Furfural Oxidation on Gold Supported on MnO2: Influence of the Support Structure on the Catalytic Performances
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Catalytic Tests
3. Results
3.1. Structural properties
3.2. Catalytic Activity
3.3. Post-Test Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Gravel, E.; Hagege, A.; Li, H.; Jawale, D.V.; Verma, D.; Namoboothiri, I.N.N.; Doris, E. Carbon nanotube-gold nanohybrids for selective catalytic oxidation of alcohols. Nanoscale 2013, 5, 6491–6497. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, R.; Ferraz, C.P.; Sha, J.; Houda, S.; Rossi, L.M.; Paul, S. Advances in Base free oxidation of bio-based compounds on supported gold catalysts. Catalysts 2017, 7, 352–375. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Cuccovia, I.M.; Silva, M.A.; Rossi, L.M. Selective oxidation of glucose to glucuronic acid by cesium-promoted gold nanoparticle catalyst. J. Mol. Catal. A Chem. 2016, 422, 35–42. [Google Scholar] [CrossRef]
- Solmi, S.; Morreale, C.; Ospitali, F.; Agnoli, S.; Cavani, F. Oxidation of d-glucose to glucaric acid using Au/C catalysts. ChemCatChem 2017, 9, 2797–2806. [Google Scholar] [CrossRef]
