Emissions from Combustion of Second-Generation Biodiesel Produced from Seeds of Date Palm Fruit (Phoenix dactylifera L.)
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Biodiesel Production from Date Seeds
2.2. Experimental Settings and Engine Test Rig
3. Results and Discussion
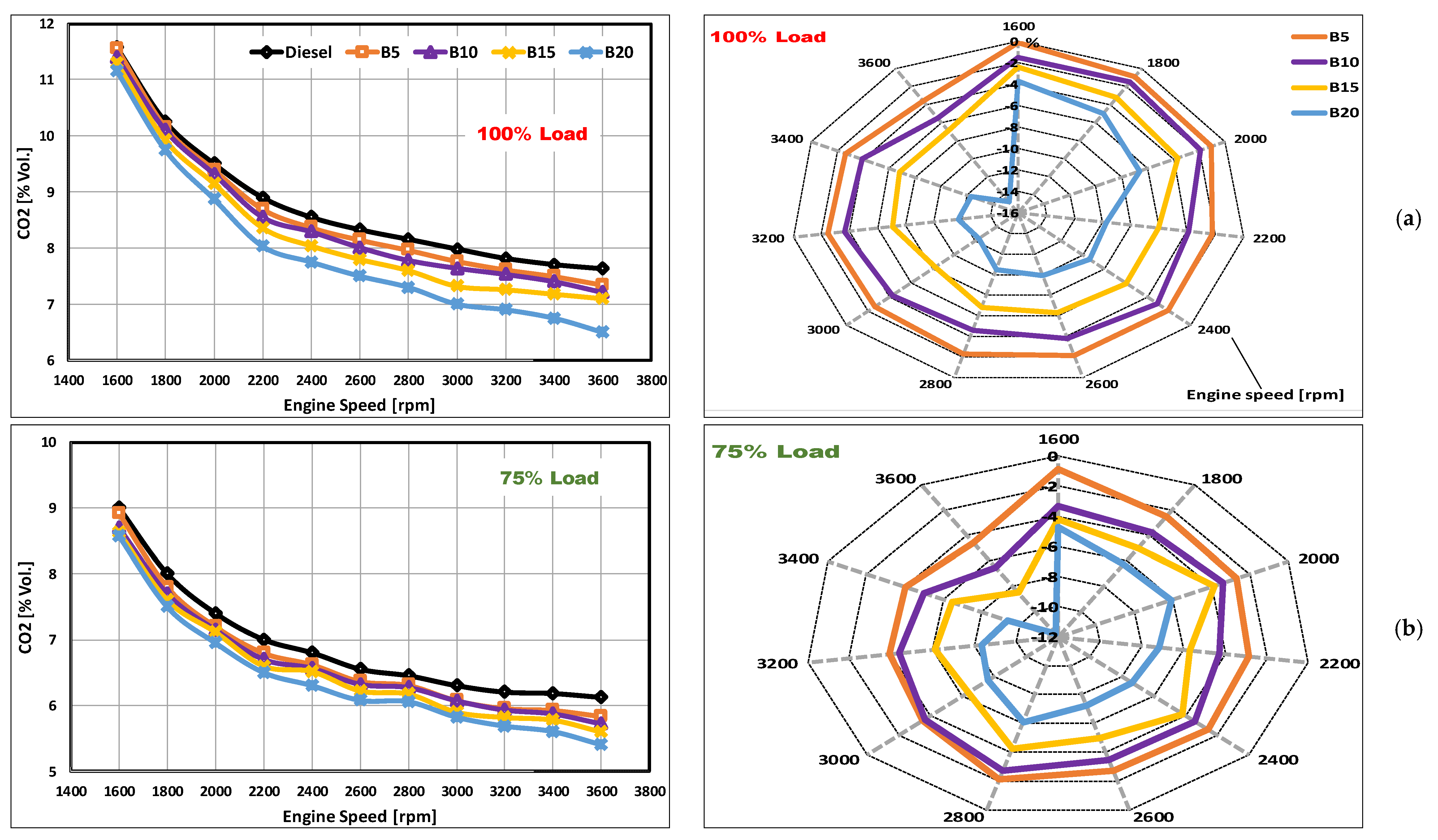
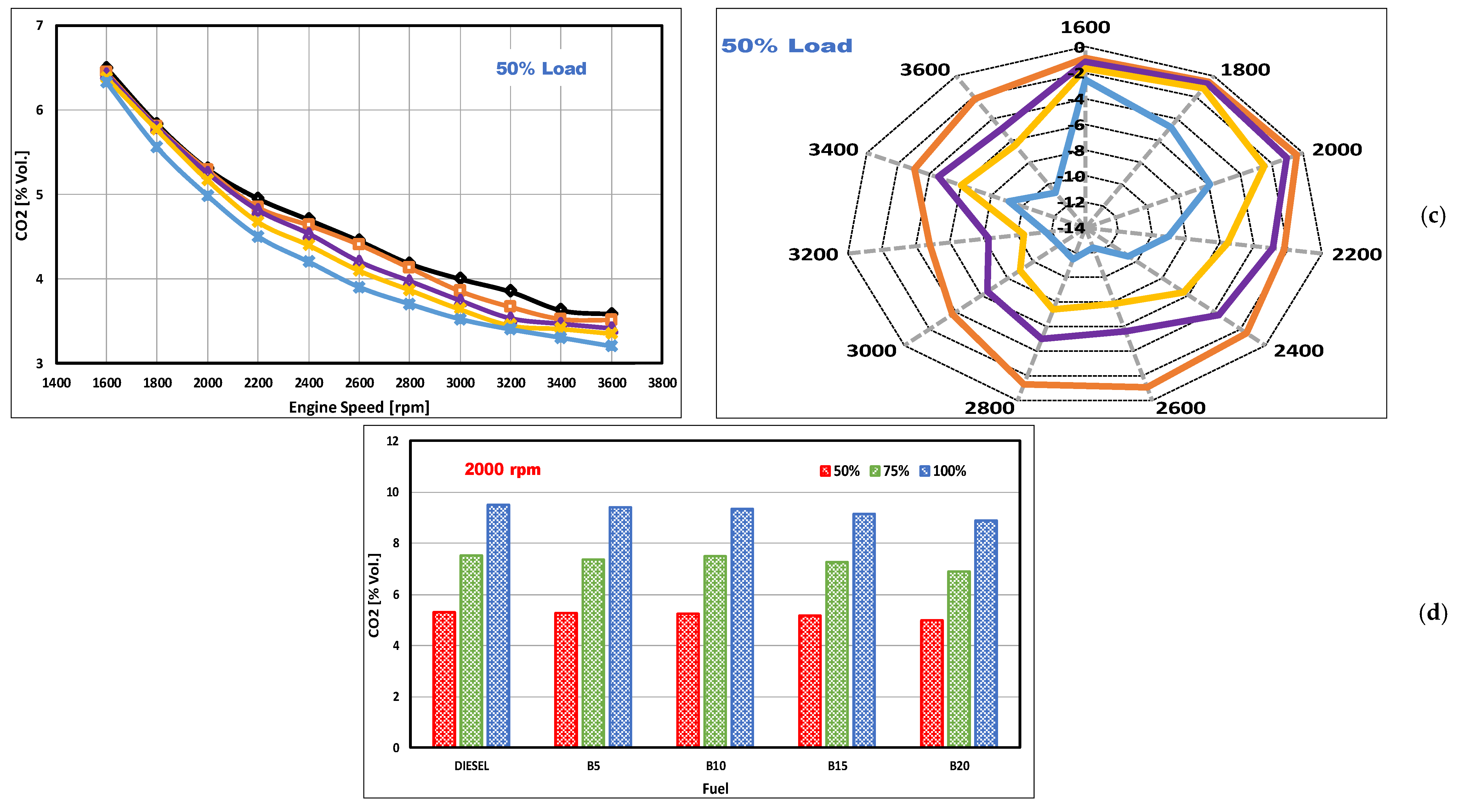
3.1. CO2 Emissions
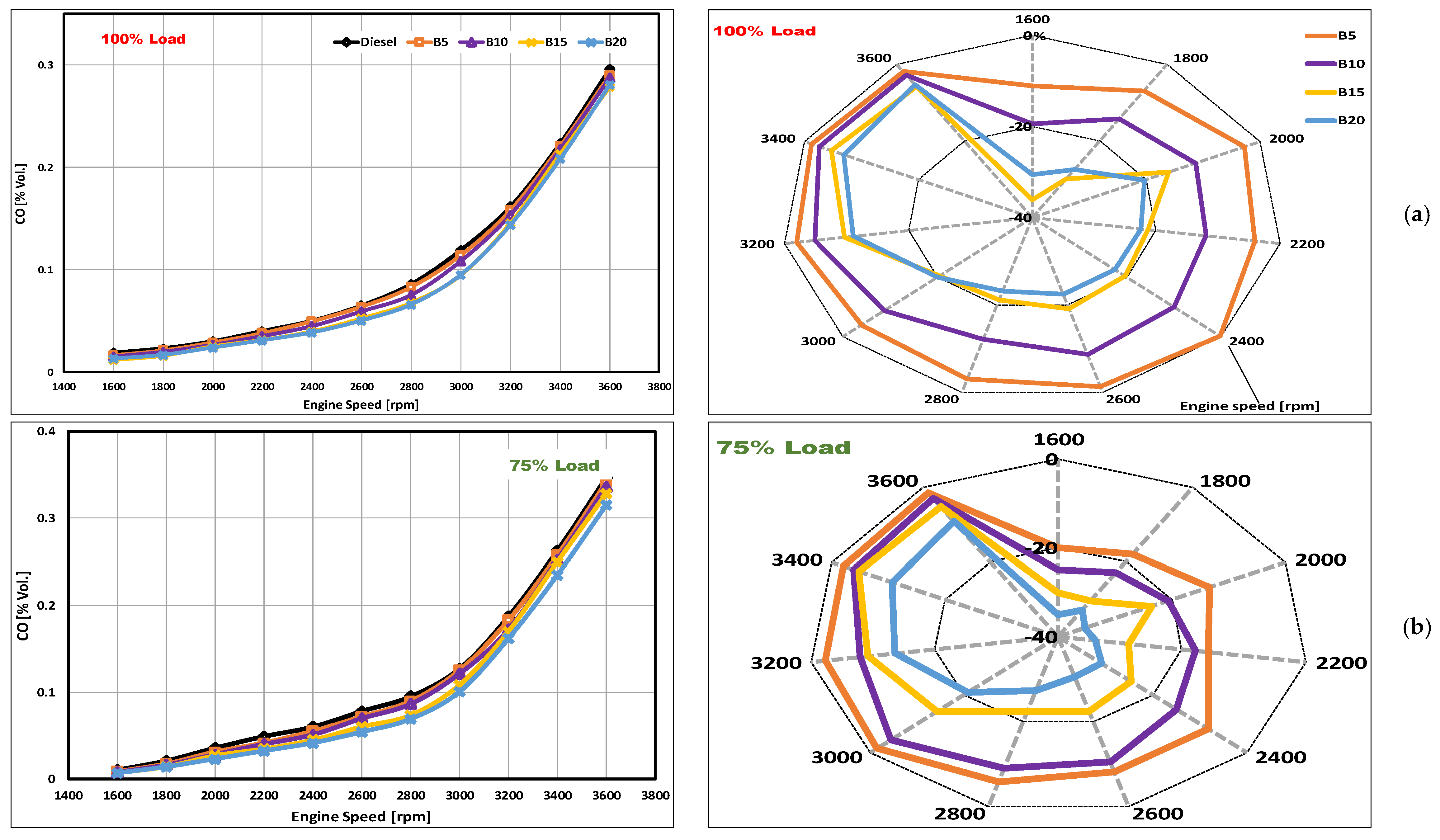
3.2. CO Emissions
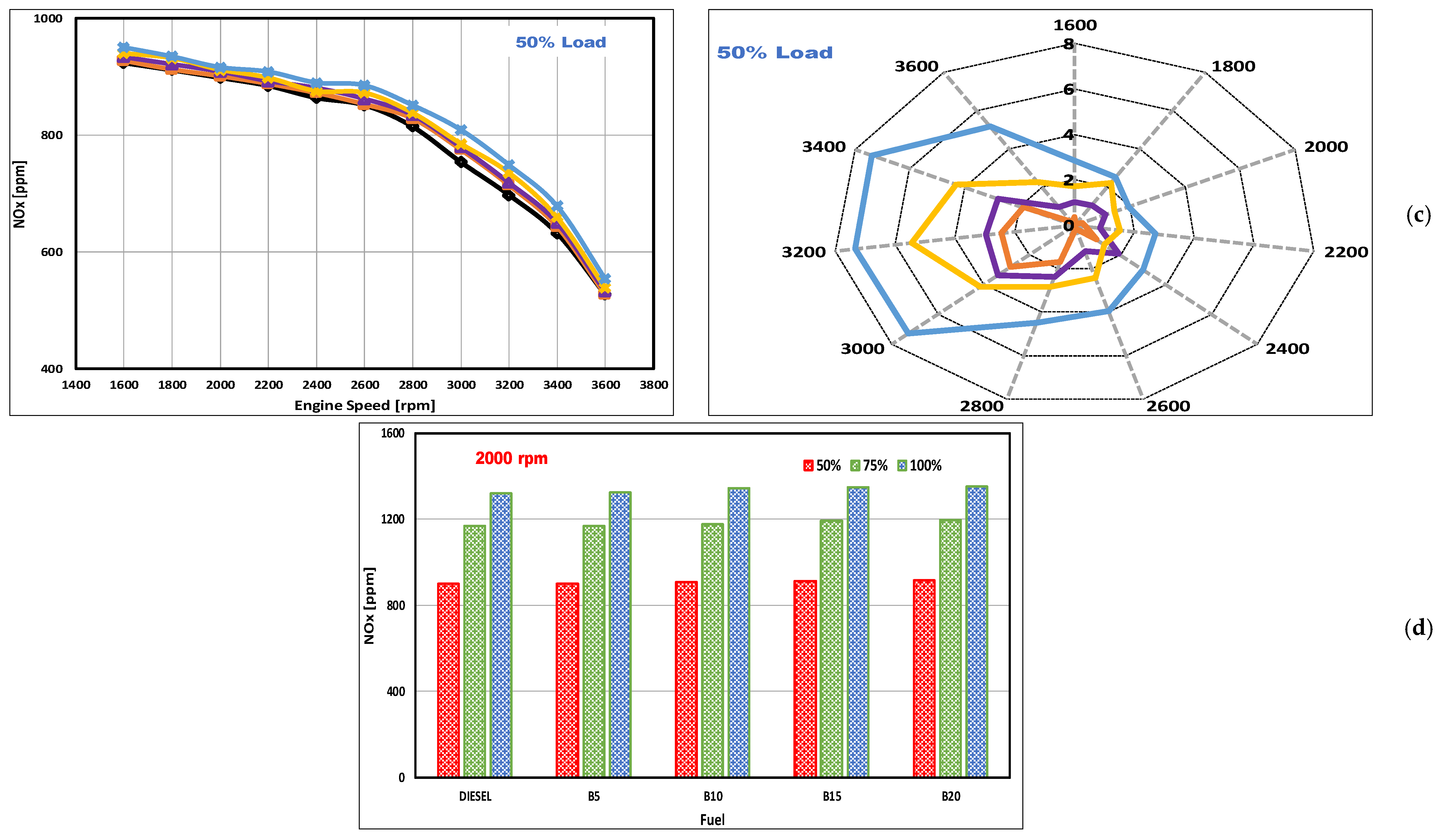
3.3. NOx Emissions
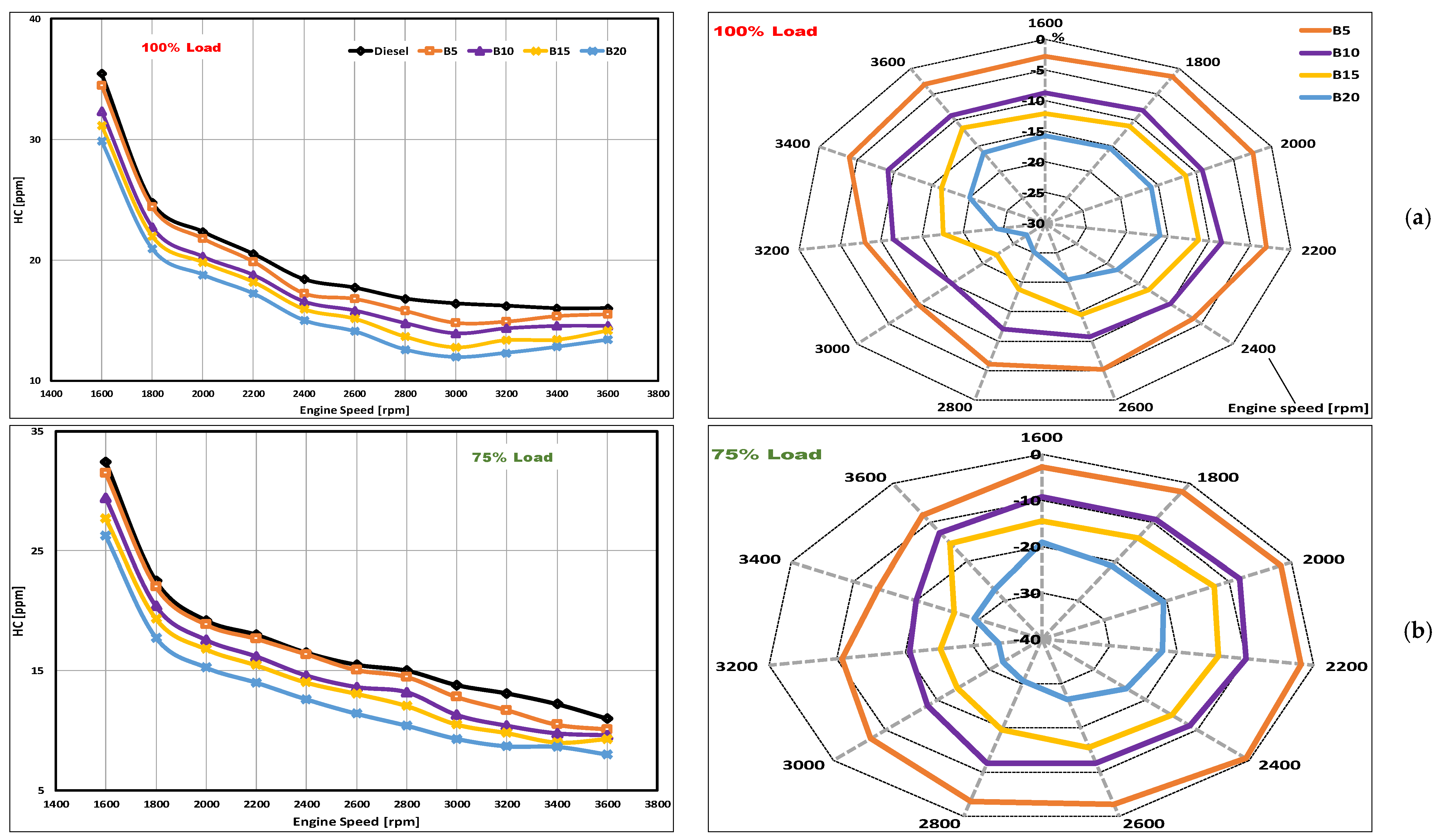
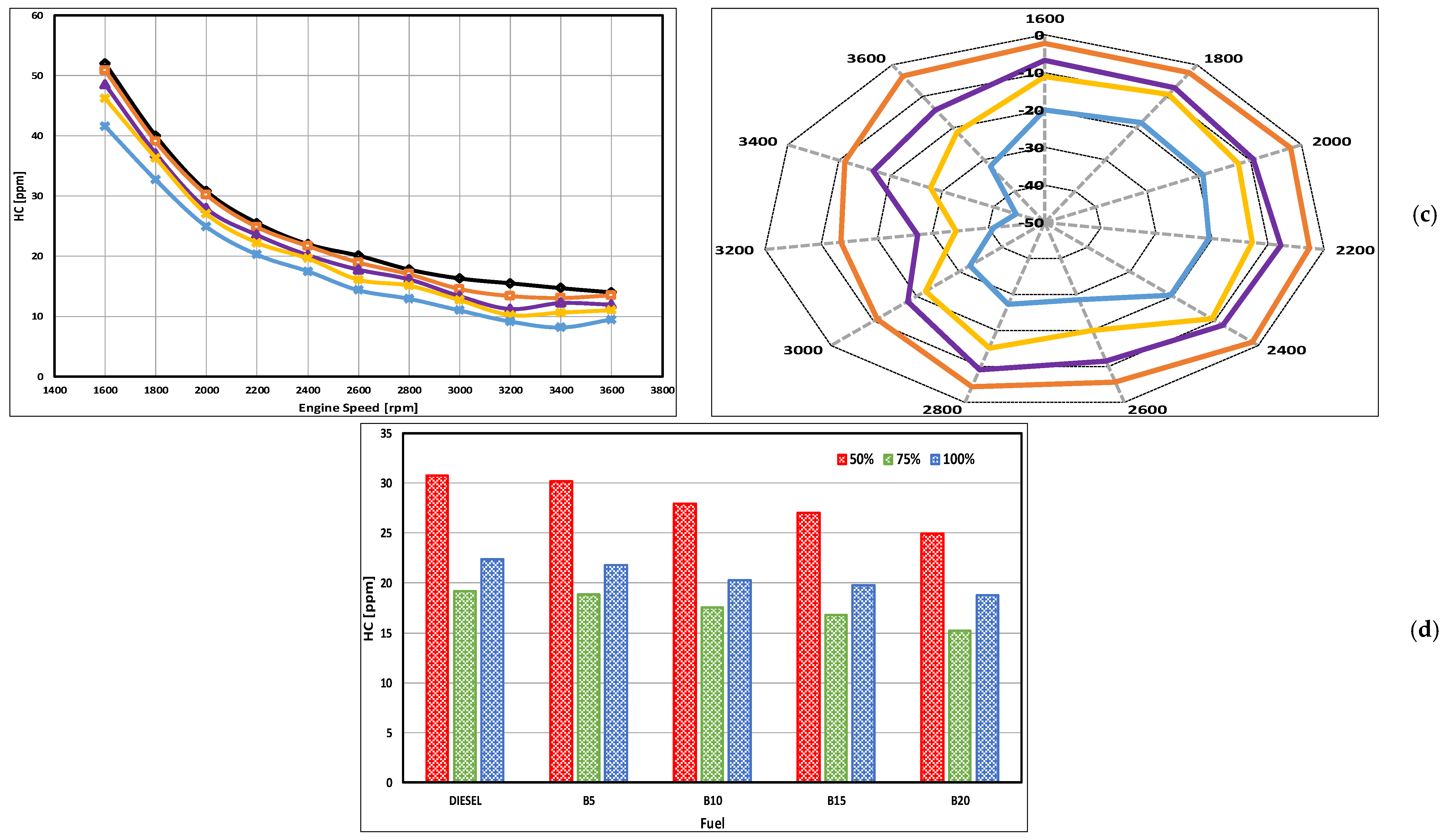
3.4. HC Emissions
4. Conclusions
- The inclusion of DSO biodiesel in the blends reduced CO2 emission levels, with a greater degree of enhancement occurring at lower engine loads and higher engine speeds. At full loading, an average reduction of emissions from B20 of 9.6% was obtained.
- The CO emissions were reduced significantly in the case of DSO biodiesel blends. At full loading, the average CO emission reductions of 3.4, 9.7, 18.6, and 19.2% were obtained using B5, B10, B15, and B20, respectively.
- The presence of inherent O2 within the DSO biodiesels resulted in greater concentrations of NOx emissions at all loads and speeds, with the peak (worst) increments of 2.8, 3.3, 5.4, and 7.4% obtained for blends B5, B10, B15, and B20, respectively. All of these peaks occurred under 50% loading. At higher speeds, the increases diminished, and the emissions approached the levels produced by fossil diesel.
- There were remarkable decreases in HC emission levels that were observed for the DSO biodiesel. The peak reductions of 13.9, 27.2, 34, and 44.4% were obtained at 75, 100, 100, and 100% loading for B5, B10, B15, and B20, respectively.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arunkumar, M.; Kannan, M.; Murali, G. Experimental Studies on Engine Performance and Emission Characteristics Using Castor Biodiesel as Fuel in CI Engine. Renew. Energy 2019, 131, 737–744. [Google Scholar] [CrossRef]
- The British Petroleum Company, BP. BP Statistical Review of World Energy, June 2017, 66th ed.; Pureprint Group Limited: London, UK, 2017; Available online: https://www.bp.com/content/dam/bp/en/corporate/pdf/energy-economics/statistical-review-2017/bp-statistical-review-of-world-energy-2017-full-report.pdf (accessed on 23 August 2018).
