Abstract
In this report, we present a study of the quinhydrone/methanol (QHY/MeOH) organic passivation technique for a silicon (Si) surface. The roles of p-benzoquinone (BQ) and hydroquinone (HQ), which make up QHY, in controlling the uniformity and coverage of the passivation layer as well as the minority carrier lifetime (τeff) of Si were investigated. The uniformity and coverage of the passivation layer after treatment with diverse mixture ratios of BQ and HQ in MeOH were studied with two different atomic force microscope (AFM) techniques, namely tunneling mode (TUNA) and high-resolution tapping mode AFM (HR-AFM). In addition, the τeff and surface potential voltages (SPV) of passivated surfaces were measured to clarify the relationship between the morphologies of the passivation layers and degrees of surface band bending. The molecular interactions between BQ and HQ in MeOH were also analyzed using Fourier-transform infrared spectroscopy (FT-IR). In our study, we successfully demonstrated the role of each molecule for effective Si surface passivation with BQ working as a passivation agent and HQ contributing as a proton (H+) donator to BQ for accelerating the passivation rate. However, our study also clearly revealed that HQ could also hinder the formation of a conformal passivation layer, which raises an issue for passivation over complex surface geometry, especially a nanostructured surface.
1. Introduction
Certain specialized organic materials are very much of interest because of their many promising applications in various devices. Ultra-thin organic films such as self-assembled monolayers (SAMs) have been studied extensively owing to their potential to modify the surface electronic properties of metallic and semiconductor materials [1,2,3]. Recently, organic molecules or ligands have been used for Si surface passivation, improving minority carrier lifetime (τeff), and reducing surface recombination velocity (SRV) [4,5].
High quality passivation of Si is especially important for solar cells, which require minimization of the SRV of photo-generated carriers. Conventionally, dielectric thin films such as silicon dioxide (SiO2) and silicon nitride (SiNX) are commonly used to provide effective passivation. However, the deposition of these dielectric passivation layers typically requires high vacuum and/or high temperature processes, which add cost to the devices. The use of organic coatings will reduce cost; in addition, the rising interest in nanostructured devices that require ultra-thin and highly conformal passivation layers further motivates the organic molecular passivation approach regardless of its relatively low stability compared with conventional dielectric passivation approaches [6].
A large number of studies reported organic Si passivation using different organic molecules [5,7] but in most cases insufficient passivation qualities have limited their further development. However, a quinhydrone/methanol (QHY/MeOH) method initially developed by Takato [8] has been reported to result in a high τeff (3.3 ms) and a low SRV (7 cm/s) [9]. The simplicity of the passivation process in which the sample is merely dipped in a QHY/MeOH solution at room temperature is highly attractive [10,11]. Due to these attractive aspects of QHY/MeOH for Si surface passivation, many studies are currently focusing on how to utilize QHY/MeOH passivation technique for real electrical device fabrication, and recently Ye et. al. successfully demonstrated that QHY/MeOH passivation is highly effective to fabricate cost-effective and low-temperature processable dopant-free Si/organic heterojunction solar cells [12].
QHY (Figure 1a) is a well-known charge-transfer complex between p-benzoquinone (BQ; Figure 1b) and hydroquinone (HQ; Figure 1c) that was initially studied by Paul et al. in the 1980s [13,14,15]. However, the QHY charge-transfer complex is also known to be very labile in solution environment [16], and previous reports showed that QHY can exist as various formations of intermediate molecular radicals in different solvents [17,18,19]. Therefore, even though the QHY/MeOH passivation technique has been widely studied by various research groups, the contribution mechanisms of each molecule, BQ and HQ and MeOH, for passivation on Si surface dangling-bond states are not yet clearly understood. In this report, we studied the molecular contribution of BQ and HQ in MeOH solvent for production of effective Si surface passivation in terms of (1) uniformity and coverage of the passivation layer; and (2) formation of the passivation agent, semiquinone (SQ), after preparing solutions with various BQ and HQ mixture ratios in MeOH.

Figure 1.
Molecular structures of (a) quinhydrone (QHY); (b) p-benzoquinone (BQ); (c) hydroquinone (HQ); (d) semiquinone (SQ).
