Collagen/Polyethylene Oxide Nanofibrous Membranes with Improved Hemostasis and Cytocompatibility for Wound Dressing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Nanofibrous Nonwovens
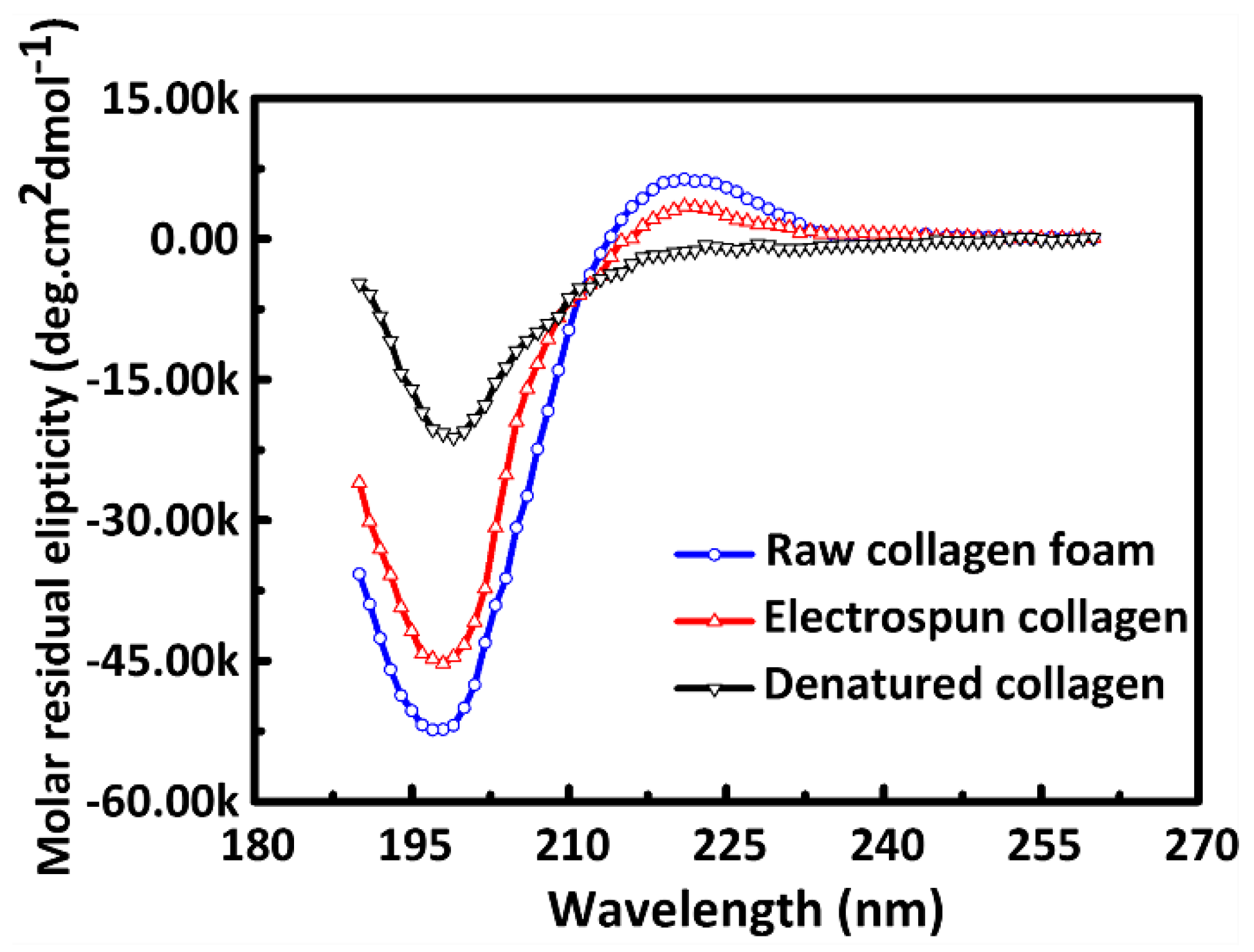
2.3. Circular Dichroism Measurement (CD)
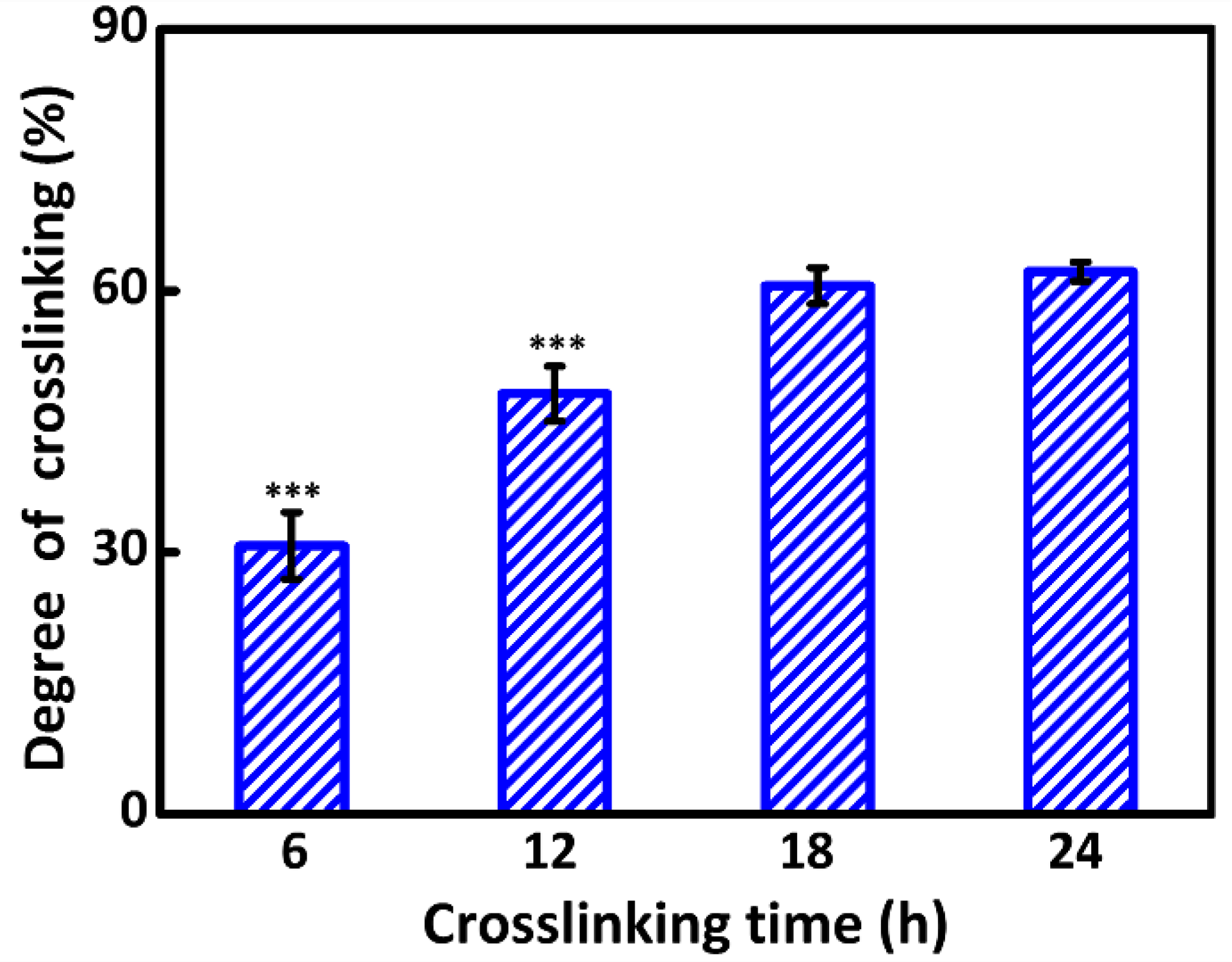
2.4. Characterizations of Crosslinking
2.5. Whole Blood Clotting Assay
2.6. Cell Proliferation Analysis
3. Results and Discussion
3.1. Triple-Helical Configuration Analysis
3.2. Degree of Crosslinking
3.3. Water Stability of Electrospun Collagen/PEO Membranes
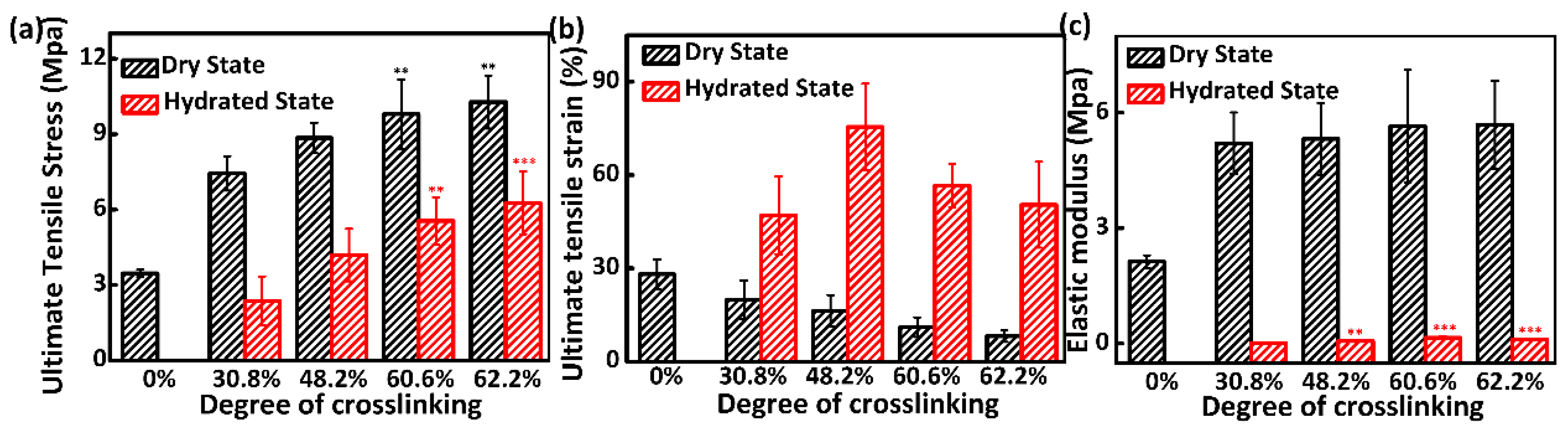
3.4. Mechanical Properties Analysis
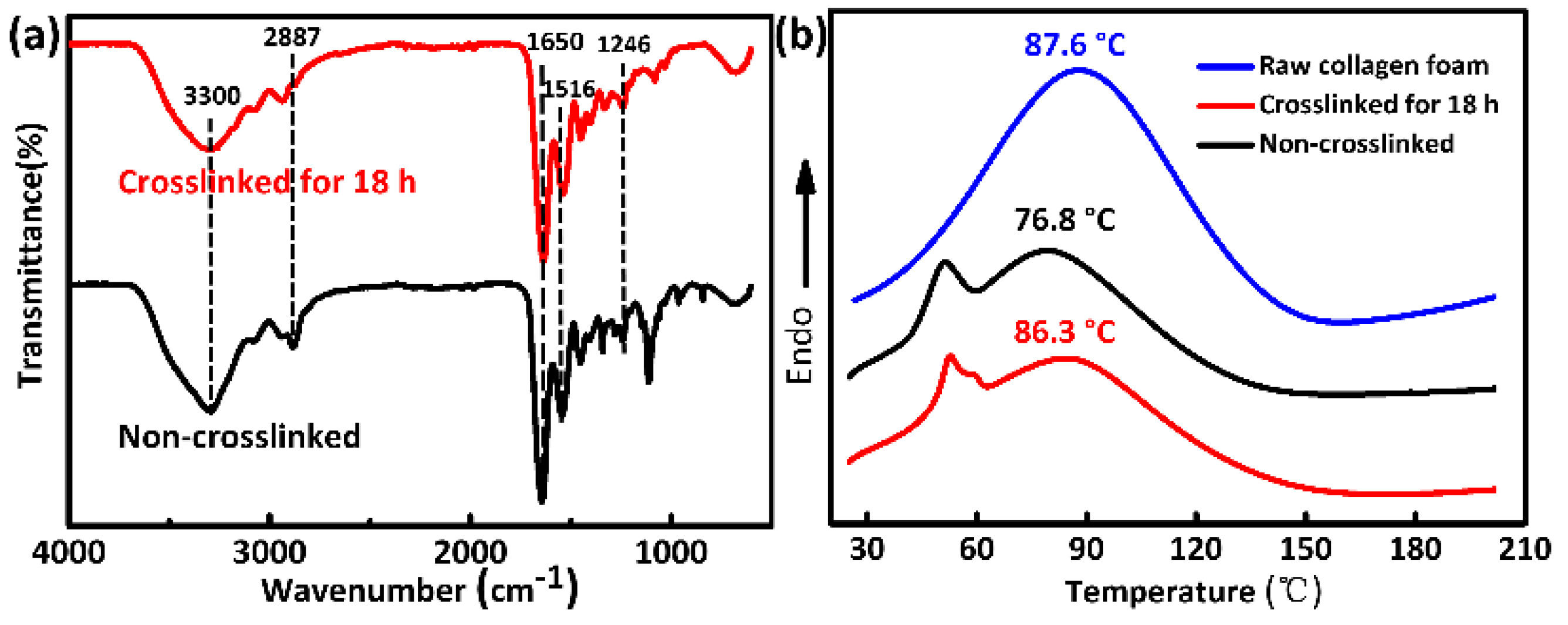
3.5. Additional Characterizations
3.6. Whole Blood Clotting Assay
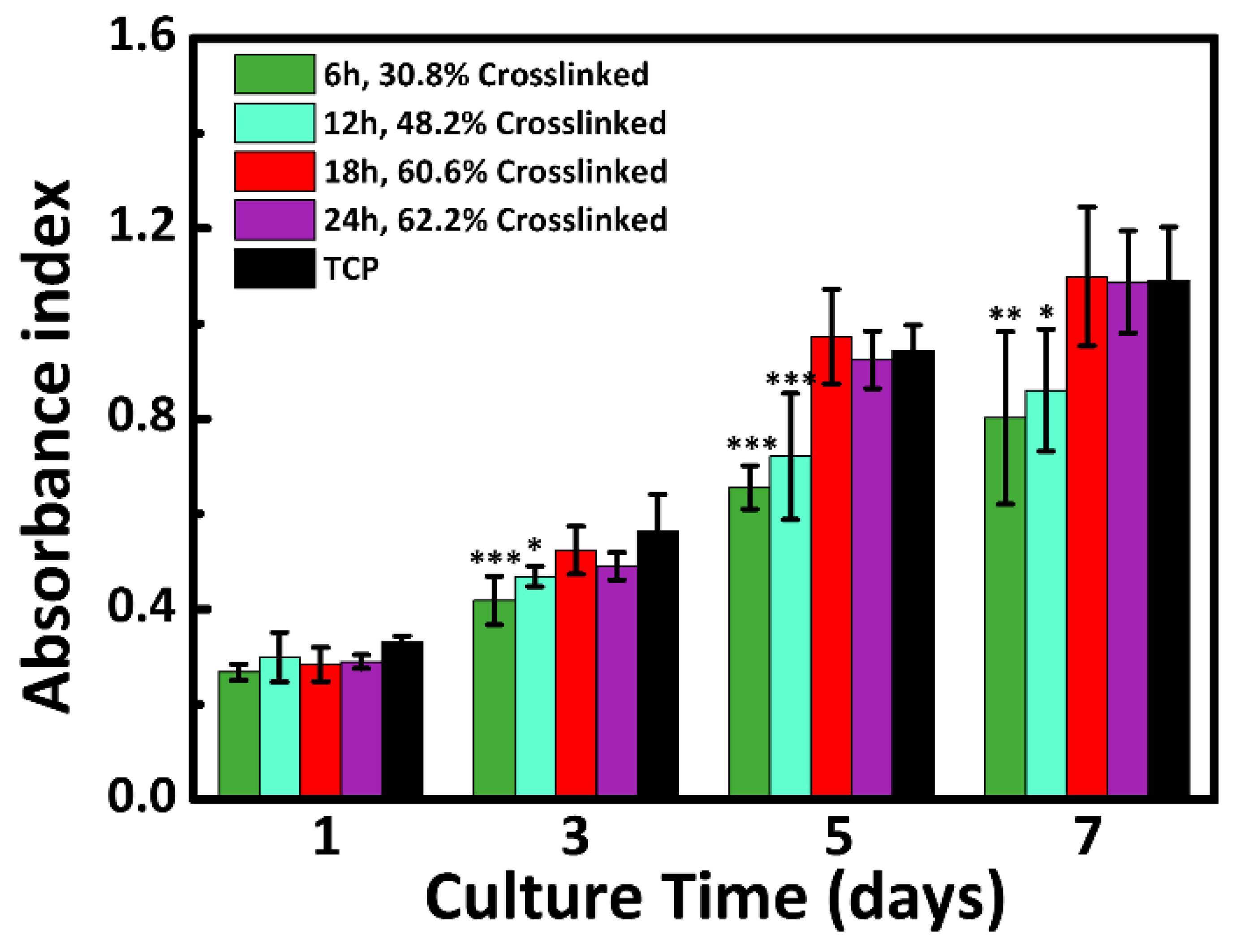
3.7. Cytocompatibility Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kanokpanont, S.; Damrongsakkul, S.; Ratanavaraporn, J.; Aramwit, P. An innovative bi-layered wound dressing made of silk and gelatin for accelerated wound healing. Int. J. Pharm. 2012, 436, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Casson, J.; O’Kane, S.; Smith, C.A.; Dalby, M.J.; Berry, C.C. Interleukin 6 plays a role in the migration of magnetically levitated mesenchymal stem cells spheroids. Appl. Sci. 2018, 8, 412. [Google Scholar] [CrossRef]
- Dhand, C.; Ong, S.T.; Dwivedi, N.; Diaz, S.M.; Venugopal, J.R.; Navaneethan, B.; Fazil, M.H.U.T.; Liu, S.; Seitz, V.; Wintermantel, E. Bio-inspired in situ crosslinking and mineralization of electrospun collagen scaffolds for bone tissue engineering. Biomaterials 2016, 104, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Smith, L.A.; Hu, J.; Ma, P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009, 30, 