Accelerated Charge Dissipation by Gas-Phase Fluorination on Nomex Paper
Abstract
:1. Introduction
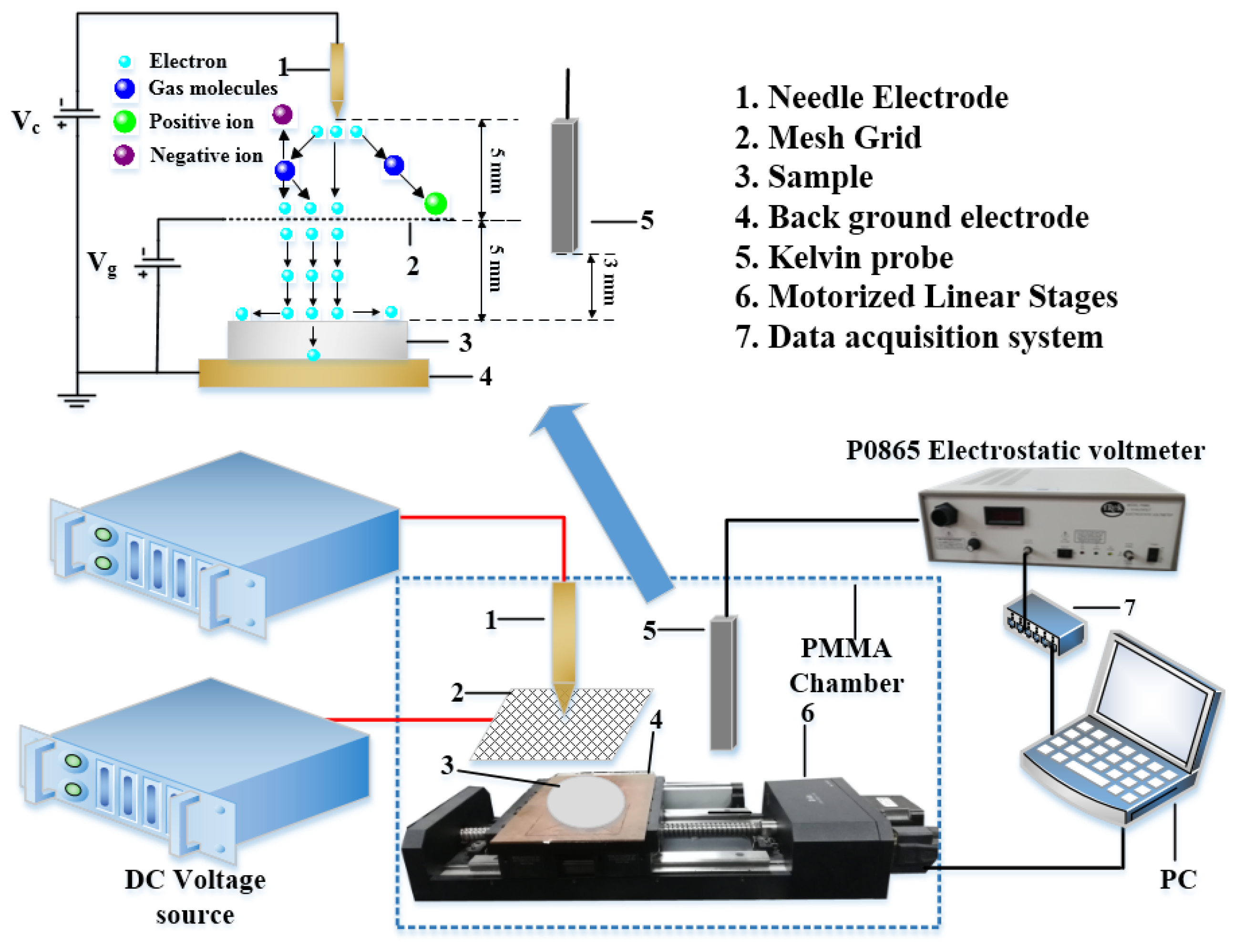
2. Experimental Section
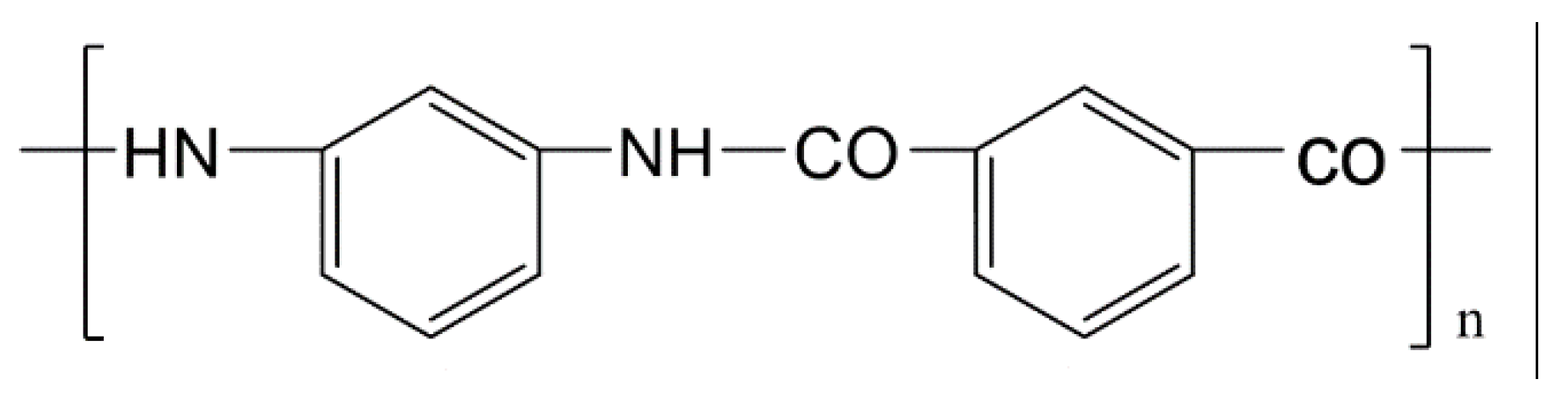
2.1. Sample Preparation
2.2. Sample Characterization
3. Results and Discussion
3.1. Surface Chemical Structure
3.2. Surface Charge Accumulation and Dissipation
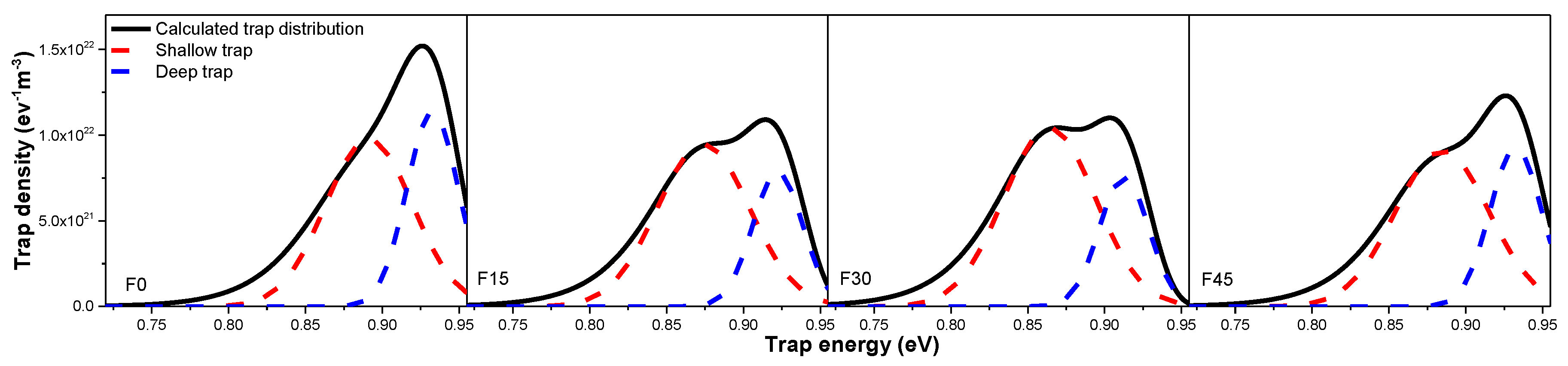
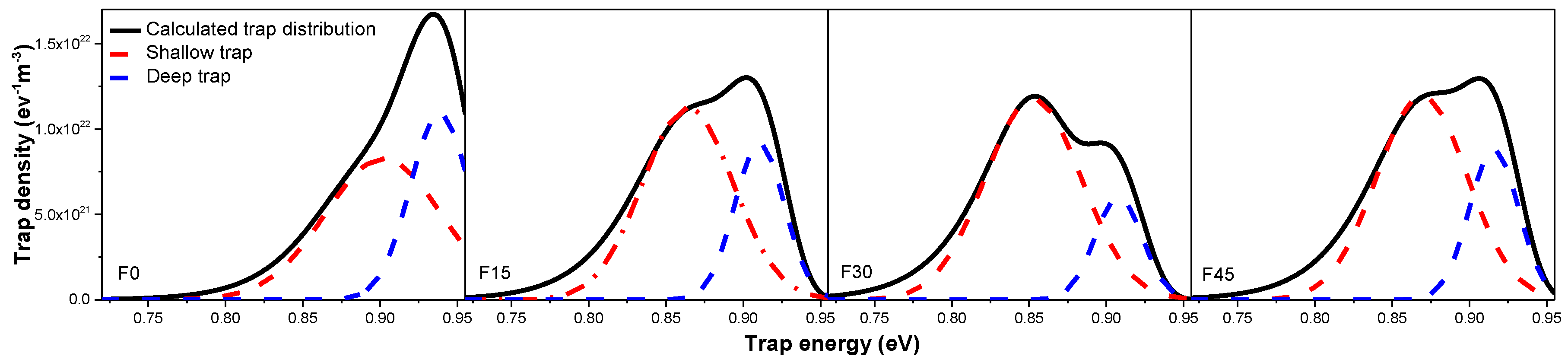
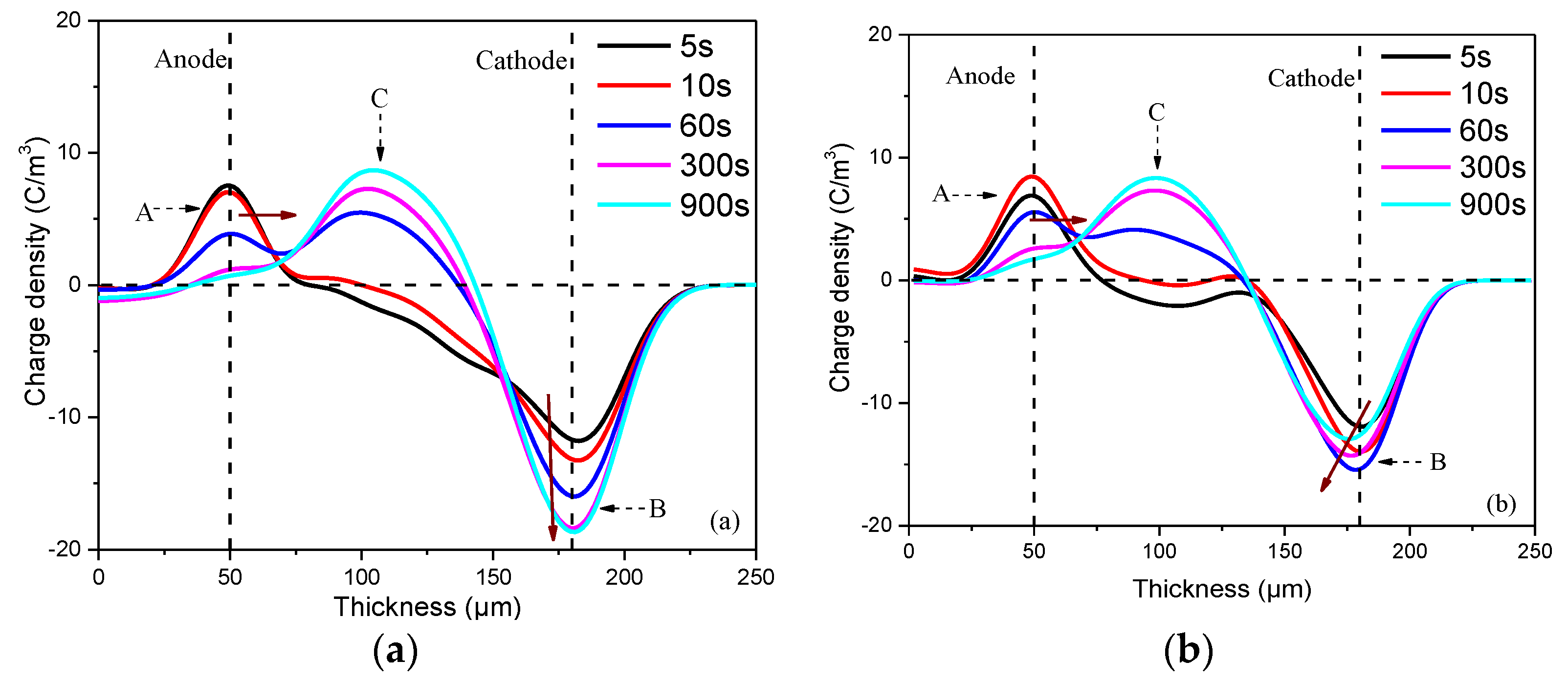
3.3. Space Charge Accumulation and Dissipation
4. Conclusions
- Gas-phase fluorination could be an effective approach to modify the surface charge dynamics of oil-impregnated Nomex paper. The considerable molecular chain scission and cleavage during fluorination are evident from ATR-IR spectra. The positive and negative surface potential decay rates are accelerated with the increase of fluorination duration and slowed down with continuous extension of fluorination duration. The samples fluorinated for 30 min show highest decay rate.
- The trap energy level and density distribution obtained by the isothermal surface potential decay method show that gas-phase fluorination exerts a great influence on the trap characteristic of oil-impregnated Nomex paper, which is mainly attributed to the competition between physical and chemical defects change caused by fluorination. The difference between the hole trap and electron trap energy level and density of the original and the fluorinated samples are mainly caused by the different conduction path of hole and electron.