- Haruta, M. When Gold Is Not Noble: Catalysis by Nanoparticles. Chem. Rec. 2003, 3, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Gonçalves, R.; Vono, L.; Wojcieszak, R.; Dias, C.; Wendere, H.; Teixeira-Neto, E.; Rossi, L. Selective hydrogenation of CO2 into CO on a highly dispersed nickel catalyst obtained by magnetron sputtering deposition: A step towards liquid fuels. Appl. Catal. B 2017, 209, 240–246. [Google Scholar] [CrossRef]
- Önal, Y.; Schimpf, S.; Claus, P. Structure sensitivity and kinetics of d-glucose oxidation to d-gluconic acid over carbon-supported gold catalysts. J. Catal. 2004, 223, 122–133. [Google Scholar] [CrossRef]
- Stephen, A.; Hashmi, K. Gold-Catalyzed Organic Reactions. Chem. Rev. 2007, 107, 3180–3211. [Google Scholar] [CrossRef]
- Okatsu, H.; Kinoshita, N.; Akita, T.; Ishida, T.; Haruta, M. Deposition of gold nanoparticles on carbons for aerobic glucose oxidation. Appl. Catal. A 2009, 369, 8–14. [Google Scholar] [CrossRef]
- Ishida, T.; Kinoshita, N.; Okatsu, H.; Akita, T.; Takei, T.; Haruta, M. Influence of the Support and the Size of Gold Clusters on Catalytic Activity for Glucose Oxidation. Angew. Chem. Int. Ed. 2008, 120, 9405–9408. [Google Scholar] [CrossRef]