2252–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.M.; Park, Y.J.; Hong, S.D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Gimeno-Alcaniz, J.V.; Ocio, M.J.; Lagaron, J.M. Comparative performance of electrospun collagen nanofibers cross-linked by means of different methods. ACS Appl. Mater. Interfaces 2009, 1, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Y.; Liang, M.; Ke, Q.; Fang, Y.; Xu, H.; Jin, X.; Huang, C. Honeycomb-like polysulphone/polyurethane nanofiber filter for the removal of organic/inorganic species from air streams. J. Hazard. Mater. 2018, 347, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Azais, T.; Robin, M.; Vallee, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.M.; Nassif, N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012, 11, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, M.; Williams, G.R.; Wu, J.; Sun, X.; Lv, Y.; Zhu, L.-M. Electrospun gelatin nanofibers loaded with vitamins a and e as antibacterial wound dressing materials. RSC Adv. 2016, 6, 50267–50277. [Google Scholar] [CrossRef]
- Huang, G.P.; Shanmugasundaram, S.; Masih, P.; Pandya, D.; Amara, S.; Collins, G.; Arinzeh, T.L. An investigation of common crosslinking agents on the stability of electrospun collagen scaffolds. J. Biomed. Mater. Res. Part A 2015, 103, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.Y.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres—Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.W.; Powell, H.M. Dehydrothermal crosslinking of electrospun collagen. Tissue Eng. Part C Methods 2011, 17, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Raiskup-Wolf, F.; Hoyer, A.; Spoerl, E.; Pillunat, L.E. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J. Cataract Refract. Surg. 2008, 34, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Madaghiele, M.; Parisi, C.; Gatti, F.; Sannino, A. Crosslinking of micropatterned collagen-based nerve guides to modulate the expected half-life. J. Biomed. Mater. Res. Part A 2014, 102, 4406–4414. