Preparation of Phthalocyanine Immobilized Bacterial Cellulose Nanocomposites for Decoloration of Dye Wastewater: Key Role of Spacers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
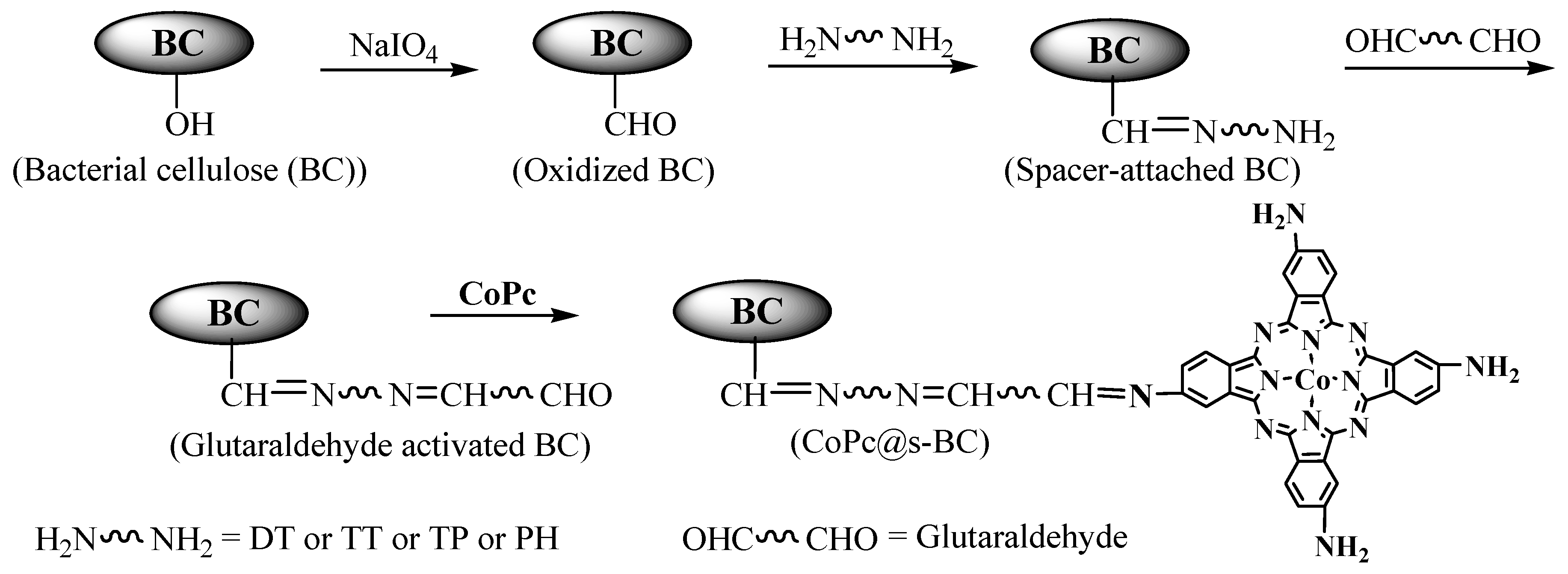
2.2. Preparation and Characterization of CoPc@s-BC
2.3. Adsorption and Catalytic Oxidation Decoloration
3. Results and Discussion
3.1. Preparation and Characterization of CoPc@s-BC
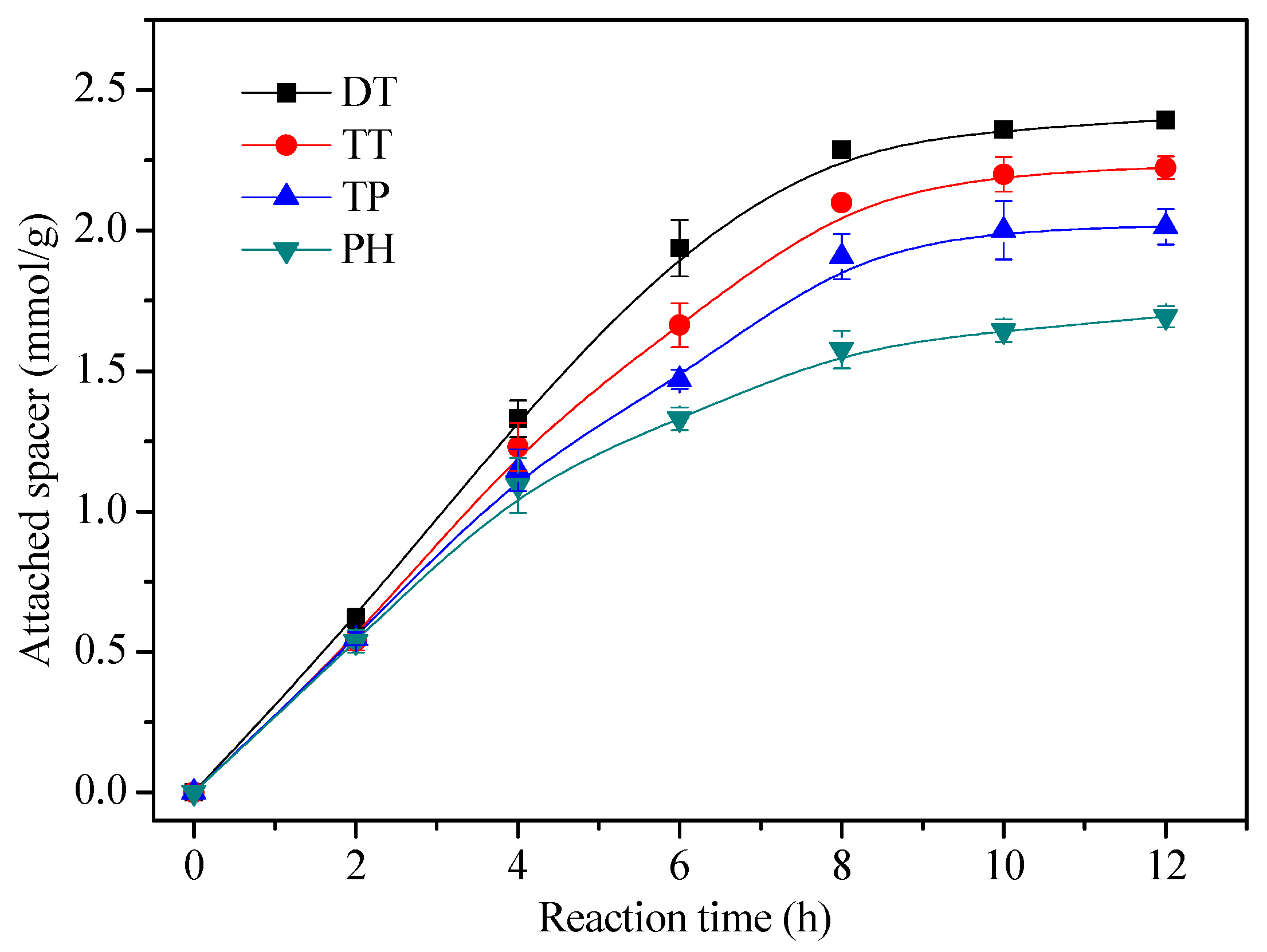
3.2. Optimization of CoPc Immobilization Conditions
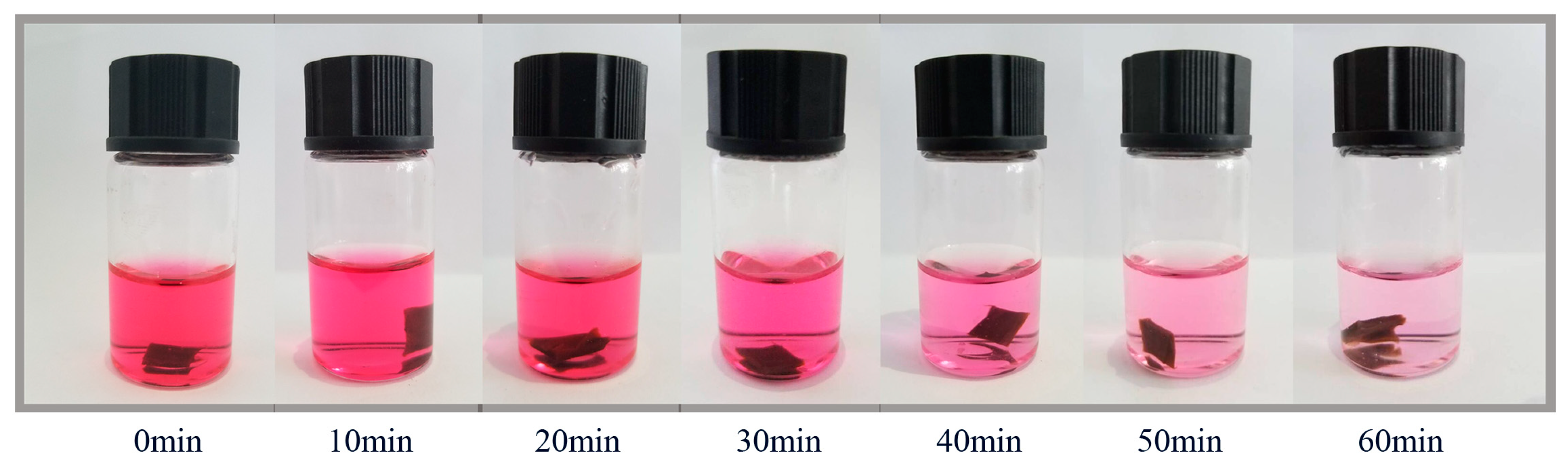
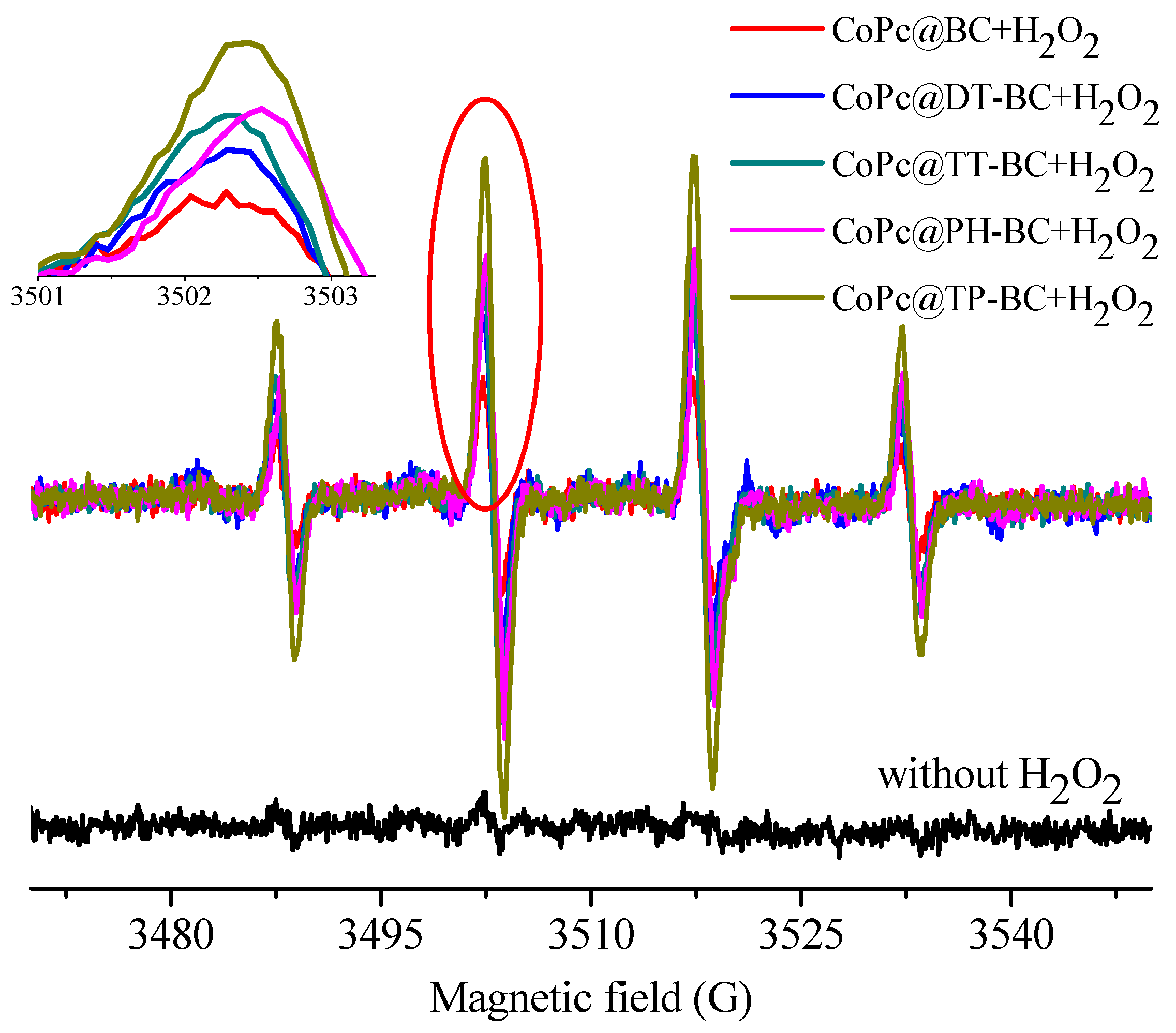
3.3. Adsorption and Catalytic Oxidation Performance of CoPc@s-BC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Wang, J.Y.; Jia, H.B.; Zhang, J.J.; Ding, L.F.; Huang, Y.; Sun, D.P.; Gong, X.D. Bacterial cellulose whisker as a reinforcing filler for carboxylated acrylonitrile-butadiene rubber. J. Mater. Sci. 2014, 49, 6093–6101. [Google Scholar] [CrossRef]
- Wang, B.; Yang, D.; Zhang, H.R.; Huang, C.; Xiong, L.; Luo, J.; Chen, X.D. Preparation of esterified bacterial cellulose for improved mechanical properties and the microstructure of isotactic polypropylene/bacterial cellulose composites. Polymers 2016, 8, 129. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Capadona, J.R.; Shanmuganathan, K.; Tyler, D.J.; Rowan, S.J.; Weder, C. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science 2008, 319, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Abeer, M.M.; Amin, M.C.I.M.; Lazim, A.M.; Pandey, M.; Martin, C. Synthesis of a novel acrylated abietic acid-g-bacterial cellulose hydrogel by gamma irradiation. Carbohydr. Polym. 