- Comotti, M.; Pina, C.D.; Matarrese, R.; Rossi, M.; Siani, A. Oxidation of alcohols and sugars using Au/C catalysts: Part 2. Sugars. Appl. Catal. A 2005, 291, 204–209. [Google Scholar] [CrossRef]
- Eggleston, G.; Vercellotti, J.R. Degradation of Sucrose, Glucose and Fructose in Concentrated Aqueous Solutions Under Constant pH Conditions at Elevated Temperature. Carbohydr. Res. 2000, 19, 1305–1318. [Google Scholar] [CrossRef]
- Biella, S.; Prati, L.; Rossi, M. Selective Oxidation of D-Glucose on Gold Catalyst. J. Catal. 2002, 206, 242–247. [Google Scholar] [CrossRef]
- Comotti, M.; Della Pina, C.; Matarrese, R.; Rossi, M. The catalytic activity of “naked” gold particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef] [PubMed]
- Bettahar, M.; Wojcieszak, R.; Monteverdi, S. NiAg catalysts prepared by reduction of Ni2+ ions in aqueous hydrazine II. Support effect. J. Colloid Interface Sci. 2009, 332, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Fukuoka, A.; Nakajima, K. Metal-free and selective oxidation of furfural to furoic acid with an N-heterocyclic carbene catalyst. ACS Sus. Chem. Eng. 2018, 6, 3434–3442. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Santarelli, F.; Paul, S.; Dumeignil, F.; Cavani, F.; Gonçalves, R.V. Recent developments in maleic acid synthesis from bio-based chemicals. Sustain. Chem. Process. 2015, 3, 9–20. [Google Scholar] [CrossRef]
- Banerjee, A.; Dick, G.; Yoshino, T.; Kanan, M. Carbon dioxide utilization via carbonate-promoted C–H carboxylation. Nature 2016, 531, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, E.; Komanoya, T.; Kamata, K.; Hara, M. Heterogeneously-Catalyzed Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid with MnO2. ChemSusChem 2017, 22, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y. Selected-Control Hydrothermal Synthesis of α- and β-MnO2 Single Crystal Nanowires. J. Am. Chem. Soc. 2002, 124, 2880–2881. [Google Scholar] [CrossRef] [PubMed]
- Realcat Platform. Available online: www.realcat.fr (accessed on 25 May 2018).
- Deraedt, Ch.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, R.; Ruiz, J.; Astruc, D. Sodium borohydride stabilizes very active gold nanoparticle catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-C.; Liu, Y.-M.; Chen, M.; Cao, Y.; He, H.Y.; Fan, K.-N. MnO2 Nanorod Supported Gold Nanoparticles with Enhanced Activity for Solvent-free Aerobic Alcohol Oxidation. J. Phys. Chem. C 2008, 112, 6981–6987. [Google Scholar] [CrossRef]