- The space charge measured by the pulsed electro-acoustic (PEA) system shows that the charge mobility of the fluorinated samples is higher than that of the original sample. The negative charge of the fluorinated samples accumulated near the cathode shows obvious inward migration tendency, while the peak value is smaller compared to the non-fluorinated samples. The space charge decay rate of the fluorinated samples is also faster than the non-fluorinated one. Nevertheless, the influence of gas-phase fluorination on other electrical properties of oil-impregnated Nomex paper will be explored, because previous research has shown that gas-phase fluorination brings about remarkable improvement of flashover strength, partial discharge resistance or corona resistance of the polymer such as polyimide and epoxy resin.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wen, M.; Song, J.; Song, Y.; Liu, Y.; Li, C.; Wang, P. Reliability assessment of insulation system for dry type transformers. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 1998–2008. [Google Scholar] [CrossRef]
- Carrascal, I.A.; Fernández-Diego, C.; Casado, J.A.; Diego, S.; Fernández, I.; Ortiz, A. Quantification of Kraft paper ageing in mineral oil impregnated insulation systems through mechanical characterization. Cellulose 2018, 25, 3583–3594. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zahn, M.K. Kerr electro-optic field mapping study of the effect of charge injection on the impulse breakdown strength of transformer oil. Appl. Phys. Lett. 2013, 103, 162906. [Google Scholar] [CrossRef]
- Du, B.X.; Li, X.L.; Jiang, J.P. Surface charge accumulation and decay on directfluorinated oil-impregnated paper. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3094–3101. [Google Scholar] [CrossRef]