2014, 110, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Foresti, M.L.; Vazquez, A.; Boury, B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: A review of recent advances. Carbohydr. Polym. 2017, 157, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Rodriguez, K.; Renneckar, S.; Vikesland, P.J. Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ. Sci.-Nano 2014, 1, 302–316. [Google Scholar] [CrossRef]
- De la Torre, G.; Claessens, C.G.; Torres, T. Phthalocyanines: Old dyes, new materials. Putting color in nanotechnology. Chem. Commun. 2007, 2000–2015. [Google Scholar] [CrossRef]
- Sorokin, A.B. Phthalocyanine metal complexes in catalysis. Chem. Rev. 2013, 113, 8152–8191. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.; Seris, J.L.; Meunier, B. Efficient oxidative dechlorination and aromatic ring-cleavage of chlorinated phenols catalyzed by iron sulfophthalocyanine. Science 1995, 268, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.B.; Kudrik, E.V. Phthalocyanine metal complexes: Versatile catalysts for selective oxidation and bleaching. Catal. Today 2011, 159, 37–46. [Google Scholar] [CrossRef]
- Chen, X.; Lu, W.Y.; Xu, T.F.; Li, N.; Qin, D.D.; Zhu, Z.X.; Wang, G.Q.; Chen, W.X. A bio-inspired strategy to enhance the photocatalytic performance of g-c3n4 under solar irradiation by axial coordination with hemin. Appl. Catal. B Environ. 