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, J.; Sionkowska, A. Effects of different crosslinking methods on the properties of collagen-calcium phosphate composite materials. Int. J. Biol. Macromol. 2015, 74, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, K.; Zheng, X. Electrospinning and crosslinking of col/pva nanofiber-microsphere containing salicylic acid for drug delivery. J. Bionic Eng. 2016, 13, 143–149. [Google Scholar] [CrossRef]
- Everaerts, F.; Torrianni, M.; Hendriks, M.; Feijen, J. Biomechanical properties of carbodiimide crosslinked collagen: Influence of the formation of ester crosslinks. J. Biomed. Mater. Res. Part A 2008, 85, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Tamura, K.; Mineguchi, R.; Ishikawa, Y.; Haraguchi, Y.; Shimizu, T.; Hara, Y. In situ cross-linked electrospun fiber scaffold of collagen for fabricating cell-dense muscle tissue. J. Artif. Organs Off. J. Jpn. Soc. Artif. Organs 2016, 19, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Geng, X.; Ye, L.; Zhang, A.; Feng, Z.; Guo, L.; Gu, Y. A vascular tissue engineering scaffold with core-shell structured nano-fibers formed by coaxial electrospinning and its biocompatibility evaluation. Biomed. Mater. 2016, 11, 035007. [Google Scholar] [CrossRef] [PubMed]
- Fessel, G.; Cadby, J.; Wunderli, S.; van Weeren, R.; Snedeker, J.G. Dose- and time-dependent effects of genipin crosslinking on cell viability and tissue mechanics—Toward clinical application for tendon repair. Acta Biomater. 