- da Silva, A.G.M.; Kisukuri, C.M.; Rodrigues, T.S.; Candido, E.G.; de Freitas, I.C.; da Silva, A.H.M.; Assaf, J.M.; Oliveira, D.C.; Andrade, L.H.; Camargo, P.H.C. MnO2 nanowires decorated with Au ultrasmall nanoparticles for the green oxidation of silanes and hydrogen production under ultralow loadings. Appl. Catal. B 2016, 184, 35–43. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products; Elsevier: Amsterdam, The Netherlands, 2000; pp. 159–163. ISBN 9780444503510. [Google Scholar]
- Taarning, E.; Nielsen, I.S.; Egeblad, K.; Madsen, R.; Christensen, C.H. Chemicals from Renewables: Aerobic Oxidation of Furfural and Hydroxymethylfurfural over Gold Catalysts. ChemSusChem 2008, 1, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Douthwait, M.; Huang, X.; Iqbal, S.; Miedziak, P.J.; Brett, G.L.; Kondrat, S.A.; Edwards, J.K.; Sankar, M.; Knight, D.W.; Bethell, D.; Hutchings, G.J. The controlled catalytic oxidation of furfural to furoic acid using AuPd/M(OH)2. Catal. Sci. Technol. 2017, 7, 5284–5293. [Google Scholar] [CrossRef]