- World Health Organization (WHO); Regional Office for Europe (OECD). Economic Cost of the Health Impact of Air Pollution in Europe: Clean Air, Health and Wealth; WHO Regional Office Publications: Copenhagen, Denmark, 2015. [Google Scholar]
- Höök, M.; Tang, X. Depletion of Fossil Fuels and Anthropogenic Climate Change—A Review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Ge, J.; Yoon, S.; Choi, N.; Ge, J.C.; Yoon, S.K.; Choi, N.J. Using Canola Oil Biodiesel as an Alternative Fuel in Diesel Engines: A Review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef]
- Liu, S.; Chen, W.; Zhu, Z.; Jiang, S.; Ren, T.; Guo, H.; Liu, S.; Chen, W.; Zhu, Z.; Jiang, S.; et al. A Review of the Developed New Model Biodiesels and Their Effects on Engine Combustion and Emissions. Appl. Sci. 2018, 8, 2303. [Google Scholar] [CrossRef]
- Nigam, P.S.; Singh, A. Production of Liquid Biofuels from Renewable Resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Johnston, M.; Holloway, T. A Global Comparison of National Biodiesel Production Potentials. Environ. Sci. Technol. 2007, 41, 7967–7973. [Google Scholar] [CrossRef] [Green Version]
- Bart, C.J.; Palmeri, N.; Cavallro, S. Biodiesel Science and Technology: From Soil to Oil; TJ International Limited: Cornwall, UK, 2010. [Google Scholar]
- Giakoumis, E.G.; Rakopoulos, C.D.; Dimaratos, A.M.; Rakopoulos, D.C. Exhaust Emissions of Diesel Engines Operating under Transient Conditions with Biodiesel Fuel Blends. Prog. Energy Combust. Sci. 2012, 38, 691–715. [Google Scholar] [CrossRef]
- Hughes, S.R.; Qureshi, N. Biofuel Demand Realization. In Biomass to Biofuels; Vertes, A., Qureshi, N., Blaschek, P., Yukawa, H., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2010; pp. 55–69. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Hazrat, M.A.; Liaquat, A.M.; Shahabuddin, M.; Varman, M. Prospects of Biodiesel from Jatropha in Malaysia. Renew. Sustain. Energy Rev. 2012, 16, 5007–5020. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E. Evaluation of Biodiesel Blending, Engine Performance and Emissions Characteristics of Jatropha Curcas Methyl Ester: Malaysian Perspective. Energy 2013, 55, 879–887. [Google Scholar] [CrossRef]