2. Experimental Section
2.1. Si Surface Passivation
To prepare samples for organic passivation, 2-inch × 2-inch (or 1-inch × 1-inch) samples were used that were cut from n-type FZ Si <100>, 100 Ω-cm resistivity, and 450 μm thick double side polished wafers. The wafers were cleaned in piranha solution (H2O2 and H2SO4 mixed in a 1:4 ratio) for 15 min followed by a 10 min rinse in DI-water and then dipped in a 10 vol% HF solution for 2 min with a 5 min DI-water rinse thereafter. Passivation solution was prepared with 10 mM of QHY/BQ/HQ in 500 mL MeOH. The passivation treatments in the solutions were carried out at various dipping times in a glass beaker, sealed to prevent solution concentration changes during the process.
2.2. Characterizations
Tunneling mode AFM (TUNA) and high resolution tapping mode AFM (HR-AFM) scans were performed with a Veeco DI-3100 using a platinum (Pt) coated conductive probe and super-sharp probe (probe diameter < 2 nm, Veeco, Plainview, New York, USA), respectively. The τeff was measured using a photoconductivity decay lifetime tester, WCT-120 (Sinton instrument, Boulder, Colorado, USA). Contact potential difference (CPD) measurements were performed with a Kelvin Probe system (KP-6500) under inert nitrogen gas atmosphere. A highly oriented pyrolytic graphite (HOPG, Veeco) was used for the reference work function, which is known to be ~4.6 eV. The surface potential voltage (SPV) was measured by comparison of the CPDs between dark and illumination conditions. Molecular interaction and formation change of BQ and HQ in MeOH were also measured with Fourier transform infrared spectroscopy (FT-IR, Varian 670, Palo Alto, California, USA) with solution samples.
3. Results and Discussion
The τeff with various solution conditions was measured to distinguish between BQ and HQ as the dominant passivation molecule. The results of the measurement of the τeff of Si samples after passivation with BQ/MeOH and HQ/MeOH solutions (Figure 2a) show that BQ/MeOH produced ~1.7 ms at a 2.0 × 10−15 cm−3 carrier injection level after a 1-h treatment while with the sample with HQ/MeOH only 68 µs was measured. From repeated measurements, we found that the τeff of HQ/MeOH treated samples never exceeded that from the HF pre-cleaned-only sample, which indicates that HQ did not make any contribution to passivation under our experimental conditions. However, when we measured the τeff while varying HQ ratios with a fixed 10 mM of BQ (Figure 2b), the highest τeff of 3.2 ms was achieved at a 1:1 mixture ratio of BQ and HQ, while the τeff of the other BQ:HQ ratios (1:0.5 and 1:1.5) was lower at 2.0 ms and 1.6 ms, respectively. These results lead us to assume that while HQ has little involvement in the passivation reaction when it exists alone, it can enhance the effectiveness of passivation under an optimal mixture ratio with BQ.
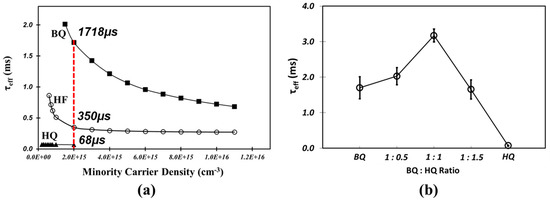
Figure 2.
(a) Measured τeff of BQ (solid-square) and HQ (solid-triangle) treated Si compared with HF pre-cleaned-only sample (open-circle); and (b) measured τeff at a minority carrier density of 2.0 × 1015 cm−3 with 100% BQ, HQ, and various HQ mixture ratios into a fixed 10 mM BQ in 500 mL MeOH.
In order to understand how HQ affects the Si surface passivation, the Si surface morphologies after passivation treatments in the solutions with different HQ mixture ratios were investigated using tapping mode AFM, which is a direct way to observe the uniformity of the passivation layer. Because the molecular passivation layer is ultra-thin, AFM scans were carried out with a super-sharp probe (probe diameter <2 nm) to generate high resolution AFM (HR-AFM) images. The HR-AFM images shown in Figure 3a–d were obtained from the samples passivated with (a) pure BQ, (b) BQ:HQ = 1:0.5, (c) BQ:HQ = 1:1, and (d) BQ:HQ = 1:1.5, which had 10 mM of fixed BQ in 500 mL MeOH.