2014, 10, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Sisson, K.; Zhang, C.; Farachcarson, M.C.; Chase, D.B.; Rabolt, J.F. Evaluation of cross-linking methods for electrospun gelatin on cell growth and viability. Biomacromolecules 2009, 10, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Powell, H.M.; Boyce, S.T. Edc cross-linking improves skin substitute strength and stability. Biomaterials 2006, 27, 5821–5827. [Google Scholar] [CrossRef] [PubMed]
- Fullana, M.J.; Wnek, G.E. Electrospun collagen and its applications in regenerative medicine. Drug Deliv. Transl. Res. 2012, 2, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, R.; Ke, Q.; Morsi, Y.; Zhang, K.; Mo, X. Electrospun collagen-chitosan-Tpu nanofibrous scaffolds for tissue engineered tubular grafts. Colloids Surf. B Biointerfaces 2011, 82, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Newton, D.; Mahajan, R.; Ayres, C.; Bowman, J.R.; Bowlin, G.L.; Simpson, D.G. Regulation of material properties in electrospun scaffolds: Role of cross-linking and fiber tertiary structure. Acta Biomater. 2009, 5, 518–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omobono, M.A.; Zhao, X.; Furlong, M.A.; Kwon, C.H.; Gill, T.J.; Randolph, M.A.; Redmond, R.W. Enhancing the stiffness of collagen hydrogels for delivery of encapsulated chondrocytes to articular lesions for cartilage regeneration. J. Biomed. Mater. Res. Part A 2015, 103, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Sanami, M.; Sweeney, I.; Shtein, Z.; Meirovich, S.; Sorushanova, A.; Mullen, A.M.; Miraftab, M.; Shoseyov, O.; O’Dowd, C.; Pandit, A.; et al. The influence of poly(ethylene glycol) ether tetrasuccinimidyl glutarate on the structural, physical, and biological properties of collagen fibers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Awang, M.A.; Firdaus, M.A.; Busra, M.B.; Chowdhury, S.R.; Fadilah, N.R.; Wan Hamirul, W.K.; Reusmaazran, M.Y.; Aminuddin, M.Y.; Ruszymah, B.H. Cytotoxic evaluation of biomechanically improved crosslinked ovine collagen on human dermal fibroblasts. Bio-Med. Mater. Eng. 2014, 24, 1715–1724. [Google Scholar]
