVs). However, the major barrier for BEV compared to conventional vehicles with internal combustion engines is the range of a single charge, which is much lower than a fully fueled conventional automobile (generally 480 km to 640 km). For example, popular electric vehicles can travel up to 400 km after fully charged [
1]. Besides, due to the limited space in BEV, it is necessary to achieve higher volumetric energy density. At the same time, the costs per kilowatt-hour are decisive factors to ensure a successful BEV integration into the automobile market.
Over the last decades, LIBs have become more and more popular and are considered to be an important potential power source for electric vehicles due to their high energy density and low weight compared to conventional batteries [
2]. One of the commercially successful cathode materials for LIBs is Li(Ni
1/3Mn
1/3Co
1/3)O
2 (nickel-manganese-cobalt oxide, NMC 111), with a gravimetric capacity of 160 mAh/g [
3]. However, much attention is focused on increasing the capacity by increasing nickel content. Most recently, Li(Ni
0.6Mn
0.2Co
0.2)O
2 (NMC 622), with a gravimetric capacity of 187 mAh/g [
3], has become the mainstream material for cathodes, due to its higher capacity and energy density compared to NMC 111. Besides, cobalt is a very expensive component in comparison to nickel and manganese. Hence, the cost for cathode material is reduced by lowering the cobalt content. In addition, the overall cost of the cell will be decreased using NMC 622 instead of NMC 111, since about 1/4 of the cell cost comes from the cathode side [
4].
The most practical approach to increase the energy density of a battery is to manufacture a thick electrode, which will increase the ratio of active material to inactive materials, such as the current collector and separator foils. Therefore, less energy consumption is required for processing steps, such as electrode sheet cutting and stacking. However, according to Ragone chart, specific energy is inversely correlated to specific power [
5], which means the power of battery declines with increasing energy. That indicates that C-rate has a great impact on Li-ion diffusion inside electrode during charging and discharging. The conventional approach to enhance energy density by simply increasing cathode thickness leads to a deterioration of rate capability and long-term cycling performance, especially at high discharge rates, mainly due to the high internal resistance, poor mechanical integrity of thick electrode, and diffusion overpotential [
6]. In order to compensate for the capacity loss of thick cathodes at higher C-rates, three-dimensional (3D) architectures were generated on electrodes using ultrafast laser processing technique. It was proven that the generated 3D architectures improved battery performance parameters, such as capacity retention and cycle stability, especially for high C-rates [
7,
8,
9]. Therefore, it is of great significance to investigate the effect of electrode thickness and the laser processing pattern on battery performance, and to determine the possibility of realizing high energy and high power operations through combining ultrafast laser structuring approach with thick film electrode concept.
4. Discussion
Laser structuring of advanced NMC 622 cathode materials using ultrafast laser ablation was investigated, regarding an improvement of cycling performances, such as capacity retention as a function of C-rate [
7,
8,
9]. With ultrafast laser radiation, almost an ideal cold ablation process can be realized due to the well-known time-delay coupling of electron and phonon system [
11,
12].