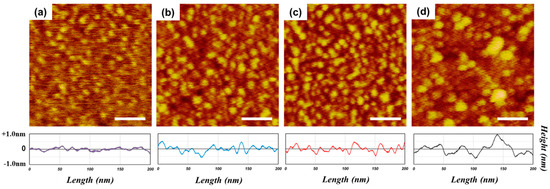
Figure 3.
High-resolution tapping mode atomic force microscope (HR-AFM) images of (a) pure BQ; (b) BQ:HQ = 1:0.5; (c) BQ:HQ = 1:1; and (d) BQ:HQ = 1:1.5 mixture ratios (scan scale = 200 nm × 200 nm; scale bar = 50 nm).
It is evident that the higher HQ mixture ratios produced more prominent and larger molecular islands on the surface, which indicates higher molecular densities on the local surface (Figure 3). However, the uniformity of the islands deteriorated with the 1.5 HQ mixture ratio (Figure 3d). The cross-section profile directly below the AFM image in Figure 3 also supports the idea that excess HQ degrades the uniformity of the passivation layer, and this corresponds with the decrease in the τeff shown in Figure 2b.
To better understand the reasons for the variation of the passivation layer morphologies and τeff with different HQ mixture ratios, the molecular interaction among BQ, HQ, and MeOH must be clarified. Therefore, FT-IR measurements were taken for the various solution conditions and the results, including pure MeOH for comparison, are shown in Figure 4a. In particular the characteristic absorptions for the alcohol O–H bond at 3390 cm−1 (stretch) and 1400 cm−1 (bending) are apparent and have been marked with vertical lines in Figure 4a. BQ in MeOH produces a highly hydroxyl (O–H) deficient solution because BQ is a strong oxidizing agent, which deprives a proton (H+) from MeOH as shown in Figure 4b. Considering that there is only 10 mM of BQ in MeOH, the reaction between BQ and MeOH must be extremely fast and dynamic because the O–H peaks at 3390 cm−1 and ~1400 cm−1 have nearly disappeared. (Note: the C–H peak at ~2900 cm−1 is fairly ubiquitous, so the origin of the C–H peak intensity change is hard to specify) [20]. Further, the FT-IR results show that the addition of HQ to BQ/MeOH solution increased the O–H peak intensity up to the 1:1 ratio with BQ. This is because the oxidation potential of HQ in MeOH is ~0.29 V [21], and this is lower than that of MeOH itself of ~0.38 V [22]. Therefore, when HQ co-exists with BQ in MeOH, HQ acts as a strong reducing agent for BQ, replacing the role of MeOH as an H+ donor as shown in Figure 4c, so that a higher fraction of MeOH molecules retain their H+. As a result, the overall absorbance of solution from O–H bonds gradually re-gained its intensity as HQ ratio increased. Above a 1 to 1 ratio the intensity of the O–H peaks at both 3390 and 1400 cm−1 became saturated, which is evidence that only a limited amount of HQ will react with BQ. This is because the BQ–HQ redox system is known to produce a stabilized intermediate molecule of semiquinone (SQ) radical by exchanging one H+ in a non-aqueous and weak hydrogen bonding agent like MeOH [17,23].
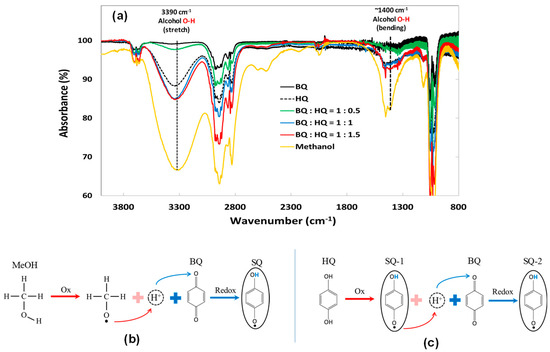
Figure 4.
(a) Fourier transform infrared spectroscopy (FT-IR) results of BQ (black-solid line), HQ (black-dash line), and various HQ mixture ratios into fixed BQ in MeOH (green, blue, red-solid line) and pure MeOH (orange-solid line); (b) reducing reaction of BQ into one SQ in BQ/MeOH; and (c) effect of HQ to produce two SQs formation in QHY (BQ:HQ = 1:1)/MeOH.