- Barnes, C.P.; Pemble, C.W.; Brand, D.D.; Simpson, D.G.; Bowlin, G.L. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007, 13, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Le Nihouannen, D.; Hacking, S.A.; Tran, S.; Li, J.; Murshed, M.; Doillon, C.J.; Ghezzi, C.E.; Zhang, Y.L.; Nazhat, S.N.; et al. Newly identified interfibrillar collagen crosslinking suppresses cell proliferation and remodelling. Biomaterials 2015, 54, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Reddy, N.; Zhang, S.; Roscioli, N.; Yang, Y. Water-stable electrospun collagen fibers from a non-toxic solvent and crosslinking system. J. Biomed. Mater. Res. Part A 2013, 101, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Teng, W.K.; Chan, B.P.; Chew, S.Y. Photochemical crosslinked electrospun collagen nanofibers: Synthesis, characterization and neural stem cell interactions. J. Biomed. Mater. Res. Part A 2010, 95, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yunoki, S.; Suzuki, T.; Sakata, M.; Tajima, K.; Munekata, M. Application of cross-linked salmon atelocollagen to the scaffold of human periodontal ligament cells. J. Biosci. Bioeng. 2004, 97, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Seon, G.M.; Lee, M.H.; Kwon, B.J.; Kim, M.S.; Koo, M.A.; Kim, D.; Seomun, Y.; Kim, J.T.; Park, J.C. Functional improvement of hemostatic dressing by addition of recombinant batroxobin. Acta Biomater. 2017, 48, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Arnoult, O.; Smith, M.; Wnek, G.E. Electrospinning of in situ crosslinked collagen nanofibers. J. Mater. Chem. 2012, 22, 19412–19417. [Google Scholar] [CrossRef]