- Bhuiya, M.M.K.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Azad, A.K. Prospects of 2nd Generation Biodiesel as a Sustainable Fuel—Part: 1 Selection of Feedstocks, Oil Extraction Techniques and Conversion Technologies. Renew. Sustain. Energy Rev. 2016, 55, 1109–1128. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M.; Kale, P.; Geremew, B.; Adeloju, S.B.; Nizami, M.; Ayoub, M. Optimization of Process Variables for Biodiesel Production by Transesterification of Flaxseed Oil and Produced Biodiesel Characterizations. Renew. Energy 2019, 139, 1272–1280. [Google Scholar] [CrossRef]
- Luque, R.; Lin, C.S.K.; Wilson, K.; Clark, J.H. Handbook of Biofuels Production: Processes and Technologies, 2nd ed.; Woodhead Publishing: Duxford, UK, 2016. [Google Scholar]
- Hawi, M.; Elwardany, A.; Ismail, M.; Ahmed, M.; Hawi, M.; Elwardany, A.; Ismail, M.; Ahmed, M. Experimental Investigation on Performance of a Compression Ignition Engine Fueled with Waste Cooking Oil Biodiesel–Diesel Blend Enhanced with Iron-Doped Cerium Oxide Nanoparticles. Energies 2019, 12, 798. [Google Scholar] [CrossRef]
- Kamil, M.; Ramadan, K.M.; Awad, O.I.; Ibrahim, T.K.; Inayat, A.; Ma, X. Environmental Impacts of Biodiesel Production from Waste Spent Coffee Grounds and Its Implementation in a Compression Ignition Engine. Sci. Total Environ. 2019, 675, 13–30. [Google Scholar] [CrossRef]
- Azeem, M.W.; Hanif, M.A.; Al-Sabahi, J.N.; Khan, A.A.; Naz, S.; Ijaz, A. Production of Biodiesel from Low Priced, Renewable and Abundant Date Seed Oil. Renew. Energy 2016, 86, 124–132. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations Organization, FAO. FAO Statistical Year Book, World Food and Agriculture. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 16 May 2019).
- Mrabet, A.; Ferchichi, A.; Chaira, N.; Mohamed, B.S.; Baaziz, Z.; Penny, T.M. Physico-Chemical Characteristics and Total Quality of Date Palm Varieties Grown in the Southern of Tunisia. Pakistan J. Biol. Sci. PJBS 2008, 11, 1003–1008. [Google Scholar] [CrossRef]
- Afiq, A.; Rahman, A.; Man, C. Date Seed and Date Seed Oil 1. Int. Food Res. J. 2013, 20, 2035–2043. [Google Scholar]
- Al-Farsi, M.; Alasalvar, C.; Al-Abid, M.; Al-Shoaily, K.; Al-Amry, M.; Al-Rawahy, F. Compositional and Functional Characteristics of Dates, Syrups, and Their By-Products. Food Chem. 2007, 104, 943–947. [Google Scholar] [CrossRef]
- Besbes, S.; Drira, L.; Blecker, C.; Deroanne, C.; Attia, H. Adding Value to Hard Date (Phoenix Dactylifera L.): Compositional, Functional and Sensory Characteristics of Date Jam. Food Chem. 2009, 112, 406–411. [Google Scholar] [CrossRef]
