Molecular Detection of Cordycepin-Induced HeLa Cell Apoptosis with Surface-Enhanced Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Drug Treatment
2.2. Differential Interference Contrast (DIC) and Fluorescence Microscopic Imaging
2.3. SERS Measurement and Analysis
3. Results and Discussion
3.1. Morphological Changes of Cells Undergoing Apoptosis
3.2. Analysis of SERS Spectra
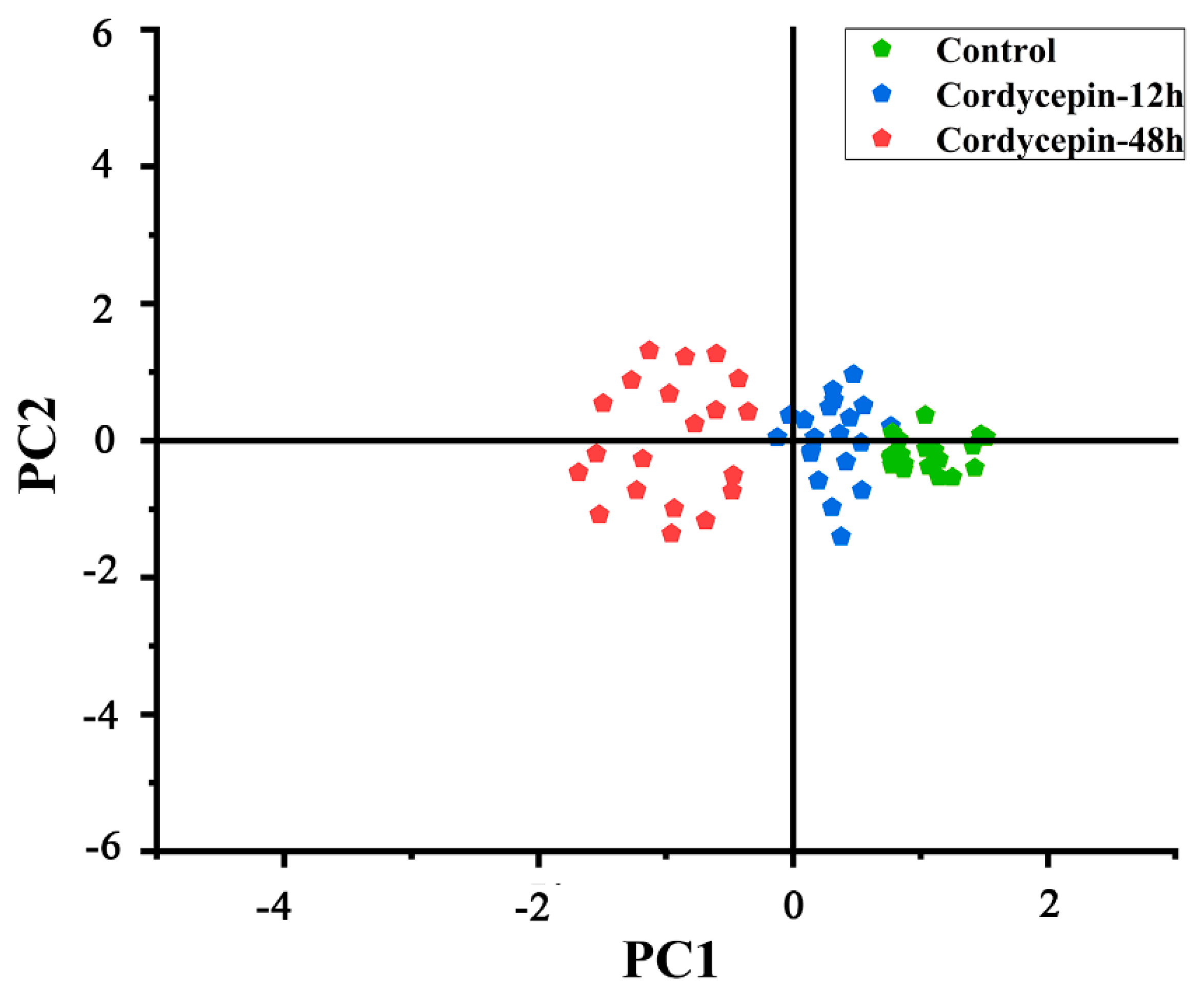
3.3. Principal Component Analysis of SERS Spectra
3.4. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer in women: Burden and trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Longoria, T.C.; Tewari, K.S. Pharmacologic Management of Advanced Cervical Cancer: Antiangiogenesis Therapy and Immunotherapeutic Considerations. Drugs 2015, 75, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Sun, Z.; He, Q.; Yan, F.; Wang, Y.; Zhang, J. Aspirin inhibits ErbB2 to induce apoptosis in cervical cancer cells. Med. Oncol. 2010, 27, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Adhikary, A.; Ray, P.; Saha, S.; Chakraborty, S.; Mohanty, S.; Das, K.; Mukherjee, S.; Mazumdar, M.; Lahiri, L.; et al. Restoration of tumor suppressor p53 by differentially regulating pro- and anti-p53 networks in HPV-18-infected cervical cancer cells. Oncogene 2012, 31, 173–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lowe, S.W.; Lin, A.W. Apoptosis in Cancer. Carcinogenesis 2000, 21, 485. [Google Scholar] [CrossRef] [PubMed]
- Torriglia, A.; Negri, C.; Chaudun, E.; Prosperi, E.; Courtois, Y.; Counis, M.F.; Scovassi, A.I. Differential involvement of DNases in HeLa cell apoptosis induced by etoposide and long term-culture. Cell Death Differ. 1999, 6, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, R.V.; Murugan, R.S.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef]
- Cao, J.-T.; Zhu, Y.-D.; Rana, R.K.; Zhu, J.-J. Microfluidic chip integrated with flexible PDMS-based electrochemical cytosensor for dynamic analysis of drug-induced apoptosis on HeLa cells. Biosens. Bioelectron. 2014, 51, 97–102. [Google Scholar] [CrossRef]