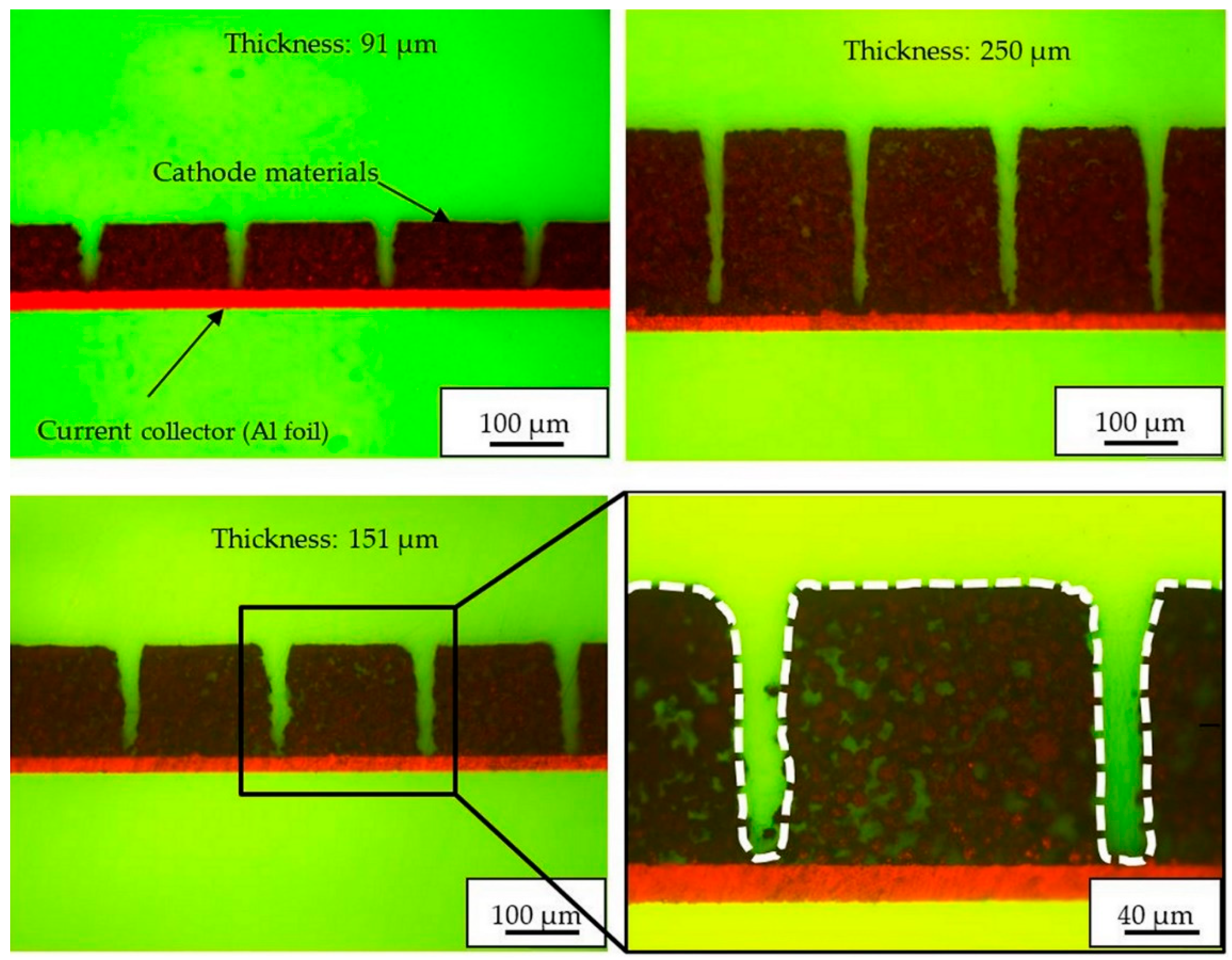
Table 1 shows that structured 91 µm cathodes with a pitch distance of 200 µm provided the highest mass loss of about 14%, while electrodes with higher film thickness showed a mass loss of about 8%. This is due to the wide crater opening of the established line structures which could be stated from the cross-section analyses (
Figure 2). It was observed that within a length of about 50 µm beneath the electrode surface, the side-walls of the generated groove structures had a slight inclination of about 5°, while for length larger than 50 µm, the channel geometry tended to become rectangularly shaped. This means that for thin film electrodes (≤ 50 µm), the mass loss could increase rapidly, while for higher film thicknesses, the slope of increase of active mass loss is decreased and converges to a linear rise as a function of thickness. In addition, it was found that the groove walls were not always ideally smoothly contoured, which might be due to the different ablation rates for different types of materials, such as binder and NMC 622 particles.