- Vuyyuru, K.R.; Strasser, P. Oxidation of biomass derived 5-hydroxymethylfurfural using heterogeneous and electrochemical catalysis. Catal. Today 2012, 195, 144–154. [Google Scholar] [CrossRef]
- Piccolo, A.; Conte, P.; Cozzolino, A. Effects of mineral and monocarboxylic acids on the molecular association of dissolved humic substances. Eur. J. Soil Sci. 1999, 50, 687–694. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, M.; Xia, Y.; Lu, M. Au/MnO2 nanostructured catalysts and their catalytic performance for the oxidation of 5-(hydroxymethyl)furfural. Catal. Commun. 2015, 64, 37–43. [Google Scholar] [CrossRef]
- Yang, M.; Allard, L.F. Flytzani-Stephanopoulos, Atomically Dispersed Au–(OH)x Species Bound on Titania Catalyze the Low-Temperature Water-Gas Shift Reaction M. J. Am. Chem. Soc. 2013, 135, 3768–3771. [Google Scholar] [CrossRef] [PubMed]
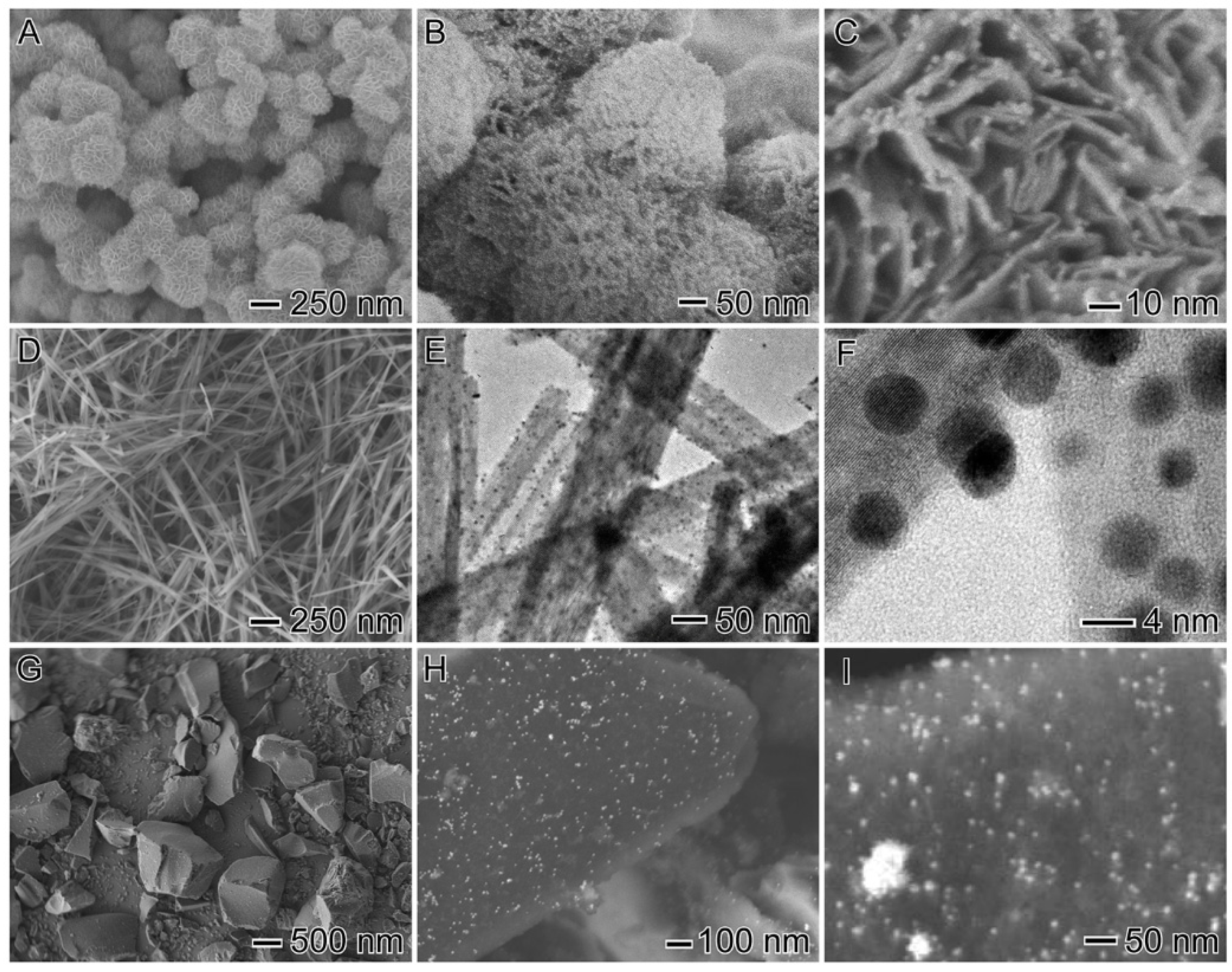
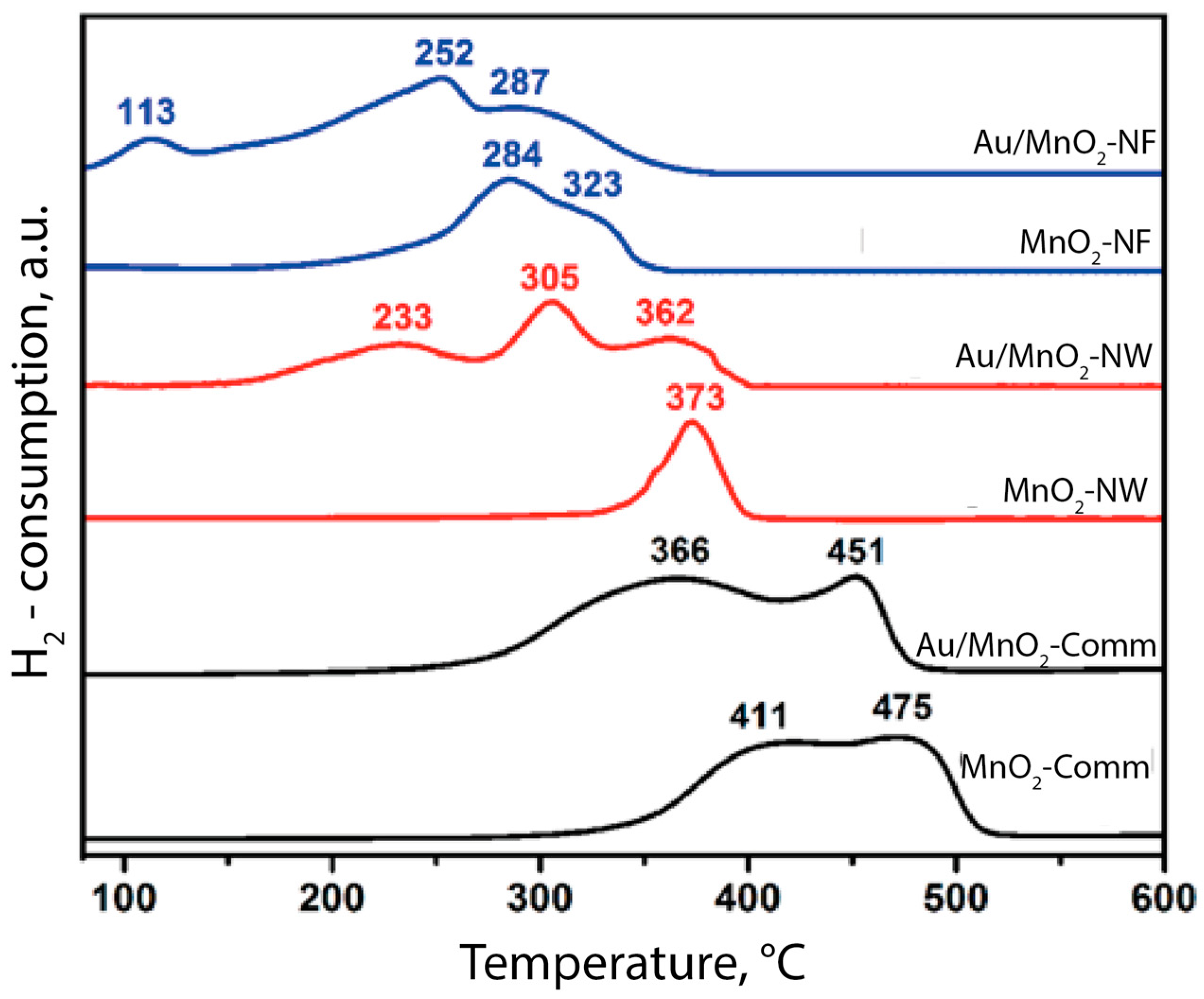
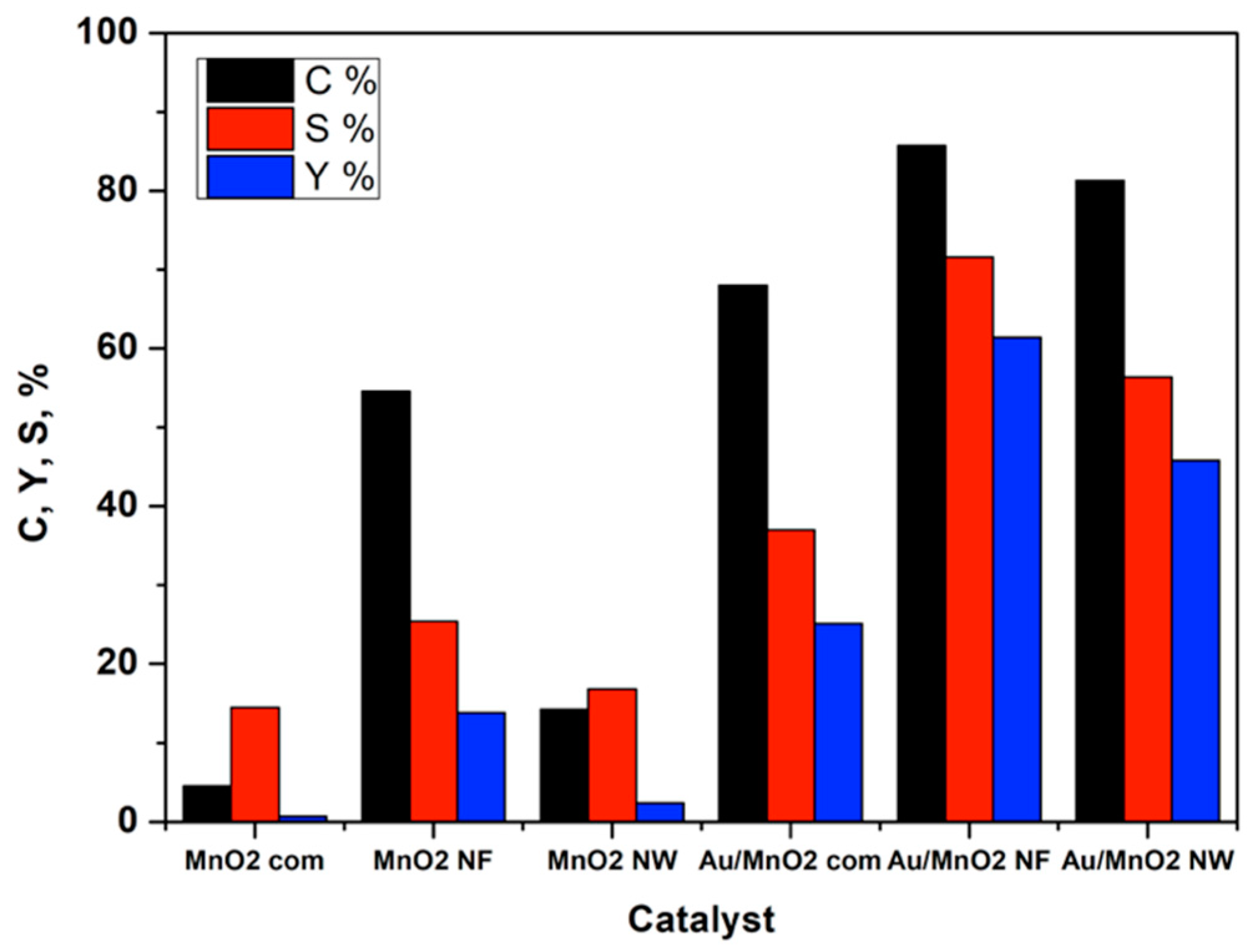
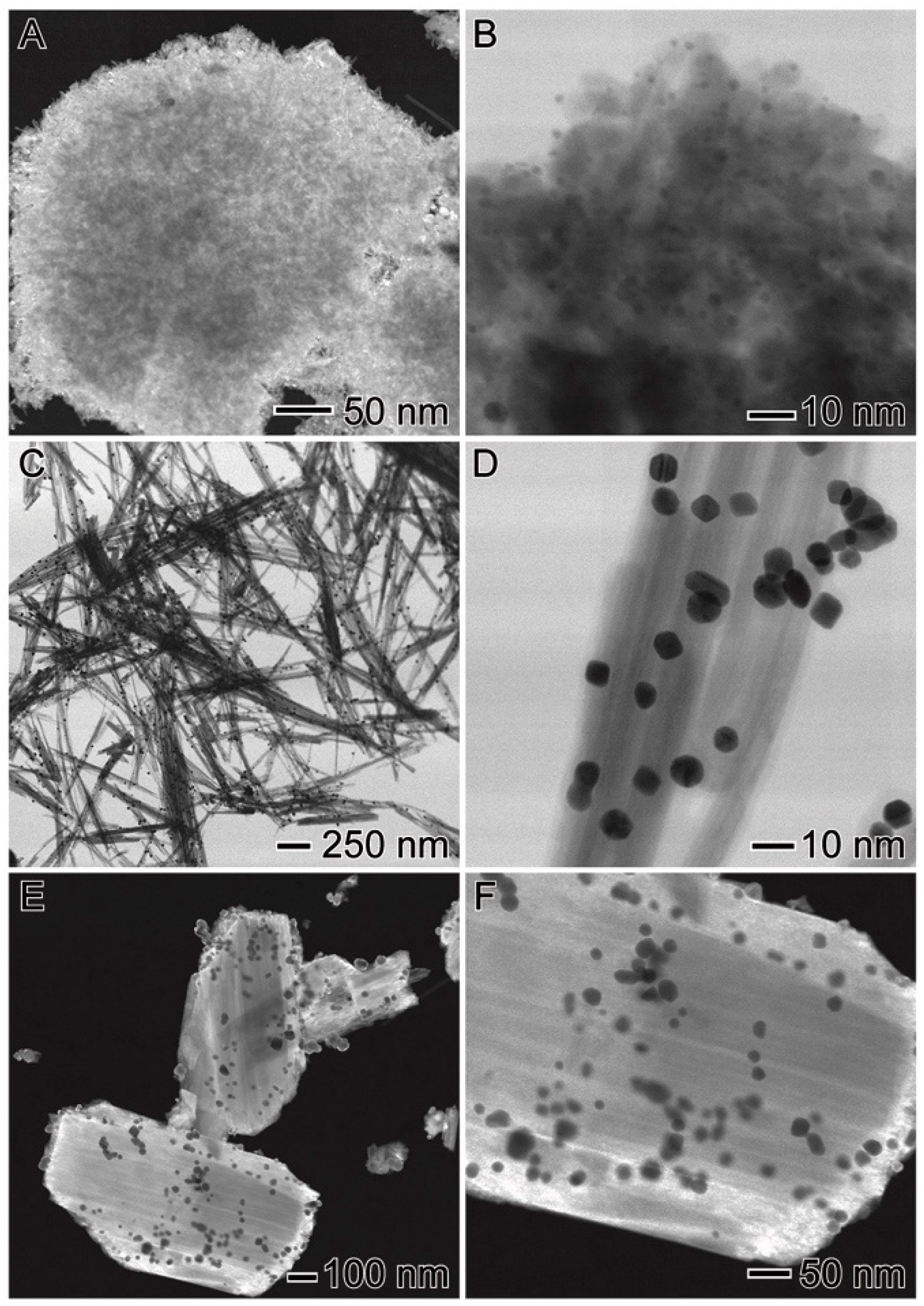
| Catalyst | Au (wt %) | Particle size (nm) | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (Å) |
|---|---|---|---|---|---|
| Au/MnO2-NF | 1.3 | 2.6 | 196 | 0.42 | 6.1 |
| MnO2 NF | - | - | 194 | 0.41 | 6.1 |
| Au/MnO2-NW | 1.3 | 3.2 | 125 | 0.16 | 6.9 |