- Tang, C.; Chen, G.; Fu, M.; Liao, R. Space charge behavior in multi-layer oil-paper insulation under different DC voltages and temperatures. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Chen, G.; Liao, R.; Yang, L.; Tang, C. Influence of Moisture on Space Charge Dynamics in Multilayer Oil-Paper Insulation. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1456–1464. [Google Scholar] [CrossRef]
- Kai, W.; Zhu, Q.; Wang, H.; Xia, W.; Li, S. Space charge behavior in the sample with two layers of oil-immersed-paper and oil. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1857–1865. [Google Scholar]
- Wang, S.Q.; Zhang, G.J.; Mu, H.B.; Wang, D.; Lei, M.; Suwarno, S.; Tanaka, Y.; Takada, T. Effects of paper-aged state on space charge characteristics in oil-impregnated paper insulation. IEEE Trans. Dielectr. Electr. Insul. 2013, 19, 1871–1878. [Google Scholar] [CrossRef]
- Meng, H.; Zhou, Y.; Chen, W.; Sha, Y.; Jin, F. Influence of Voltage Reversal on Space Charge Behavior in Oil-paper Insulation. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 331–339. [Google Scholar]
- Du, B.X.; Jiang, J.P.; Zhang, J.G.; Liu, D.S. Dynamic behavior of surface charge on double-layer oil-paper insulation under pulse voltage. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2712–2719. [Google Scholar] [CrossRef]
- Sun, P.; Sima, W.; Chen, J.; Zhang, D.; Jiang, X.; Chen, Q. An application area of C 60: Overall improvement of insulating oil’s electrical performance. Appl. Phys. Lett. 2018, 112, 142902. [Google Scholar] [CrossRef]