- Besbes, S.; Blecker, C.; Deroanne, C.; Drira, N.E.; Attia, H. Date Seeds: Chemical Composition and Characteristic Profiles of the Lipid Fraction. Food Chem. 2004, 84, 577–584. [Google Scholar] [CrossRef]
- Vandepopuliere, J.M.; Al-Yousef, Y.; Lyons, J.J. Dates and Date Pits as Ingredients in Broiler Starting and Coturnix Quail Breeder Diets. Poult. Sci. 1995, 74, 1134–1142. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Usage of Date (Phoenix Dactylifera L.) Seeds in Human Health and Animal Feed. In Nuts and Seeds in Health and Disease Prevention; Academic Press: Burlington, MA, USA, 2011; pp. 447–452. [Google Scholar]
- Ali-Mohamed, A.Y.; Khamis, A.S.H. Mineral Ion Content of the Seeds of Six Cultivars of Bahraini Date Palm (Phoenix Dactylifera). J. Agric. Food Chem. 2004, 52, 6522–6525. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kasapis, S.; Al-Kharusi, N.S.Z.; Al-Marhubi, I.M.; Khan, A.J. Composition Characterisation and Thermal Transition of Date Pits Powders. J. Food Eng. 2007, 80, 1–10. [Google Scholar] [CrossRef]
- Devshony, S.; Eteshola, E.; Shani, A. Characteristics and Some Potential Applications of Date Palm (Phoenix Dactylifera L.) Seeds and Seed Oil. J. Am. Oil Chem. Soc. 1992, 69, 595–597. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Muhtaseb, A.H.; Al-Haj, L.; Al-Hinai, M.A.; Hellier, P.; Rashid, U. Optimization of Oil Extraction from Waste “Date Pits” for Biodiesel Production. Energy Convers. Manag. 2016, 117, 264–272. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Taher, H.; Al Dhaheri, S.; Wajeeh, S.; Nour, M.; El-Najjar, E. Biodiesel Production from Oils Extracted from Date Pits. Green Sustain. Chem. 2017, 07, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Nehdi, I.; Omri, S.; Khalil, M.I.; Al-Resayes, S.I. Characteristics and Chemical Composition of Date Palm (Phoenix Canariensis) Seeds and Seed Oil. Ind. Crop. Prod. 2010, 32, 360–365. [Google Scholar] [CrossRef]
- Yousuf, R.G.; Winterburn, J.B. Waste Date Seed Oil Extract as an Alternative Feedstock for Poly(3-Hydroxybutyrate) Synthesis. Biochem. Eng. J. 2017, 127, 68–76. [Google Scholar] [CrossRef]
- Akaagerger, S.M.; Giwa, S.O.; Ibrahim, M.G. Production of Biodiesel from Desert Date Seed Oil. Int. J. ChemTech Res. 2016, 9, 453–463. [Google Scholar]
- Sanford, S.; White, J.; Shah, P. Feedstock and Biodiesel Characteristics Report; Renewable Energy Group: Ames, IA, USA, 2009. [Google Scholar]