Figure 4 shows the rate capability of cells containing structured and unstructured NMC 622 cathodes. For the formation process at C/10, cells with structured cathodes have lower discharge capacity compared to unstructured cathodes. During formation, a larger solid electrolyte interface (SEI) was formed due to the increase of interface between electrode and electrolyte [
13]. This procedure consumes more active materials and electrolyte. Therefore, fewer lithium-ions are available for the intercalation process (discharge). Cells with structured NMC 622 cathodes begin to show superior performance at discharge rates from C/2 to 3C. Especially for electrodes with thicknesses of 151 µm and 250 µm, laser-structured cathodes with a pitch distance of 200 µm provide 40 mAh/g more discharge capacity at C/2 and 1C. The diffusion-controlled transport of lithium-ions in the cathode is considered a bottleneck, which strongly influences the discharge rate. The laser generated line structures lead to an increase in active surface area. With reduced pitch distance, the number of the line structures across the cathode increases, which results in a shorter length of average diffusion path for lithium-ions from electrolyte to bulk material. Therefore, this leads to a reduced cell polarization and an improved high rate capability. It is verified that the lithium concentration along the contour of 3D architectures in electrodes is significantly increased using laser-induced breakdown spectroscopy, which indicates an improvement of lithium mobility by providing additional lithium diffusion pathways [
14]. However, as the discharge rate increased from C/2 to 5C, all cells suffered from successive loss in discharge capacity. The decreasing of the discharge curve at higher C-rates corresponds to an enhanced cell polarization induced by the internal cell resistance [
6]. When being cycled again at C/5, the cell with 250 µm cathode shows an irreversible capacity loss of 5%, while other cells have 2%–3% loss. According to Zhang et al. [
15], during electrochemical cycling, side reactions can be induced, such as electrolyte oxidation with metallic lithium, which can promote the growth of the resistive surface layer. Another factor is the mechanical stability. Upon cycling, active materials suffer from a volume expansion and contraction with lithium intercalation and deintercalation. The change in volume can induce severe strain accumulation in the thick electrode, leading to increased internal stress [
16]. Especially at high C-rates above 1C, the rapid volume change will lead to crack formation or a fracture in the cathode laminate. Those cracks can cause particle isolation, resulting in capacity loss of the electrode.
For commercial applications, power density and energy density are more practical to characterize electrochemical cell performance. Since cathodes with 200 µm pitch distance show great improvement of rate capability for each thickness, the volumetric energy density of cells containing structured cathodes with 200 µm pitch distance and cells with unstructured cathodes were calculated from galvanostatic measurements and were plotted versus the power density (
Figure 7a). In order to be more consistent with the practical situation in the battery industry, the calculation of volumetric energy density with respect to cathode side was carried out using the total volume of the electrode material plus half of the volume of current collector and separator, since double-coated cathodes and anodes are mostly used in industry. This Ragone chart reveals that the energy density decreases with higher power density, especially for thick electrodes, and the energy density drops dramatically with increasing power. Clearly, there is a trade-off between energy density and power density controlled by the electrode thickness. At 100 W/L to 400 W/L, a cell with a 250 µm unstructured cathode has the highest energy density of about 1400 Wh/L, which is due to the decrease of current collector fraction with increasing electrode thickness. Meanwhile, all unstructured cathodes have 100 to 200Wh/L more energy density compared to structured cathodes, which means that for low power application (C-rate below C/2), the improvement of lithium-ion diffusion with 3D structures in electrode is insufficient to compensate the mass loss of electrode materials owing to laser structuring. However, laser structured cathodes show higher energy density with increased power when power density reached a threshold. For cathodes with 151 µm and 250 µm thicknesses, the turning point is 400 W/L, and 600 W/L, respectively. The cells with structured cathode of 91 µm thickness begin to show higher energy densities, at 3000 W/L, which is significantly delayed in comparison to cells with other cathodes, indicating that laser structuring technique is very suitable for improving the electrochemical performance of cells with thick cathodes at higher C-rates.
Furthermore, when the inactive components, such as current collector and separator are taken into account, the production cost of battery with thick film electrode will be significantly reduced.
Table 2 shows the total area and quantity of NMC 622 cathodes that are required for the production of 52-Ah automobile cells using an electrode design of 21 cm × 24 cm, as suggested by Wood et al. [
17]. The area of cathodes decreases from 1.45 m
2 to 0.56 m
2 with increasing film thickness from 91 µm to 250 µm, allowing for using half of the separators as well as the current collectors. For cells with 91 µm cathodes, 14 to 17 cathode sheets are needed, while only six sheets will be packed into the cells with cathodes having a thickness of 250 µm. As a result, the cost of electrode cutting and welding will be decreased. Besides, the difference of required area for 52-Ah cell between structured NMC 622 cathodes and unstructured NMC 622 cathodes is less distinct (the differences for cathodes with thicknesses of 91 µm and 250 µm are 0.12 m
2–0.02 m
2 and 0.02 m
2–0.01 m
2, respectively).