- Du, B.; Jian, L.; Wang, F.; Wei, Y.; Yao, S. Influence of Monodisperse Fe3O4 Nanoparticle Size on Electrical Properties of Vegetable Oil-Based Nanofluids. J. Nanomater. 2015, 2015, 3. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, Z.; Jian, L.; Wu, L.; Xiang, C. Enhanced Electrical Insulation and Heat Transfer Performance of Vegetable Oil Based Nanofluids. J. Nanomater. 2018, 2018, 4504208. [Google Scholar]
- Abd-Elhady, A.M.; Ibrahim, M.E.; Taha, T.A.; Izzularab, M.A. Dielectric and Thermal Properties of Transformer Oil Modified by Semiconductive CdS Quantum Dots. J. Electron. Mater. 2016, 45, 1–7. [Google Scholar] [CrossRef]
- Amiri, A.; Shanbedi, M.; Ahmadi, G.; Rozali, S. Transformer oils-based graphene quantum dots nanofluid as a new generation of highly conductive and stable coolant. Int. Commun. Heat Mass Transf. 2017, 83, 40–47. [Google Scholar] [CrossRef]
- Song, Z.; Chao, T.; Jian, H.; Wang, X. Thermal stability and dielectric properties of nano-SiO2 doped cellulose. Appl. Phys. Lett. 2017, 111, 012902. [Google Scholar]
- Jian, H.; Li, Y.; Liao, R.; Liu, G.; Qiang, L.; Chao, T. Fabrication of Al2O3 Nano-Structure Functional Film on a Cellulose Insulation Polymer Surface and Its Space Charge Suppression Effect. Polymers 2017, 9, 502. [Google Scholar] [Green Version]
- Sahin, H.T.; Manolache, S.; Young, R.A.; Denes, F. Surface fluorination of paper in CF4 -RF plasma environments. Cellulose 2002, 9, 171–181. [Google Scholar] [CrossRef]
- Vaswani, S.; Koskinen, J.; Hess, D.W. Surface modification of paper and cellulose by plasma-assisted deposition of fluorocarbon films. Surf. Coat. Technol. 2005, 195, 121–129. [Google Scholar] [CrossRef]
- Tan, I.H.; Silva, M.L.P.D.; Demarquette, N.R. Paper surface modification by plasma deposition of double layers of organic silicon compounds. J. Mater. Chem. 2001, 11, 1019–1025. [Google Scholar] [CrossRef]