2017, 201, 518–526. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Chen, Y.; Gu, Y.; Wu, F.; Lu, W.Y.; Xu, T.F.; Chen, W.X. Catalytic degradation of recalcitrant pollutants by fenton-like process using polyacrylonitrile-supported iron (ii) phthalocyanine nanofibers: Intermediates and pathway. Water Res. 2016, 93, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.H.; de Souza, T.F.M.; Ribeiro, A.O.; Calefi, P.S.; Ciuffi, K.J.; Nassar, E.J.; Molina, E.F.; Hamer, P.; de Faria, E.H. New strategies for synthesis and immobilization of methalophtalocyanines onto kaolinite: Preparation, characterization and chemical stability evaluation. Dyes Pigments 2016, 134, 41–50. [Google Scholar] [CrossRef]
- Yang, W.X.; Zhang, R.L.; Luo, K.; Zhang, W.P.; Zhao, J.S. Electrocatalytic performances of multi-walled carbon nanotubes chemically modified by metal phthalocyanines in li/socl2 batteries. RSC Adv. 2016, 6, 75632–75639. [Google Scholar] [CrossRef]
- De Wael, K.; Adriaens, A. Phthalocyanines and porphyrins linked to gold adatoms and their catalytic property towards hydroxide oxidation. Electrochim. Acta 2008, 53, 2355–2361. [Google Scholar] [CrossRef]
- Balkus, K.J.; Eissa, M.; Levado, R. Oxidation of alkanes catalyzed by zeolite-encapsulated perfluorinated ruthenium phthalocyanines. J. Am. Chem. Soc. 1995, 117, 10753–10754. [Google Scholar] [CrossRef]