- Yue, G.G.-L.; Lee, J.K.-M.; Chan, B.C.-L.; Kwok, H.-F.; Hoi, S.W.-H.; Sze, D.M.-Y.; Fung, K.-P.; Leung, P.-C.; Lau, C.B.-S. An innovative anti-cancer Chinese herbal formula exhibited multi-targeted efficacies in metastatic breast cancer mouse model. Chin. Med. 2018, 13, 64. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Ji, Y.; An, P.; Zhang, S.; Li, Z. Traditional Herbal Medicine: A Review of Potential of Inhibitory Hepatocellular Carcinoma in Basic Research and Clinical Trial. Evid. Based Complement. Altern. Med. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sharma, A.K.; Sandhu, S.S.; Kashyap, D. Cordycepin: A bioactive metabolite with therapeutic potential. Life Sci. 2013, 93, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Overgaard-Hansen, K. The inhibition of 5-phosphoribosyl-1-pyrophosphate formation by cordycepin triphosphate in extracts of Ehrlich ascites tumor cells. BBA Spec. 1964, 80, 504–507. [Google Scholar] [CrossRef]
- Rottman, F.; Guarino, A.J. The inhibition of phosphoribosyl-pyrophosphate amidotransferase activity by cordycepin monophosphate. Biochim. Biophys. Acta (BBA) Spéc. Sect. Enzym. Subj. 1964, 89, 465–472. [Google Scholar] [CrossRef]
- Holbein, S.; Wengi, A.; Decourty, L.; Freimoser, F.M.; Jacquier, A.; Dichtl, B. Cordycepin interferes with 3′ end formation in yeast independently of its potential to terminate RNA chain elongation. RNA 2009, 15, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.Y.; Moon, A.; Duffin, R.; Barthet-Barateig, A.; Meijer, H.A.; Clemens, M.J.; de Moor, C.H. Cordycepin inhibits protein synthesis and cell adhesion through effects on signal transduction. J. Biol. Chem. 2010, 285, 2610–2621. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-W.; Park, C.; Cha, H.-J.; Hong, S.H.; Park, S.-H.; Kim, G.-Y.; Kim, W.J.; Kim, C.H.; Song, K.S.; Choi, Y.H. Cordycepin inhibits lipopolysaccharide-induced cell migration and invasion in human colorectal carcinoma HCT-116 cells through down-regulation of prostaglandin E2 receptor EP4. BMB Rep. 2018, 51, 532–537. [Google Scholar] [CrossRef]