- Farooq, M.; Ramli, A.; Naeem, A.; Mahmood, T.; Ahmad, S.; Humayun, M.; Islam, M.G.U. Biodiesel Production from Date Seed Oil (Phoenix Dactylifera L.) via Egg Shell Derived Heterogeneous Catalyst. Chem. Eng. Res. Des. 2018, 132, 644–651. [Google Scholar] [CrossRef]
- Majewski, W.A.; Khair, M.K. Diesel Emissions and Their Control; Society of Automotive Engineers (SAE): Wallendale, PA, USA, 2006. [Google Scholar]
- Chauhan, B.S.; Kumar, N.; Cho, H.M. A Study on the Performance and Emission of a Diesel Engine Fueled with Jatropha Biodiesel Oil and Its Blends. Energy 2012, 37, 616–622. [Google Scholar] [CrossRef]
- Aydin, H.; Bayindir, H. Performance and Emission Analysis of Cottonseed Oil Methyl Ester in a Diesel Engine. Renew. Energy 2010, 35, 588–592. [Google Scholar] [CrossRef]
- An, H.; Yang, W.M.; Maghbouli, A.; Li, J.; Chou, S.K.; Chua, K.J. Performance, Combustion and Emission Characteristics of Biodiesel Derived from Waste Cooking Oils. Appl. Energy 2013, 112, 493–499. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Kumar, N.; Cho, H.M.; Lim, H.C. A Study on the Performance and Emission of a Diesel Engine Fueled with Karanja Biodiesel and Its Blends. Energy 2013, 56, 1–7. [Google Scholar] [CrossRef]
- Horner, J.M. Anthropogenic Emissions of Carbon Monoxide. Rev. Environ. Health 2000, 15, 289–298. [Google Scholar] [CrossRef]
- Ulusoy, Y.; Arslan, R.; Kaplan, C. Emission Characteristics of Sunflower Oil Methyl Ester. Energy Sources Part A Recover. Util. Environ. Eff. 2009, 31, 906–910. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Yusaf, T. Engine Performance and Emissions Using Jatropha Curcas, Ceiba Pentandra and Calophyllum Inophyllum Biodiesel in a CI Diesel Engine. Energy 2014, 69, 427–445. [Google Scholar] [CrossRef]
- Richards, P. Automotive Fuels Reference Book, 3rd ed.; SAE International: Wallendale, PA, USA, 2014. [Google Scholar]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of Biodiesel on Engine Performances and Emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Suresh, M.; Jawahar, C.P.; Richard, A. A Review on Biodiesel Production, Combustion, Performance, and Emission Characteristics of Non-Edible Oils in Variable Compression Ratio Diesel Engine Using Biodiesel and Its Blends. Renew. Sustain. Energy Rev. 2018, 92, 38–49. [Google Scholar] [CrossRef]