- Han, Z.B.; Han, X.; Zhao, X.M.; Yu, J.T.; Xu, H. Iron phthalocyanine supported on amidoximated pan fiber as effective catalyst for controllable hydrogen peroxide activation in oxidizing organic dyes. J. Hazard. Mater. 2016, 320, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, Y. Bacterial cellulose nanofibers decorated with phthalocyanine: Preparation, characterization and dye removal performance. Mater. Lett. 2015, 142, 235–237. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Huang, J. Novel preparation of multiwalled carbon nanotubes/bacterial cellulose nanocomposite for phthalocyanine immobilization. Funct. Mater. Lett. 2017, 10, 1750038. [Google Scholar] [CrossRef]
- Chen, S.; Teng, Q. Quantitative immobilization of phthalocyanine onto bacterial cellulose for construction of a high-performance catalytic membrane reactor. Materials 2017, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Dyer, P.W.; Handa, S.; Reeve, T.B.; Suhard, S. The synthesis and catalytic application of spacer-modified diol-functionalised merrifield resins. Tetrahedron Lett. 2005, 46, 4753–4756. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X.; Xu, Z. Effect of a spacer on phthalocyanine functionalized cellulose nanofiber mats for decolorizing reactive dye wastewater. Cellulose 2012, 19, 1351–1359. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Z.G.; Cui, Z.G.; Liu, L.M. Comparison of covalent immobilization of amylase on polystyrene pellets with pentaethylenehexamine and pentaethylene glycol spacers. Bioresour. Technol. 2011, 102, 9374–9379. [Google Scholar] [CrossRef] [PubMed]