- An, Z.; Qiang, Y.; Chen, X.; Yue, J.; Zheng, F.; Zhang, Y. Suppression effect of surface fluorination on charge injection into linear low density polyethylene. J. Appl. Phys. 2009, 105, 13–69. [Google Scholar] [CrossRef]
- An, Z.; Yao, J.; Mao, M.; Zhang, Y.; Xia, Z. Significantly improved charge stability of cellular polypropylene films by fluorination and subsequent annealing. J. Electrost. 2010, 68, 523–527. [Google Scholar] [CrossRef]
- Du, B.X.; Jie, L.; Du, H.; Yi, Y. Effect of surface fluorination on space charge behavior in multilayered polyimide films. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1817–1823. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, G.; Qiang, W.; Li, C.; He, J.; An, Z. Suppression of surface charge accumulation on Al2O3-filled epoxy resin insulator under dc voltage by direct fluorination. Aip Adv. 2015, 5, 77–241. [Google Scholar] [CrossRef]
- Du, B.X.; Zhu, W.B.; Li, X.L. Effects of direct fluorination on charge coupling behavior of oil-paper insulation under DC and pulse voltages. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 947–955. [Google Scholar] [CrossRef]
- Maeno, T.; Futami, T.; Kushibe, H.; Takada, T.; Cooke, C.M. Measurement of spatial charge distribution in thick dielectrics using the pulsed electroacoustic method. IEEE Trans. Electr. Insul. 1988, 23, 433–439. [Google Scholar] [CrossRef]
- Wang, H.H.; Lin, M.F. Modification of nylon-6 with wholly rigid poly(m-phenylene isophthalamide). J. Appl. Polym. Sci. 1991, 43, 259–269. [Google Scholar] [CrossRef]
- Li, J.; Zhou, F.; Min, D.; Li, S.; Rong, X. The Energy Distribution of Trapped Charges in Polymers Based on Isothermal Surface Potential Decay Model. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1723–1732. [Google Scholar] [CrossRef]