From the report by Har-Lavan et al. it was confirmed the final molecular formation bonding with Si surface is the SQ molecule [24]. In Figure 5, the schematic illustration is shown for SQ and Si surface dangling bonds to produce Si–SQ bond for passivation.

Figure 5.
Schematic illustration for Si–SQ formation.
For SQ formation, only one H+ exchange is required; therefore, a 1 to 1 interaction between BQ and HQ should be the maximum reaction ratio, which indicates that excess HQ above the 1:1 ratio with BQ will remain un-reacted in the solution. It was shown that HQ alone is not involved in the passivation reaction (Figure 2). Based on the HR-AFM images in Figure 3d, we assumed that the un-reacted HQ had hindered an intimate interaction between the passivation molecule and Si surface dangling-bond states followed by deteriorated uniformity of the passivation layer.
To further clarify the role of HQ, TUNA was used to provide surface conductivity mapping with high current sensitivity (<1 pA). With a TUNA scan the densely packed molecular area and non-passivated area can be distinguished because of the high resistivity of native oxide formed on the non-passivated surface, in contrast with the passivation area. (Note: it is known that the densely packed molecule area on the Si surface delays formation of native oxide) [25,26]. The TUNA scan results with various HQ mixture ratios with BQ are shown in Figure 6. From the TUNA image of the excess 1.5 HQ ratio (Figure 6c), a large number of dark areas (white circle) on the surface were observed, which indicates that the excess mixture ratio of HQ would hinder formation of a uniform passivation layer followed by sparse oxidation for non-passivated areas; however, with lower HQ ratios of 0.5 (Figure 6a) and 1.0 (Figure 6b), fewer dark areas were revealed. The large contrast between 0.5 HQ and 1.0 HQ also can be explained by the different density of SQ produced by H+ exchange between BQ and HQ because SQ is the actual molecule passivating the Si surface, as confirmed by Cahen et al. [24]. From the HR-AFM and TUNA results, it is clear that HQ affects the uniformity and coverage of the passivation layer on the Si surface.
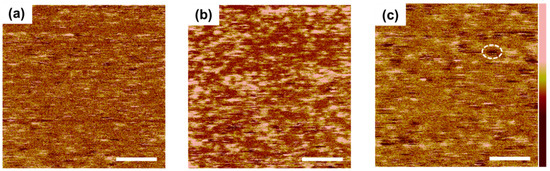
Figure 6.
Tunneling mode atomic force microscope (TUNA) images of (a) 0.5 HQ; (b) 1.0 HQ; and (c) 1.5 HQ mixture ratios with 10 mM of BQ in MeOH. (scan scale = 200 nm × 200 nm; scale bar - 50 nm).
The dipping time effect of passivation was also investigated in both pure BQ and BQ:HQ = 1:1 (QHY) in MeOH with τeff measurements. The measured τeff values for samples dipped in the solutions from 10 to 180 min (Figure 7a) show a dramatic increase of τeff with QHY/MeOH compared with pure BQ/MeOH for dipping times less than 50 min. This rapid increase of τeff can be explained by the higher density of SQ in QHY/MeOH accelerating the passivation reaction between SQ and the Si surface as discussed earlier in relation to Figure 4. However, after 50 min a gradual decrease of τeff with QHY/MeOH was observed, but the τeff from BQ/MeOH continued to increase. From repeated τeff measurements for various dipping times, 1 h and 3 h were determined to be the optimal treatment times for QHY/MeOH and BQ/MeOH, respectively. To help understand the negative effects of over-treatment, HR-AFM images of the sample surfaces from various treatment times were obtained (Figure 7b–e). After 180-min passivation in QHY/MeOH (Figure 7c), the scan shows highly severe molecular aggregations compared with that of pure BQ (Figure 7e). The QHY/MeOH passivation is known to induce positive surface charge on the Si surface by the strong dipole moment of the SQ molecule producing Si surface band-bending (field effect passivation) [24]; therefore, the molecular aggregation after excess dipping time weakened the molecular dipole moment [12]. This is because, although dipole moment is a vector quantity produced by charge asymmetry within molecules, random stacking of molecules would encourage π–π interaction between stacked molecules, thereby reducing charge asymmetry and consequently producing a weaker dipole moment within the molecular layer [27].