- Usta, N. An Experimental Study on Performance and Exhaust Emissions of a Diesel Engine Fuelled with Tobacco Seed Oil Methyl Ester. Energy Convers. Manag. 2005, 46, 2373–2386. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rahman, M.M. Performance and Emission Characteristics of Biodiesel–Diesel Blend and Environmental and Economic Impacts of Biodiesel Production: A Review. Renew. Sustain. Energy Rev. 2017, 74, 938–948. [Google Scholar] [CrossRef]
- Qi, D.H.; Geng, L.M.; Chen, H.; Bian, Y.Z.; Liu, J.; Ren, X.C. Combustion and Performance Evaluation of a Diesel Engine Fueled with Biodiesel Produced from Soybean Crude Oil. Renew. Energy 2009, 34, 2706–2713. [Google Scholar] [CrossRef]
- Kim, H.; Choi, B. The Effect of Biodiesel and Bioethanol Blended Diesel Fuel on Nanoparticles and Exhaust Emissions from CRDI Diesel Engine. Renew. Energy 2010, 35, 157–163. [Google Scholar] [CrossRef]
- Giakoumis, E.; Sarakatsanis, C.; Giakoumis, E.G.; Sarakatsanis, C.K. A Comparative Assessment of Biodiesel Cetane Number Predictive Correlations Based on Fatty Acid Composition. Energies 2019, 12, 422. [Google Scholar] [CrossRef]
- Carslaw, D.C.; Rhys-Tyler, G. New Insights from Comprehensive On-Road Measurements of NOx, NO2 and NH3 from Vehicle Emission Remote Sensing in London, UK. Atmos. Environ. 2013, 81, 339–347. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Nitrogen Oxides (NOx), Why and How They Are Controlled; Clean Air Technology Center Report (MD-12): Research Triangle Park, NC, USA, 1999.
- Heywood, J. Internal Combustion Engine Fundamentals, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Pundir, B.P. Engine Emissions: Fundamentals and Advances in Control, 2nd ed.; Alpha Science International Ltd.: Oxford, UK, 2008. [Google Scholar]
- Lin, C.Y.; Lin, H.A. Diesel Engine Performance and Emission Characteristics of Biodiesel Produced by the Peroxidation Process. Fuel 2006, 85, 298–305. [Google Scholar] [CrossRef]
- Lin, C.Y.; Li, R.J. Engine Performance and Emission Characteristics of Marine Fish-Oil Biodiesel Produced from the Discarded Parts of Marine Fish. Fuel Process. Technol. 2009, 90, 883–888. [Google Scholar] [CrossRef]
- Tat, M.E.; Van Gerpen, J.H. Measurement of Biodiesel Speed of Sound and Its Impact on Injection Timing; Report 4 in a Series of 6 (No. NREL/SR-510-31462); National Renewable Energy Lab.: Golden, CO, USA, 2003.