- Yang, L.; Fitie, C.F.; van der Werf, K.O.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Mechanical properties of single electrospun collagen type i fibers. Biomaterials 2008, 29, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.P.; Nezarati, R.M.; Radzicki, C.M.; Renfro, A.L.; Robinson, J.L.; Whitely, M.E.; Cosgriff-Hernandez, E.M. In situ crosslinking of electrospun gelatin for improved fiber morphology retention and tunable degradation. J. Mater. Chem. B 2015, 3, 7930–7938. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, S.; Lin, Z.; Du, C. Reactive electrospinning of composite nanofibers of carboxymethyl chitosan cross-linked by alginate dialdehyde with the aid of polyethylene oxide. Carbohydr. Polym. 2016, 148, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Krebs, M.D.; Bonino, C.A.; Khan, S.A.; Alsberg, E. Electrospun alginate nanofibers with controlled cell adhesion for tissue engineering. Macromol. Biosci. 2010, 10, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, F.; Gao, J.; Wang, L. Antibacterial wound dressing from chitosan/polyethylene oxide nanofibers mats embedded with silver nanoparticles. J. Biomater. Appl. 2015, 29, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Bedran-Russo, A.K.; Castellan, C.S.; Shinohara, M.S.; Hassan, L.; Antunes, A. Characterization of biomodified dentin matrices for potential preventive and reparative therapies. Acta Biomater. 2011, 7, 1735–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Zhong, S.P.; Teo, W.E.; Zhu, X.; Beuerman, R.; Ramakrishna, S.; Yung, L.Y.L. Development of a novel collagen–GAG nanofibrous scaffold via electrospinning. Mater. Sci. Eng. C 2007, 27, 262–266. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, characterization and evaluation of collagen from jellyfish rhopilema esculentum kishinouye for use in hemostatic applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef] [PubMed]