The energy density of an entire cell tends to be lower when anode and separator are taken into account, with about 370 Wh/L to 430 Wh/L energy density for cells with 100 µm to 250 µm cathodes [
18]. The cathode of 91 µm thickness has almost 1300 Wh/L, about 300 Wh/L more energy density, compared to the results from Zheng et al., of NMC 111 [
6], which is due to the higher specific capacity of NMC 622.
Figure 7b shows the improvement of energy density for cells with structured cathodes in comparison to cells with unstructured cathodes having the same thicknesses. For structured cathodes, a 100% to 400% higher energy density is observed at 1C to 5C. As for 151 µm cathode, the enhancement is more significant at 1C to 3C. Although cells with structured cathodes having a line pitch distance of 200 µm showed higher discharge capacity with increasing discharge rate comparing to cells having other line pitch distances, it is unnecessary to pursue higher power simply by decreasing the pitch distance, because that leads to a dramatic active mass loss, which in turn would increases the total battery manufacture cost. Therefore, the selection of pitch distance and electrode thickness should be related to specific demands of applications. For the stationary storage application, unstructured thick film electrodes are preferred, while for high powered usage, such as in electrical vehicles, laser structuring has great potential. Besides, thick film electrode and 3D electrode concepts ensure higher capacity as well as higher power, at intermediate C-rates around C/2.
The CV analyses of cells with NMC 622 cathodes in
Figure 5 show only one redox couple from 3 V to 4.3 V, which corresponds to the oxidation and reduction of Ni
2+/Ni
4+ [
19]. The oxidation peak of NMC 622 cathode appears at 3.8 V under 0.02 mV/s scan rate and gradually rises with increasing scan rate. The result is similar to the study by Cao et al. [
20]. Furthermore, CV is used to determine the chemical diffusion coefficient of lithium-ions (D
CV) in cathode materials during charge and discharge process using the Randles–Sevcik equation [
21]:
In this equation,
Ip is the maximum current from CV measurement during charge or discharge,
F is Faraday’s constant,
R is the gas constant,
T is the absolute temperature,
z0 is the number of transferred electrons,
A is the electrode area, and
C0 is the total amount of lithium-ions in an NMC 622 particle before delithiation. Since NMC 622 has a molar mass of 203.5 g/mol and a bulk density of 4.76 g/cm
3, the Li concentration
C0 was calculated to be 0.0234 mol/cm
3. According to the study from Denis et al. [
22], in order to calculate the chemical diffusion coefficient of lithium-ion in LiFeO
4 (LFP), electrode area
A in Equation (2) was substituted by 1/3 of the of the total Brunauer–Emmett–Teller (BET) surface area, because the lithium-ion diffusion path in LFP is one-dimensional. Since NMC 622 has a two-dimensional diffusion path for lithium-ion along (111) plane [
23], the electrode area
A is calculated as two-third of the total BET surface area for structured and unstructured electrodes. The BET surface equals the specific surface area multiplied by the mass of active material in the electrode, thus the electrode area is calculated with following equation:
The chemical diffusion coefficients of cells with different cathodes were calculated combining the Equations (2) and (3), as well as the slope from
Figure 6, and are shown in
Table 3. The diffusion coefficient of lithium-ions in NMC 622 electrode during discharge at room temperature varies from 2.7 × 10
−13 cm
2/s to 8.6 × 10
−13 cm
2/s. However, the values are lower compared to the diffusion coefficient acquired from galvanostatic intermittent titration technique (GITT) of 8 × 10
−11 cm
2/s [
24].