- Choi, S.; Lim, M.-H.; Kim, K.M.; Jeon, B.H.; Song, W.O.; Kim, T.W. Cordycepin-induced apoptosis and autophagy in breast cancer cells are independent of the estrogen receptor. Toxicol. Appl. Pharmacol. 2011, 257, 165–173. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Jin, C.-Y.; Park, C.; Hong, S.H.; Kim, G.-Y.; Jeong, Y.K.; Lee, J.-D.; Yoo, Y.H.; Choi, Y.H. Induction of apoptosis by cordycepin via reactive oxygen species generation in human leukemia cells. Toxicol. Vitr. 2011, 25, 817–824. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.-C.; Lin, Y.-T.; Huang, S.-H.; Wang, S.-M. Cordycepin Induces Apoptosis of CGTH W-2 Thyroid Carcinoma Cells through the Calcium-Calpain-Caspase 7-PARP Pathway. J. Agric. Food Chem. 2010, 58, 11645–11652. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, S.-H.; Hueng, D.-Y.; Syu, J.-P.; Liao, C.-C.; Wu, Y.-C. Cordycepin induces apoptosis of C6 glioma cells through the adenosine 2A receptor-p53-caspase-7-PARP pathway. Chem. Interact. 2014, 216, 17–25. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Park, S.J.; Jo, E.; Hwang, I.H.; Lee, K.B.; Kim, S.W.; Kim, D.J.; Joo, J.C.; Hong, S.H.; Lee, M.G.; et al. Cordycepin induces apoptosis of human ovarian cancer cells by inhibiting CCL5-mediated Akt/NF-kappaB signaling pathway. Cell Death Discov. 2018, 4, 62. [Google Scholar] [CrossRef]
- Liu, X.; Fu, R.; Pan, Y.; Meza-Sosa, K.F.; Zhang, Z.; Lieberman, J. PNPT1 Release from Mitochondria during Apoptosis Triggers Decay of Poly(A) RNAs. Cell 2018, 174, 187–201. [Google Scholar] [CrossRef]
- Hickman, J.A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992, 11, 121–139. [Google Scholar] [CrossRef]
- Jiang, Q.L.; Zhang, S.; Tian, M.; Zhang, S.Y.; Xie, T.; Chen, D.Y.; Chen, Y.J.; He, J.; Liu, J.; Ouyang, L.; et al. Plant lectins, from ancient sugar-binding proteins to emerging anti-cancer drugs in apoptosis and autophagy. Cell Prolif. 2015, 48, 17–28. [Google Scholar] [CrossRef]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective assessment of changes in nuclear morphology and cell distribution following induction of apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Vashishtha, S.C.; Nazarali, A.J.; Dimmock, J.R. Application of Fluorescence Microscopy to Measure Apoptosis in Jurkat T Cells After Treatment with a New Investigational Anticancer Agent (N.C.1213). Cell. Mol. Neurobiol. 1998, 18, 437–445. [Google Scholar] [CrossRef]