| MnO2-NW | - | - | 122 | 0.16 | 6.8 |
| Au/MnO2-Comm | 1.4 | 2.6 | 15 | 0.05 | 17.8 |
| MnO2-Comm | - | - | 14 | 0.05 | 17.8 |
| Entry | T [°C] | Time [min] | XFUR [%] | SFA [%] | CB [%] |
|---|---|---|---|---|---|
| 1 | 110 | 30 | 45 | 82 | 93 |
| 2 | 110 | 70 | 61 | 80 | 93 |
| 3 | 110 | 120 | 86 | 72 | 92 |
| 4 | 110 | 240 | 100 | 82 | 93 |
| 5 | 80 | 120 | 31 | 92 | 92 |
| 6 | 80 | 240 | 65 | 89 | 96 |
| 7 | 150 | 120 | 100 | 67 | 75 |
| Entry | Catalyst | XFUR [%] | SFA [%] | CB [%] |
|---|---|---|---|---|
| 1 | Au/MnO2 -NF | 82 | 74 | 92 |
| 2 | Au/MnO2-NW | 75 | 64 | 93 |
| 3 | Au/MnO2-Comm | 53 | 37 | 91 |
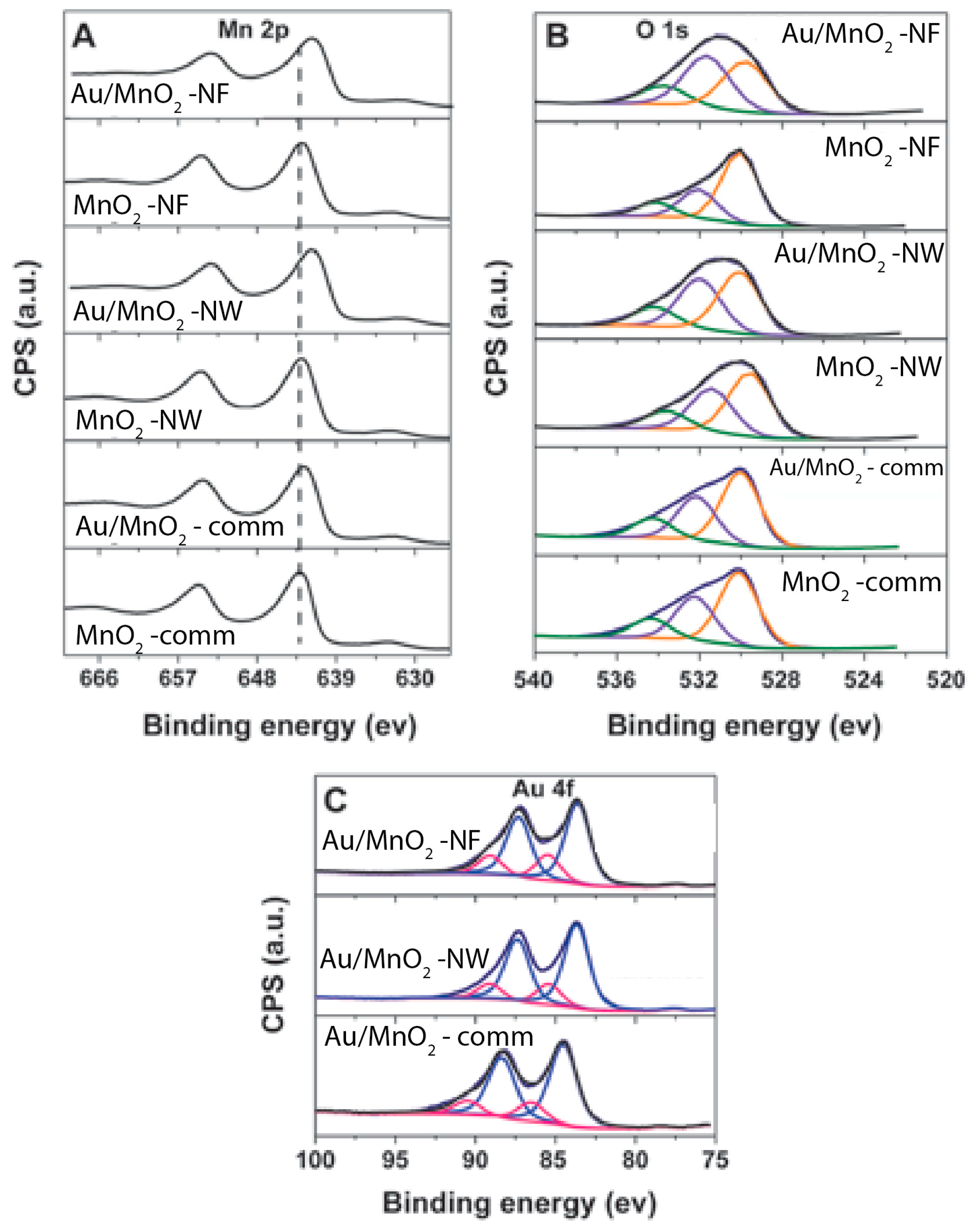
| Sample | BE of Mn 2p3/2 (eV) | BE of O 1s (eV) | Au 4f (eV) | |
|---|---|---|---|---|
| OL | OS | Auδ+ | ||
| Au/MnO2-NW | 641.7 | 530.1 (46) * | 532.0 (39) * | 85.4 (20) * |
| MnO2-NW | 642.6 | 529.5 (52) * | 531.5 (34) * | no signal |
| Au/MnO2-comm | 642.0 | 530.1 (56) * | 532.0 (30) * | 86.5 (18) * |
| MnO2-comm | 642.7 | 530.0 (56) * | 532.2 (29) * | no signal |
| Au/MnO2-NF | 641.7 | 529.8 (41) * | 531.6 (47) * | 85.5 (26) * |