Table 3 shows that the diffusion coefficients of lithium-ions during charge process are higher comparing to discharge process, indicating that the during deintercalation, lithium-ions require less activation energy to diffuse from one octahedral site to the next site through an intermediate tetrahedral site [
24]. The lithium-ion diffusivity in the NMC 622 electrode decreases with increasing electrode thickness, which corresponds to the deterioration of rate capability for thick electrodes. Although structured cathodes show higher discharge capacity at C/2 to 2C in galvanostatic measurement, the diffusion coefficients of structured and unstructured cathodes are at the same level (
Table 3). However, it should be pointed out that the electrode area
A can only be controversially discussed. In recent research from Smyrek et al., it was shown that the sidewalls of laser generated patterns play a dominant role regarding lithium-ion diffusion [
14]. Therefore, it is justified to replace the electrode area
A (Equation (3)) by the electrode surface-shell envelope, assuming that the direct contact area of active material to free liquid electrolyte provides the main contribution to lithium-ion diffusion kinetics. After laser structuring, the surface of electrode can expand 100–300%. The generated surface leads to a shortened diffusion path for lithium-ions inside the bulk electrode, and therefore improves energy at high power. In addition, the electrode wetting ability of laser structured electrodes with liquid electrolytes can be accelerated and become more homogeneous due to the capillary effect, which is a benefit regarding cell production costs and safety issues [
25].
5. Conclusions
NMC 622 cathodes were assembled versus lithium using coin cell design. The influence of film thickness and laser structuring pattern on rate capability, as well as the diffusion coefficient of lithium-ions in electrodes were investigated. Galvanostatic measurements and cyclic voltammetry were applied to study the discharge capacity and energy density of the cells with structured and unstructured cathodes. An ultrafast laser with a wavelength of 1030 nm was used to generate line patterns down to the current collector in cathodes with film thicknesses of 91 µm to 250 µm. The pitch distance of line pattern was varied from 200 µm to 600 µm. Despite the mass loss due to the laser ablation of electrode material during laser processing, the cells containing structured cathodes with 200 µm pitch distance showed great improvement in specific capacity with discharge rates from C/2 to 3C. Comparing that to a cell with unstructured cathode, an increase of energy density from 50% to 300% was observed in galvanostatic measurements. At higher discharge rates of above 2C, the cells with 91 µm cathodes showed better electrochemical performance in terms of the volumetric energy density compared to cells with thicker cathode. Besides, volumetric energy density can be improved by increasing the electrode thickness at low C-rates (C/10 and C/5), due to a decrease of Al foil fraction inside the cathode. The increased surface area after laser processing provides new lithium diffusion pathways, which guarantees a high discharge capacity at elevated C-rates. However, a deterioration of energy density with increasing cathode thickness and increasing discharge rate was observed, which is due to the increase of internal resistance and the damage of the mechanical integrity of thick cathode material. Besides, the qualities of NMC 622 powder, such as particle sizes and purity, have impacts on the electrochemical performance, which were observed in the ongoing study using NMC 622 powder from another supplier. Cyclic voltammetry verifies that the chemical diffusion coefficient of lithium-ions in NMC 622 electrode decreases with increasing electrode film thickness. However, the diffusion coefficients of lithium-ions in structured and unstructured cathodes were almost the same. To explain the improved rate capability of structured cathodes, the expansion of the electrode’s surface must be taken into account. With line pattern, up to 400% surface expansion is applied to cathode, resulting in new diffusion paths for lithium-ions. The Ragone chart reveals a trade-off between power and energy for NMC 622 cathodes, and laser structuring shows an improvement of energy at higher power. Besides, the production cost of cells could be reduced, since less cathode sheets and other inactive components, such as separators and current collectors, are required for a 52-Ah automobile pouch cell with increasing electrode film thickness. Therefore, proper film thickness and laser structuring pattern should be selected related to the specific application-oriented demand. Thick electrodes (> 200 µm) without laser structuring are suitable for applications such as energy storage at low discharge rates of C/10 and C/5. While structured electrodes with thicknesses below 100 µm are more suitable for high-power (≥ 1000 W/L). Meanwhile, the combination of laser structuring and thick film electrode has potential in the automobile industry, where high power and high energy are simultaneously required.