- Alptekin, O.; Tukel, S.S.; Yildirim, D.; Alagoz, D. Covalent immobilization of catalase onto spacer-arm attached modified florisil: Characterization and application to batch and plug-flow type reactor systems. Enzym. Microb. Technol. 2011, 49, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Ozyilmaz, G. The effect of spacer arm on hydrolytic and synthetic activity of candida rugosa lipase immobilized on silica gel. J. Mol. Catal. B Enzym. 2009, 56, 231–236. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Palomo, J.M.; Cabrera, Z.; Guisan, J.M.; Fernandez-Lafuente, R. Specificity enhancement towards hydrophobic substrates by immobilization of lipases by interfacial activation on hydrophobic supports. Enzym. Microb. Technol. 2007, 41, 565–569. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, G. Preparation and photo-oxidative functions of poly(ethylene-co-methacrylic acid) (pe-co-maa) nanofibrous membrane supported porphyrins. J. Mater. Chem. 2012, 22, 10581–10588. [Google Scholar] [CrossRef]
- Cho, W.S.; Kim, S.H.; Kim, D.J.; Mun, S.D.; Kim, R.; Go, M.J.; Park, M.H.; Kim, M.; Lee, J.; Kim, Y. Zirconium complexes with pendant aryloxy groups attached to the metallocene moiety by ethyl or hexyl spacers. Polyhedron 2014, 67, 205–212. [Google Scholar] [CrossRef]
- Stavrakov, G.; Philipova, I.; Zheleva, D.; Atanasova, M.; Konstantinov, S.; Doytchinova, I. Docking-based design of galantamine derivatives with dual-site binding to acetylcholinesterase. Mol. Inform. 2016, 35, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Ghritlahre, B.K.; Ghosh, K.K.; Verma, R.; Verma, S.; Zhao, X.J. Influence of amine-based cationic gemini surfactants on catalytic activity of -chymotrypsin. Int. J. Chem. Kinet. 2016, 48, 779–784. [Google Scholar] [CrossRef]