- Achneck, H.E.; Sileshi, B.; Jamiolkowski, R.M.; Albala, D.M.; Shapiro, M.L.; Lawson, J.H. A comprehensive review of topical hemostatic agents: Efficacy and recommendations for use. Ann. Surg. 2010, 251, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, W.D.; Burks, S. Hemostats, sealants, and adhesives: Components of the surgical toolbox. Transfusion 2008, 48, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ha, H.J.; Ko, Y.K.; Yoon, S.J.; Rhee, J.M.; Kim, M.S.; Lee, H.B.; Khang, G. Correlation of proliferation, morphology and biological responses of fibroblasts on ldpe with different surface wettability. J. Biomater. Sci. Polym. Ed. 2007, 18, 609–622. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Gao, J.; Hu, X.; Guo, H.; Wang, F.; Qiao, Y.; Wang, L. Collagen/Polyethylene Oxide Nanofibrous Membranes with Improved Hemostasis and Cytocompatibility for Wound Dressing. Appl. Sci. 2018, 8, 1226. https://doi.org/10.3390/app8081226
Zhao X, Gao J, Hu X, Guo H, Wang F, Qiao Y, Wang L. Collagen/Polyethylene Oxide Nanofibrous Membranes with Improved Hemostasis and Cytocompatibility for Wound Dressing. Applied Sciences. 2018; 8(8):1226. https://doi.org/10.3390/app8081226
Chicago/Turabian StyleZhao, Xinzhe, Jing Gao, Xingyou Hu, Huiwen Guo, Fujun Wang, Yansha Qiao, and Lu Wang. 2018. "Collagen/Polyethylene Oxide Nanofibrous Membranes with Improved Hemostasis and Cytocompatibility for Wound Dressing" Applied Sciences 8, no. 8: 1226. https://doi.org/10.3390/app8081226
APA StyleZhao, X., Gao, J., Hu, X., Guo, H., Wang, F., Qiao, Y., & Wang, L. (2018). Collagen/Polyethylene Oxide Nanofibrous Membranes with Improved Hemostasis and Cytocompatibility for Wound Dressing. Applied Sciences, 8(8), 1226. https://doi.org/10.3390/app8081226