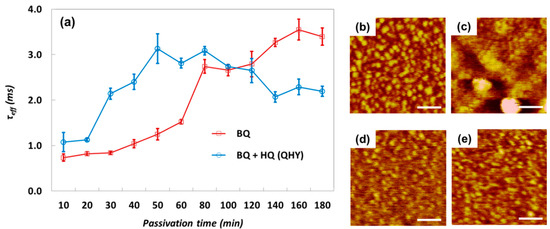
Figure 7.
(a) Measured τeff after dipping in BQ and HQ/MeOH for 10 min to 180 min. HR-AFM images of Si surface after dipping in QHY/MeOH (b,c) and BQ/MeOH (d,e) for 1 h and 3 h, respectively.
To clarify the relationship between τeff and passivation layer morphology, surface potential voltage (SPV) was also measured with two different dipping times, 1 h and 3 h, for QHY, BQ, and HQ/MeOH solutions. The measured SPV results (Table 1) show that QHY with 1 h dipping produced a stronger SPV and an increased τeff (317 meV and 2813 μs) compared with 3 h dipping (249 meV and 2195 μs). In contrast, for BQ, 3 h dipping produced a stronger SPV with a longer τeff (357 meV and 3398 μs) compared with 1 h dipping (317 meV and 1522 μs). These SPV results strongly support the relationship between dipping time and τeff that was described in relation to Figure 7. In addition, as shown in Table 1, HQ/MeOH barely produces SPV on the Si surface, thereby supporting the contention that HQ itself provides no passivation effect for Si, as noted earlier.

Table 1.
SPV values measured by Kelvin Probe system compared with τeff from different dipping times and passivation solutions.
From the results of SPV, AFM, and τeff measurements, the molecular contribution of BQ and HQ for Si surface passivation was clarified with BQ shown as a passivation agent and HQ shown as accelerating the passivation process after donating H+ to BQ. However, even though HQ offers a shorter passivation time, it has a significant effect on passivation layer uniformity, which might be a crucial factor to passivate a complex Si surface. In this paper, therefore, we also performed QHY/MeOH and BQ/MeOH passivation for a nanostructured Si surface (Figure 8a). The nanostructured Si surface was fabricated with a reactive ion etching (RIE) process by using silica nanospheres as a mask material, which was developed as a silica nanosphere lithography (SNL) technique in our previous report [28]. The nanostructures were fabricated on a 2-inch diameter round Si wafer with a 310 nm period. After nanostructure fabrication, the wafer was cut for passivation to offer an identical nanostructure environment for each passivation condition.
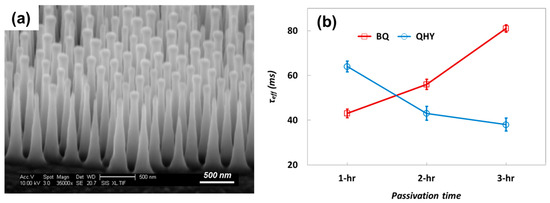
Figure 8.
(a) Si nanostructure fabricated on 1-inch × 1-inch Si surface; and (b) measured τeff after dipping in QHY/MeOH, BQ/MeOH and 10% HF solution.
For nanostructured Si surfaces, passivation was performed in QHY/MeOH and BQ/MeOH with 1 h, 2 h, and 3 h dipping. The results of the τeff measurements (Figure 8b) show that τeff from BQ/MeOH increased as the dipping time increased with the highest τeff of 81 μs with 3 h dipping. In contrast for QHY/MeOH, the best τeff of 64 μs was measured at 1 h, and τeff decreased with longer dipping times. From the experiments of nanostructured Si surface passivation, the trend of τeff with dipping time was well-matched with the earlier results from the plane Si surface passivation. Therefore, for complex Si surface passivation, HQ deteriorates surface passivation quality because, most probably, it impedes the formation of a conformal passivation layer, as discussed earlier.