- Jimenez, A.J.; Marcos, M.L.; Hausmann, A.; Rodriguez-Morgade, M.S.; Guldi, D.M.; Torres, T. Assembling phthalocyanine dimers through a platinum(ii) acetylide linker. Chem. Eur. J. 2011, 17, 14139–14146. [Google Scholar] [CrossRef] [PubMed]
- Lederer, M.; Ince, M.; Martinez-Diaz, M.V.; Torres, T.; Guldi, D.M. Photoinduced electron transfer in a zinc phthalocyanine-fullerene conjugate connected by a long flexible spacer. Chempluschem 2016, 81, 941–946. [Google Scholar] [CrossRef]
- He, D.D.; Peng, Y.R.; Yang, H.Q.; Ma, D.D.; Wang, Y.H.; Chen, K.Z.; Chen, P.P.; Shi, J.F. Single-wall carbon nanotubes covalently linked with zinc (ii) phthalocyanine bearing poly (aryl benzyl ether) dendritic substituents: Synthesis, characterization and photoinduced electron transfer. Dyes Pigments 2013, 99, 395–401. [Google Scholar] [CrossRef]
- Yang, J.; Sun, D.; Li, J.; Yang, X.; Yu, J.; Hao, Q.; Liu, W.; Liu, J.; Zou, Z.; Gu, J. In situ deposition of platinum nanoparticles on bacterial cellulose membranes and evaluation of pem fuel cell performance. Electrochim. Acta 2009, 54, 6300–6305. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X.; Xu, Z. Functionalization of cellulose nanofiber mats with phthalocyanine for decoloration of reactive dye wastewater. Cellulose 2011, 18, 1295–1303. [Google Scholar] [CrossRef]