- Sun, M.G.; Williams, J.; Munoz-Pinedo, C.; Perkins, G.A.; Brown, J.M.; Ellisman, M.H.; Green, D.R.; Frey, T.G. Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nat. Cell Biol. 2007, 9, 1057–1065. [Google Scholar] [CrossRef]
- Stine, K.C.; Warren, B.; Becton, D.L. Apoptosis Induced by Interleukin-12 Measured by DNA Electrophoresis and in Situ End Labeling in Leukemia. Ann. N. Y. Acad. Sci. 2010, 795, 420–421. [Google Scholar] [CrossRef]
- Sundaram, N.; Pahwa, A.K.; Ard, M.D.; Lin, N.; Perkins, E.; Bowles, A.P. Selenium Causes Growth Inhibition and Apoptosis in Human Brain Tumor Cell Lines. J. Neuro-Oncol. 2000, 46, 125–133. [Google Scholar] [CrossRef]
- Fouladi-Nashta, A.A.; Alberio, R.; Kafi, M.; Nicholas, B.; Campbell, K.H.; Webb, R. Differential staining combined with TUNEL labelling to detect apoptosis in preimplantation bovine embryos. Reprod. Biomed. Online 2005, 10, 497–502. [Google Scholar] [CrossRef]
- Ubai, T.; Azuma, H.; Kotake, Y.; Inamoto, T.; Takahara, K.; Ito, Y.; Kiyama, S.; Sakamoto, T.; Horie, S.; Muto, S.; et al. FTY720 induced BCL-associated and FAS-independent apoptosis in human renal cancer cells in vitro and significantly reduced in vivo tumor growth in mouse xenograft. Anticancer Res. 2007, 27, 75–88. [Google Scholar]
- Morris, M.D. Review—Modern Raman Spectroscopy: A Practical Approach. Anal. Chem. 2006, 78, 33. [Google Scholar] [CrossRef][Green Version]
- Matthäus, C.; Bird, B.; Miljković, M.; Chernenko, T.; Romeo, M.; Diem, M. Infrared and Raman microscopy in cell biology. Methods Cell Biol. 2008, 89, 275. [Google Scholar]
- Koenig, J.L. Raman spectroscopy of biological molecules: A review. J. Polym. Sci. Part A Polym. Chem. 2010, 6, 59–177. [Google Scholar] [CrossRef]