4. Conclusions
In this paper, we investigated the BQ and HQ molecular contribution for Si surface passivation and clarified the role of each molecule. Based on this report, HQ alone cannot provide passivation, but an optimal HQ mixture ratio with BQ offers a highly accelerated passivation reaction compared with pure BQ because of the increased density of SQ after H+ exchange between BQ and HQ in the solution. However, excess addition of HQ caused severe molecular aggregation and reduced coverage of the passivation layer. Even with an optimal HQ amount, excess dipping times also produced a highly non-uniform and aggregated molecular passivation layer which, subsequently, degraded τeff due to weakened SPV. Although BQ/MeOH had a slower passivation rate, a less dramatic change of passivation layer morphology was observed with BQ/MeOH as dipping time increased, producing a τeff comparable with that from QHY/MeOH. As a result of the influence on passivation layer morphology of HQ, BQ/MeOH offered an enhanced τeff compared with that of QHY/MeOH passivation, especially for a complex nanostructured Si surface that requires highly conformal passivation layer quality.
Funding
This work was supported by the Dong-A University research fund 2018.
Conflicts of Interest
The author declare no competing financial interest.
References
- Tredgold, R.H. An introduction to ultrathin organic films—From langmuir-blodgett to self-assembly-Ulman, A. Nature 1991, 354, 120. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Poirier, G.E.; Pylant, E.D. The Self-Assembly Mechanism of Alkanethiols on Au(111). Science 1996, 272, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Royea, W.J.; Juang, A.; Lewis, N.S. Preparation of air-stable, low recombination velocity Si(111) surfaces through alkyl termination. Appl. Phys. Lett. 2000, 77, 1988–1990. [Google Scholar] [CrossRef]
- Avasthi, S.; Qi, Y.; Vertelov, G.K.; Schwartz, J.; Kahn, A.; Sturm, J.C. Silicon surface passivation by an organic overlayer of 9,10-phenanthrenequinone. Appl. Phys. Lett. 2010, 96, 222109. [Google Scholar] [CrossRef]
- Chen, J.; Ge, K.; Zhang, C.; Guo, J.; Yang, L.; Song, D.; Li, F.; Xu, Z.; Xu, Y.; Mai, Y. Vacuum-Free, Room-Temperature Organic Passivation of Silicon: Toward Very Low Recombination of Micro-/Nanotextured Surface Structures. ACS Appl. Mater. Interfaces 2018, 10, 44890–44896. [Google Scholar] [CrossRef] [PubMed]
- Fleischli, F.D.; Suárez, S.; Schaer, M.; Zuppiroli, L. Organic Thin-Film Transistors: The Passivation of the Dielectric-Pentacene Interface by Dipolar Self-Assembled Monolayers. Langmuir 2010, 26, 15044–15049. [Google Scholar] [CrossRef]
- Takato, H.; Sakata, I.; Shimokawa, R. Quinhydrone/Methanol Treatment for the Measurement of Carrier Lifetime in Silicon Substrates. Jpn. J. Appl. Phys. 2002, 41, L870–L872. [Google Scholar] [CrossRef]
- Chhabra, B.; Bowden, S.; Opila, R.L.; Honsberg, C.B. High effective minority carrier lifetime on silicon substrates using quinhydrone-methanol passivation. Appl. Phys. Lett. 2010, 96, 63502. [Google Scholar] [CrossRef]
- Cornil, J.; Calbert, J.P.; Bredas, J.L. Electronic Structure of the Pentacene Single Crystal: Relation to Transport Properties. J. Am. Chem. Soc. 2001, 123, 1250–1251. [Google Scholar] [CrossRef]
- Chhabra, B.; Suzer, S.; Opila, R.L.; Honsberg, C.B. Electrical and chemical characterization of chemically passivated silicon surfaces. In Proceedings of the 2008 33rd IEEE Photovoltaic Specialists Conference, San Diego, CA, USA, 11–16 May 2008. [Google Scholar]