| MnO2-NF | 642.6 | 530.0 (61) * | 532.0 (28) * | no signal |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, C.P.; Da Silva, A.G.M.; Rodrigues, T.S.; Camargo, P.H.C.; Paul, S.; Wojcieszak, R. Furfural Oxidation on Gold Supported on MnO2: Influence of the Support Structure on the Catalytic Performances. Appl. Sci. 2018, 8, 1246. https://doi.org/10.3390/app8081246
Ferraz CP, Da Silva AGM, Rodrigues TS, Camargo PHC, Paul S, Wojcieszak R. Furfural Oxidation on Gold Supported on MnO2: Influence of the Support Structure on the Catalytic Performances. Applied Sciences. 2018; 8(8):1246. https://doi.org/10.3390/app8081246
Chicago/Turabian StyleFerraz, Camila Palombo, Anderson Gabriel Marques Da Silva, Thenner Silva Rodrigues, Pedro Henrique Cury Camargo, Sébastien Paul, and Robert Wojcieszak. 2018. "Furfural Oxidation on Gold Supported on MnO2: Influence of the Support Structure on the Catalytic Performances" Applied Sciences 8, no. 8: 1246. https://doi.org/10.3390/app8081246
APA StyleFerraz, C. P., Da Silva, A. G. M., Rodrigues, T. S., Camargo, P. H. C., Paul, S., & Wojcieszak, R. (2018). Furfural Oxidation on Gold Supported on MnO2: Influence of the Support Structure on the Catalytic Performances. Applied Sciences, 8(8), 1246. https://doi.org/10.3390/app8081246