- Ghobadian, B.; Rahimi, H.; Nikbakht, A.M.; Najafi, G.; Yusaf, T.F. Diesel Engine Performance and Exhaust Emission Analysis Using Waste Cooking Biodiesel Fuel with an Artificial Neural Network. Renew. Energy 2009, 34, 976–982. [Google Scholar] [CrossRef]
- Meng, X.; Chen, G.; Wang, Y. Biodiesel Production from Waste Cooking Oil via Alkali Catalyst and Its Engine Test. Fuel Process. Technol. 2008, 89, 851–857. [Google Scholar] [CrossRef]
- Wu, F.; Wang, J.; Chen, W.; Shuai, S. A Study on Emission Performance of a Diesel Engine Fueled with Five Typical Methyl Ester Biodiesels. Atmos. Environ. 2009, 43, 1481–1485. [Google Scholar] [CrossRef]
- Puhan, S.; Vedaraman, N.; Ram, B.V.B.; Sankarnarayanan, G.; Jeychandran, K. Mahua Oil (Madhuca Indica Seed Oil) Methyl Ester as Biodiesel-Preparation and Emission Characterstics. Biomass Bioenergy 2005, 28, 87–93. [Google Scholar] [CrossRef]
- Golimowski, W.; Krzaczek, P.; Marcinkowski, D.; Gracz, W.; Wałowski, G. Impact of Biogas and Waste Fats Methyl Esters on NO, NO2, CO, and PM Emission by Dual Fuel Diesel Engine. Sustainability 2019, 11, 1799. [Google Scholar] [CrossRef]
- Lozhkina, O.V.; Lozhkin, V.N. Estimation of Nitrogen Oxides Emissions from Petrol and Diesel Passenger Cars by Means of On-Board Monitoring: Effect of Vehicle Speed, Vehicle Technology, Engine Type on Emission Rates. Transp. Res. Part D Transp. Environ. 2016, 47, 251–264. [Google Scholar] [CrossRef]
- Duda, K.; Wierzbicki, S.; Śmieja, M.; Mikulski, M. Comparison of Performance and Emissions of a CRDI Diesel Engine Fuelled with Biodiesel of Different Origin. Fuel 2018, 212, 202–222. [Google Scholar] [CrossRef]
- Koszałka, G.; Hunicz, J. Detailed Speciation of Emissions from a Diesel Engine Fuelled with Canola Methyl Ester. In Proceedings of the BulTrans-2018, 10th International Scientific Conference on Aeronautics, Automotive and Railway Engineering and Technologies, Sozopol, Bulgaria, 15–17 September 2018; Volume 234. [Google Scholar] [CrossRef]

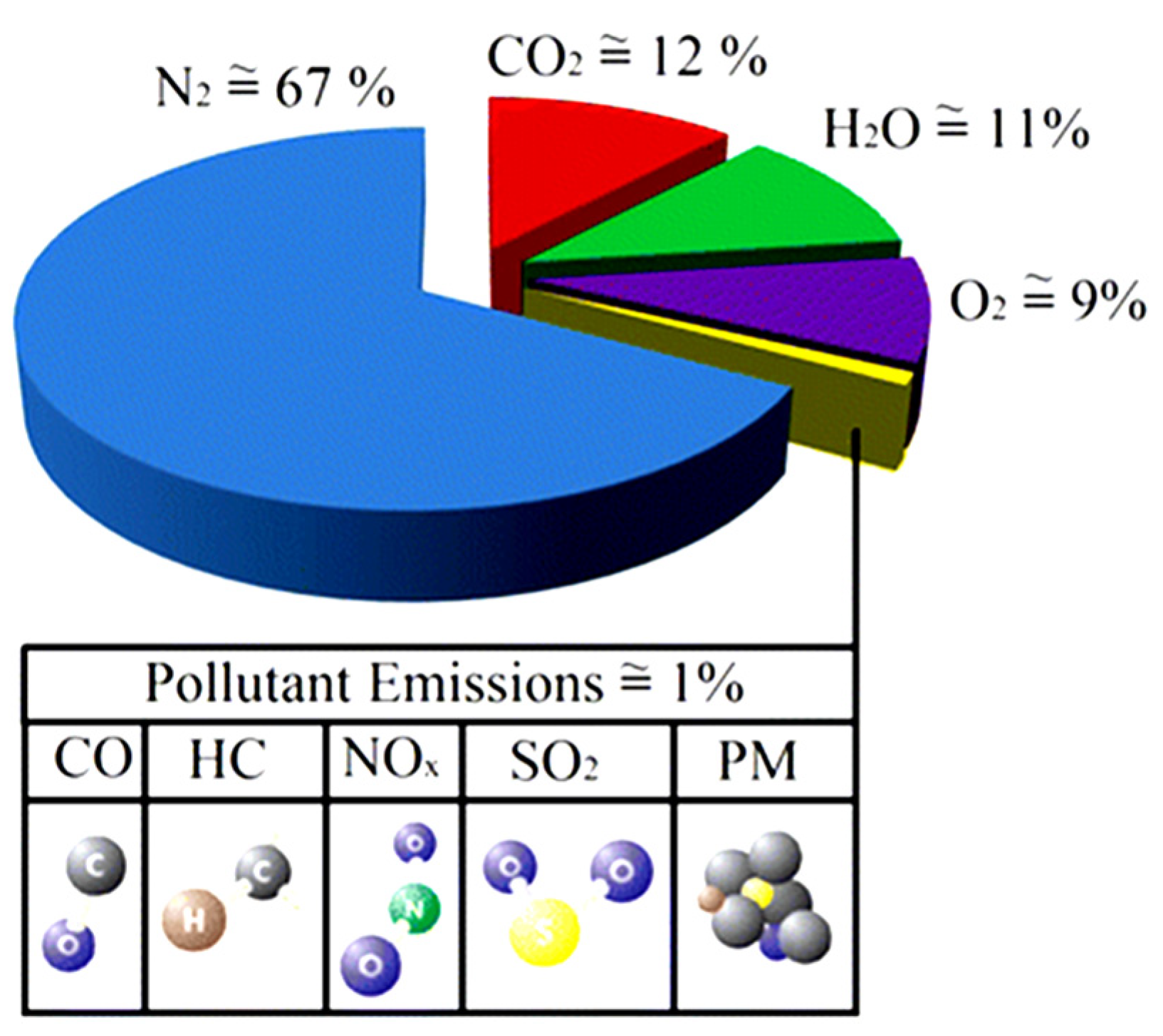

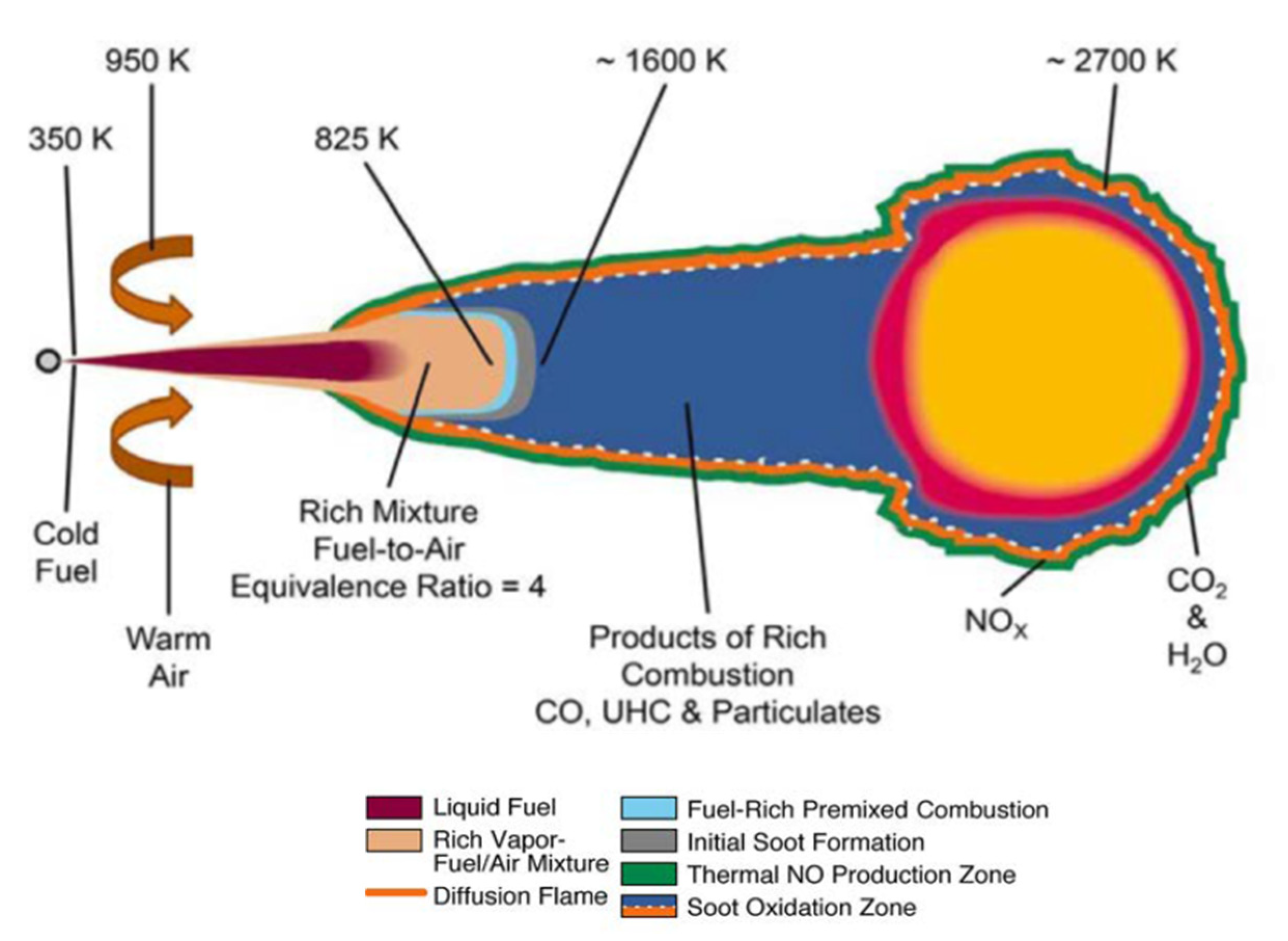

| Fatty Acid | Chemical Structure | Content in DSO (wt.%) | Molar Mass (g/mole) |
|---|---|---|---|
| Lignoceric | C24:0 | 0.24 | 368.65 |
| Erucic | C22:1 | 0.38 | 338.58 |
| Behenic | C22:0 | 0.46 | 340.59 |
| Arachidic | C20:0 | 0.21 | 312.54 |
| Linolenic | C18:3 | 0.11 | 278.44 |
| Linoleic | C18:2 | 9.95 | 280.45 |
| Oleic | C18:1 | 44.73 | 282.47 |
| Stearic | C18:0 | 4.54 | 284.48 |
| Palmitoleic | C16:1 | 0.09 | 254.41 |
| Palmitic | C16:0 | 12.01 | 256.43 |
| Myristic | C14:0 | 10.14 | 228.38 |
| Lauric | C12:0 | 16.14 | 200.32 |
| Capric | C10:0 | 0.47 | 172.27 |
| Caprylic | C8:0 | 0.34 | 144.21 |
| Property | DSO Biodiesel | Jatropha Biodiesel a | ASTM D6751 Biodiesel | ASTM D975 Diesel |
|---|---|---|---|---|
| Flash point (°C) | 164 | 182.5 | >130 | 1D: 38 |