- Zou, Z.; Liu, W.; Wang, D.; Liu, Z.; Jiang, E.; Wu, S.; Zhu, J.; Guo, W.; Sheng, J.; Ye, J. Electron-selective quinhydrone passivated back contact for high-efficiency silicon/organic heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2018, 185, 218–225. [Google Scholar] [CrossRef]
- Patil, A.O.; Curtin, D.Y.; Paul, I.C. Solid-state formation of quinhydrones from their components. Use of solid-solid reactions to prepare compounds not accessible from solution. J. Am. Chem. Soc. 1984, 106, 348–353. [Google Scholar] [CrossRef]
- Scheffer, J.R.; Wong, Y.F.; Patil, A.O.; Curtin, D.Y.; Paul, I.C. CPMAS C-13 NMR-spectra of quinones, hydroquinones, and their complexes—Use of CMR to follow a reaction in the solid-state. J. Am. Chem. Soc. 1985, 107, 4898–4904. [Google Scholar] [CrossRef]
- Patil, A.O.; Pennington, W.T.; Desiraju, G.R.; Curtin, D.Y.; Paul, I.C. Recent Studies on the Formation and Properties of Quinhydrone Complexes. Mol. Cryst. Liq. Cryst. 1986, 134, 279–304. [Google Scholar] [CrossRef]
- Toda, F. Organic Solid State Reactions; Springer Science & Business Media: Berlin, Germany, 2005; Volume 254. [Google Scholar]
- Guin, P.S.; Das, S.; Mandal, P.C. Electrochemical Reduction of Quinones in Different Media: A Review. Int. J. Electrochem. 2011, 2011, 1–22. [Google Scholar] [CrossRef]
- Eggins, B.; Chambers, J. Proton effects in the electrochemistry of the quinone hydroquinone system in aprotic solvents. J. Electrochem. Soc. 1970, 117, 186–192. [Google Scholar] [CrossRef]
- Astudillo, P.D.; Valencia, D.P.; González-Fuentes, M.A.; Díaz-Sánchez, B.R.; Frontana, C.; González, F.J. Electrochemical and chemical formation of a low-barrier proton transfer complex between the quinone dianion and hydroquinone. Electrochim. Acta 2012, 81, 197–204. [Google Scholar] [CrossRef]
- Rykaczewski, K.; Hildreth, O.J.; Wong, C.P.; Fedorov, A.G.; Scott, J.H.J. Guided Three-Dimensional Catalyst Folding during Metal-Assisted Chemical Etching of Silicon. Nano Lett. 2011, 11, 2369–2374. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Werner, P.; de Boor, J.; Gösele, U. Metal-Assisted Chemical Etching of Silicon: A Review. Adv. Mater. 2011, 23, 285–308. [Google Scholar] [CrossRef]
- Lehn, J.-M.; Ziessel, R. Photochemical generation of carbon monoxide and hydrogen by reduction of carbon dioxide and water under visible light irradiation. Proc. Natl. Acad. Sci. USA 1982, 79, 701–704. [Google Scholar] [CrossRef]
- Kotulak, N.A.; Chen, M.; Schreiber, N.; Jones, K.; Opila, R.L. Examining the free radical bonding mechanism of benzoquinone– and hydroquinone–methanol passivation of silicon surfaces. Appl. Surf. Sci. 2015, 354, 469–474. [Google Scholar] [CrossRef]
- Har-Lavan, R.; Schreiber, R.E.; Yaffe, O.; Cahen, D. Molecular field effect passivation: Quinhydrone/methanol treatment of n-Si(100). J. Appl. Phys. 2013, 113, 84909. [Google Scholar] [CrossRef]
- Haick, H.; Hurley, P.T.; Hochbaum, A.I.; Yang, P.; Lewis, N.S. Electrical Characteristics and Chemical Stability of Non-Oxidized, Methyl-Terminated Silicon Nanowires. J. Am. Chem. Soc. 2006, 128, 8990–8991. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ho, J.C.; Yerushalmi, R.; Jacobson, Z.A.; Fan, Z.; Alley, R.L.; Javey, A. Controlled nanoscale doping of semiconductors via molecular monolayers. Nat. Mater. 2008, 7, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; de Paula, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Choi, J.-Y.; Alford, T.L.; Honsberg, C.B. Fabrication of Periodic Silicon Nanopillars in a Two-Dimensional Hexagonal Array with Enhanced Control on Structural Dimension and Period. Langmuir 2015, 31, 4018–4023. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).