- Kneipp, K. Surface-enhanced Raman scattering. Phys. Today 2007, 60, 40–46. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, Z.; Xu, T.; Su, S.; Zhong, Y.; Peng, F.; Su, Y.; He, Y. Surface-Enhanced Raman Scattering-Based Sensing In Vitro: Facile and Label-Free Detection of Apoptotic Cells at the Single-Cell Level. Anal. Chem. 2013, 85, 2809–2816. [Google Scholar] [CrossRef]
- Badr, Y.; Mahmoud, M. Effect of silver nanowires on the surface-enhanced Raman spectra (SERS) of the RNA bases. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 639–645. [Google Scholar] [CrossRef]
- Das, G.; Mecarini, F.; Gentile, F.; De Angelis, F.; Kumar, H.M.; Candeloro, P.; Liberale, C.; Cuda, G.; Di Fabrizio, E. Nano-patterned SERS substrate: Application for protein analysis vs. temperature. Biosens. Bioelectron. 2009, 24, 1693–1699. [Google Scholar] [CrossRef]
- Kneipp, K.; Flemming, J. Surface enhanced Raman scattering (SERS) of nucleic acids adsrobed on colloidal silver particles. J. Mol. Struct. 1986, 145, 173–179. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D.J.J. Adsorption and Surface-Enhanced Raman of Dyes on Silver and God Sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Sato-Berrú, R.; Mejía-Uriarte, E.V.; Frausto-Reyes, C.; Villagrán-Muniz, M.; Saniger, J.M. Application of principal component analysis and Raman spectroscopy in the analysis of polycrystalline BaTiO3 at high pressure. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 66, 557–560. [Google Scholar] [CrossRef]
- Shu, Z.; Shi, X.; Nie, D.; Guan, B. Low-Molecular-Weight Fucoidan Inhibits the Viability and Invasiveness and Triggers Apoptosis in IL-1beta-Treated Human Rheumatoid Arthritis Fibroblast Synoviocytes. Inflammation 2015, 38, 1777–1786. [Google Scholar] [CrossRef]
- Moritz, T.J.; Taylor, D.S.; Krol, D.M.; Fritch, J.; Chan, J.W. Detection of doxorubicin-induced apoptosis of leukemic T-lymphocytes by laser tweezers Raman spectroscopy. Biomed. Opt. Express 2010, 1, 1138–1147. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Wek, R.C. Phosphorylation of the alpha-subunit of the eukaryotic initiation factor-2 (eIF2alpha) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J. Biol. Chem. 2005, 280, 14189–14202. [Google Scholar] [CrossRef]