| 2D: 52 | ||||
| Cloud point (°C) | +9.4 | 3 | Report | −20 |
| Cetane number (-) | 62 | 51 | 47 min. | 40 min. |
| Acid number (mg KOH·g−1) | 0.29 | 0.05 | 0.5 max. | - |
| Viscosity @ 40 °C (mm2·s−1) | 4.38 | 4.38 | 1.9–6.0 | 1D: 1.3–2.4 |
| 2D: 1.9–4.1 | ||||
| Heating value (MJ/kg) | 39.8 | 40.5 | - | 44.6 |
| Calcium & Magnesium combined (mg·kg−1) | 3.27 | - | 5 max. | - |
| Sulfur (ppm) | 0.93 | 4.59 | 15 ppm for S15 grade; 500 ppm for S500 grade | 1D and 2D |
| 15 ppm for S15 grade; 500 ppm for S500 grade | ||||
| Water & sediment (vol.%) | 0.019 | - | 0.05 max. | 0.05% max. |
| Sulphated ash (wt.%) | <0.02 | - | 0.020 max. | - |
| Carbon residue (wt.%) | 0.023 | - | 0.050 max. | 1D: 0.15 max. |
| 2D: 0.35 max. | ||||
| Phosphorous content (wt.%) | 0.0002 | - | 0.001 max. | - |
| Distillation 90% (°C) | 352.4 | - | 360 max. | 1D: 288 max. |
| 2D: 282–338 | ||||
| Sodium & potassium (ppm) | 3.2 | - | 5 max. | |
| Oxidation stability (h @ 110 °C) | 7.4 b | 3 c | 6 min. | 110 |
| Specification | Value |
|---|---|
| Engine brand | Lombardini (15-LD-225) |
| Bore (mm) | 69 |
| Stroke (mm) | 60 |
| Displacement volume (L) | 0.224 |
| Compression ratio | 21 |
| Engine power at 3600 rpm (kW) | 3.5 |
| Engine torque at 2400 rpm (N·m) | 10.4 |
| Intake valve opening (Crank angle BTDC) | 6° |
| Intake valve closure (Crank angle ABDC) | 22° |
| Exhaust valve opening (Crank angle BBDC) | 58° |
| Exhaust valve closure (Crank angle ATDC) | 10° |
| Types of Exhaust Gases | Measuring Range | Display Resolution | Accuracy |
|---|---|---|---|
| CO | 0–10% | 0.01% | 0.06% |
| CO2 | 0–20% | 0.1% | 0.3% |
| HC | 0–2000 ppm | 1 ppm | 4 ppm |
| NOx | 0–5000 ppm | 1 ppm | 25 ppm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamil, M.; Ramadan, K.; Ghenai, C.; Olabi, A.G.; Nazzal, I.T. Emissions from Combustion of Second-Generation Biodiesel Produced from Seeds of Date Palm Fruit (Phoenix dactylifera L.). Appl. Sci. 2019, 9, 3720. https://doi.org/10.3390/app9183720
Kamil M, Ramadan K, Ghenai C, Olabi AG, Nazzal IT. Emissions from Combustion of Second-Generation Biodiesel Produced from Seeds of Date Palm Fruit (Phoenix dactylifera L.). Applied Sciences. 2019; 9(18):3720. https://doi.org/10.3390/app9183720
Chicago/Turabian StyleKamil, Mohammed, Khalid Ramadan, Chaouki Ghenai, Abdul Ghani Olabi, and Ibrahim T. Nazzal. 2019. "Emissions from Combustion of Second-Generation Biodiesel Produced from Seeds of Date Palm Fruit (Phoenix dactylifera L.)" Applied Sciences 9, no. 18: 3720. https://doi.org/10.3390/app9183720
APA StyleKamil, M., Ramadan, K., Ghenai, C., Olabi, A. G., & Nazzal, I. T. (2019). Emissions from Combustion of Second-Generation Biodiesel Produced from Seeds of Date Palm Fruit (Phoenix dactylifera L.). Applied Sciences, 9(18), 3720. https://doi.org/10.3390/app9183720