- Wang, W.; Min, D.; Li, S. Understanding the Conduction and Breakdown Properties of Polyethylene Nanodielectrics: Effect of deep traps. IEEE Trans. Dielectr. Electr. Insul. 2015, 23, 564–572. [Google Scholar] [CrossRef]
- Meunier, M.; Quirke, N.; Aslanides, A. Molecular modeling of electron traps in polymer insulators: Chemical defects and impurities. J. Chem. Phys. 2001, 115, 2876–2881. [Google Scholar] [CrossRef]
- An, Z.; Chen, X.; Yue, J.; Zheng, F.; Zhang, Y. Significant suppression of space charge injection into linear low density polyethylene by surface oxyfluorination. J. Appl. Phys. 2009, 106, 11. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; He, L.; Khan, M.Z.; Zhang, T.; Zhao, Q.; He, Y.; Huang, Z.; Zhao, H.; Li, J. Accelerated Charge Dissipation by Gas-Phase Fluorination on Nomex Paper. Appl. Sci. 2019, 9, 3879. https://doi.org/10.3390/app9183879
Wang F, He L, Khan MZ, Zhang T, Zhao Q, He Y, Huang Z, Zhao H, Li J. Accelerated Charge Dissipation by Gas-Phase Fluorination on Nomex Paper. Applied Sciences. 2019; 9(18):3879. https://doi.org/10.3390/app9183879
Chicago/Turabian StyleWang, Feipeng, Li He, Muhammad Zeeshan Khan, Tao Zhang, Qi Zhao, Yushuang He, Zhengyong Huang, Haisen Zhao, and Jian Li. 2019. "Accelerated Charge Dissipation by Gas-Phase Fluorination on Nomex Paper" Applied Sciences 9, no. 18: 3879. https://doi.org/10.3390/app9183879
APA StyleWang, F., He, L., Khan, M. Z., Zhang, T., Zhao, Q., He, Y., Huang, Z., Zhao, H., & Li, J. (2019). Accelerated Charge Dissipation by Gas-Phase Fluorination on Nomex Paper. Applied Sciences, 9(18), 3879. https://doi.org/10.3390/app9183879