- Jones, B.E.; Lo, C.R.; Srinivasan, A.; Valentino, K.L.; Czaja, M.J. Ceramide induces caspase-independent apoptosis in rat hepatocytes sensitized by inhibition of RNA synthesis. Hepatology 1999, 30, 215–222. [Google Scholar] [CrossRef]
- Bushell, M.; Stoneley, M.; Sarnow, P.; Willis, A. Translation inhibition during the induction of apoptosis: RNA or protein degradation? Biochem. Soc. Trans. 2004, 32, 606–610. [Google Scholar] [CrossRef]
- Hu, S.; Feng, Y.; Zhang, D.; Lu, X.; Tian, J.; Fan, J.; Zhong, L. Raman spectral changes of Artemisinin-induced Raji cells apoptosis. Vib. Spectrosc. 2015, 81, 83–89. [Google Scholar] [CrossRef]
- Jovanovic, M.; Hengartner, M.O. miRNAs and apoptosis: RNAs to die for. Oncogene 2006, 25, 6176–6187. [Google Scholar] [CrossRef]


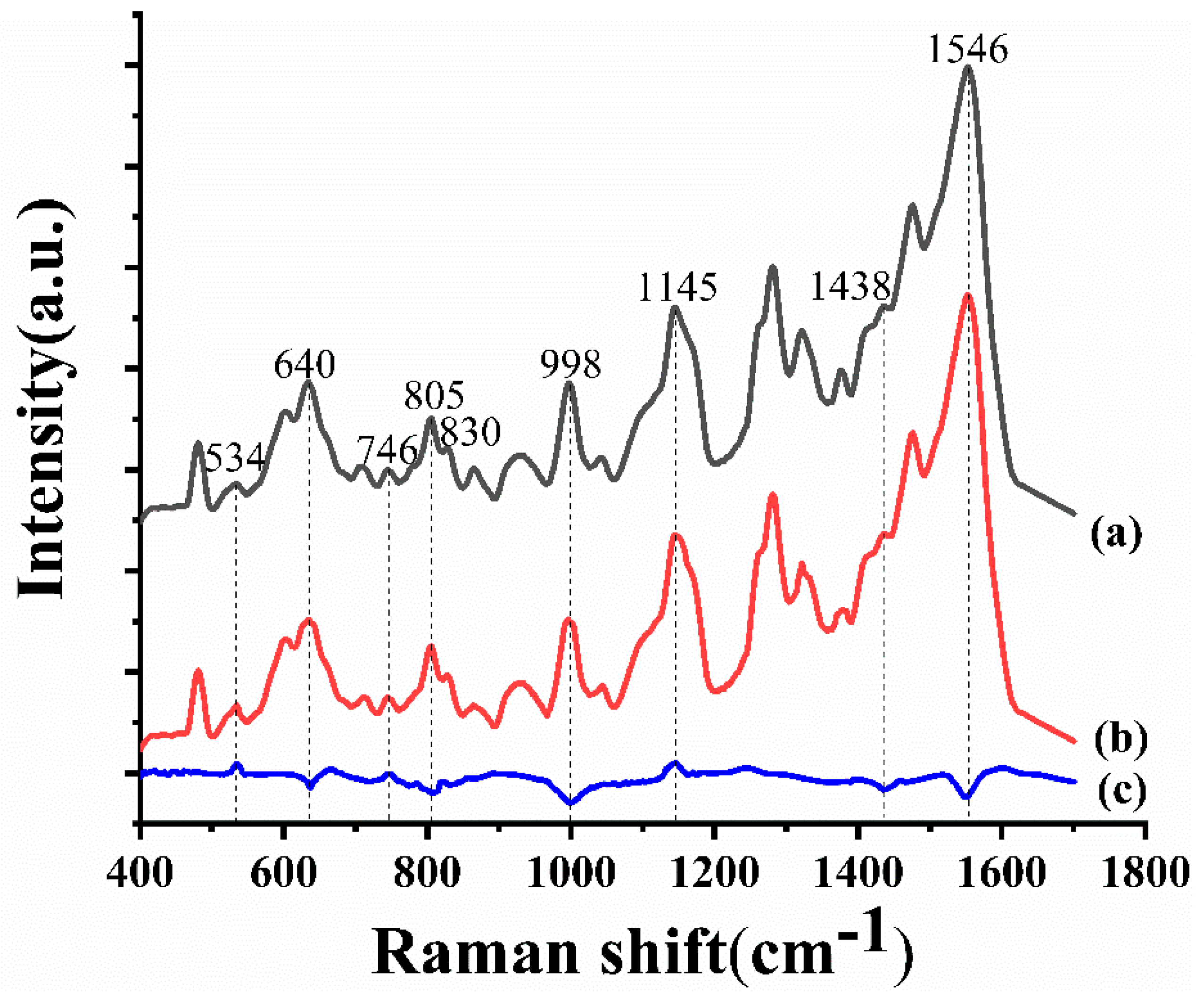


| Raman shift (cm−1) | Assignment |
|---|---|
| 534 | Adenine ring breath |
| 640 | C–C twist of tyrosine |
| 746 | Thymine ring breath |
| 805 | RNA |
| 830 | O–P–O asymmetric stretch, tyrosine ring breath |
| 998 | Phenylalanine symmetric ring breath |
| 1145 | Deoxyribose phosphate |
| 1438 | RNA |
| 1546 | Amide Ⅱ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, P.; Xu, L.; Wang, L.; Chen, N.; Zhang, X.; Chen, H.; Li, J. Molecular Detection of Cordycepin-Induced HeLa Cell Apoptosis with Surface-Enhanced Raman Spectroscopy. Appl. Sci. 2019, 9, 3990. https://doi.org/10.3390/app9193990
Ma P, Xu L, Wang L, Chen N, Zhang X, Chen H, Li J. Molecular Detection of Cordycepin-Induced HeLa Cell Apoptosis with Surface-Enhanced Raman Spectroscopy. Applied Sciences. 2019; 9(19):3990. https://doi.org/10.3390/app9193990
Chicago/Turabian StyleMa, Pei, Lei Xu, Luyao Wang, Nan Chen, Xuedian Zhang, Hui Chen, and Junying Li. 2019. "Molecular Detection of Cordycepin-Induced HeLa Cell Apoptosis with Surface-Enhanced Raman Spectroscopy" Applied Sciences 9, no. 19: 3990. https://doi.org/10.3390/app9193990
APA StyleMa, P., Xu, L., Wang, L., Chen, N., Zhang, X., Chen, H., & Li, J. (2019). Molecular Detection of Cordycepin-Induced HeLa Cell Apoptosis with Surface-Enhanced Raman Spectroscopy. Applied Sciences, 9(19), 3990. https://doi.org/10.3390/app9193990