- Achar, B.; Fohlen, G.; Parker, J.; Keshavayya, J. Synthesis and structural studies of metal (ii) 4, 9, 16, 23-phthalocyanine tetraamines. Polyhedron 1987, 6, 1463–1467. [Google Scholar] [CrossRef]
- Liu, H.Q.; Hsieh, Y.L. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2119–2129. [Google Scholar] [CrossRef]
- Huang, X.J.; Chen, P.C.; Huang, F.; Ou, Y.; Chen, M.R.; Xu, Z.K. Immobilization of candida rugosa lipase on electrospun cellulose nanofiber membrane. J. Mol. Catal. B Enzym. 2011, 70, 95–100. [Google Scholar] [CrossRef]
- Chen, W.X.; Lu, W.Y.; Yao, Y.Y.; Xu, M.H. Highly efficient decomposition of organic dyes by aqueous-fiber phase transfer and in situ catalytic oxidation, using fiber-supported cobalt phthalocyanine. Environ. Sci. Technol. 2007, 41, 6240–6245. [Google Scholar] [CrossRef] [PubMed]
- Matama, T.; Araujo, R.; Gubitz, G.M.; Casal, M.; Cavaco-Paulo, A. Functionalization of cellulose acetate fibers with engineered cutinases. Biotechnol. Prog. 2010, 26, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.P.; Lu, W.Y.; Li, N.; Chen, W.X. Enhanced removal of acid red 1 with large amounts of dyeing auxiliaries: The pivotal role of cellulose support. Cellulose 2014, 21, 2073–2087. [Google Scholar] [CrossRef]
- Yamazaki, I.; Piette, L.H. EPR spin-trapping study on the oxidizing species formed in the reaction of the ferrous ion with hydrogen-peroxide. J. Am. Chem. Soc. 1991, 113, 7588–7593. [Google Scholar] [CrossRef]
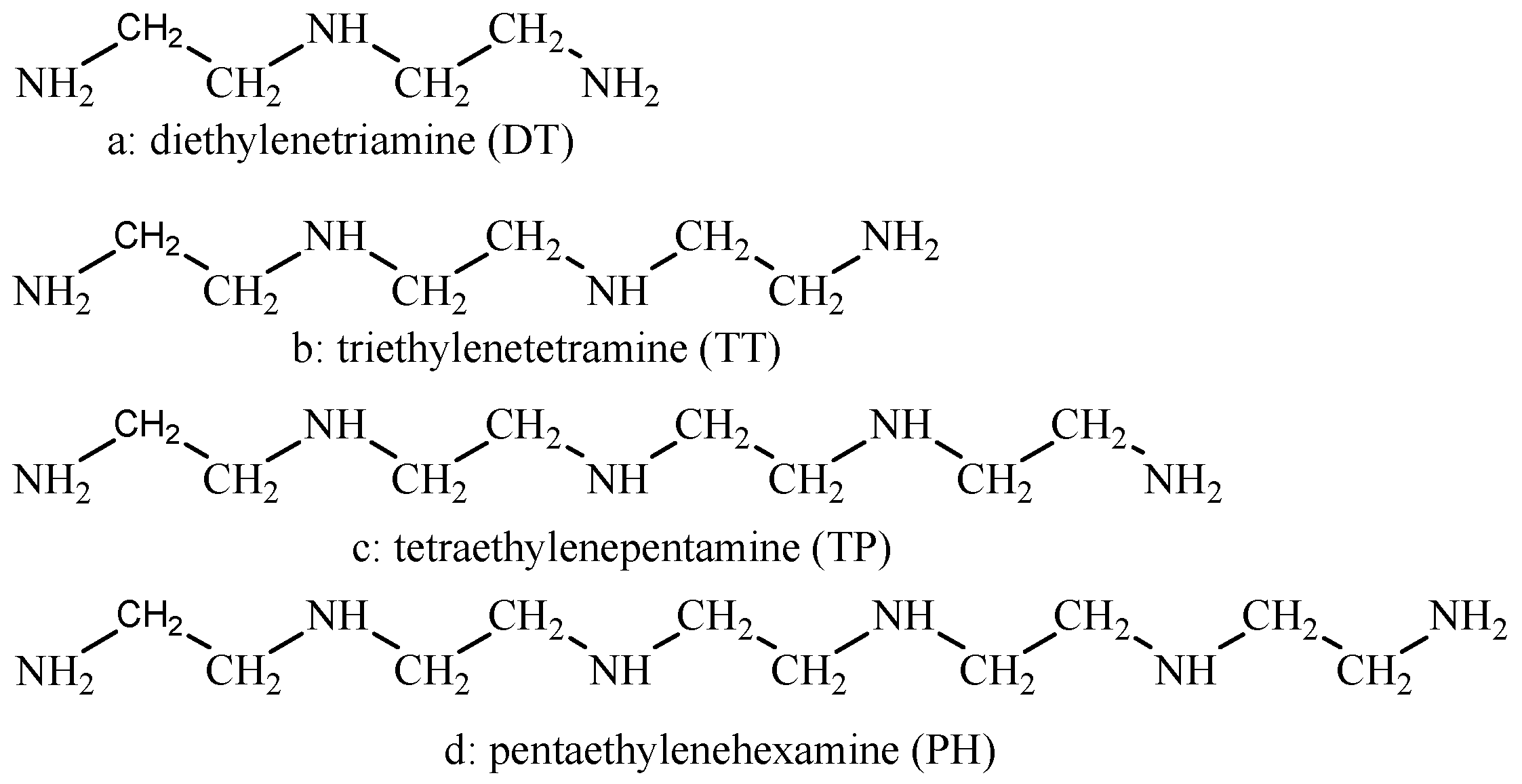
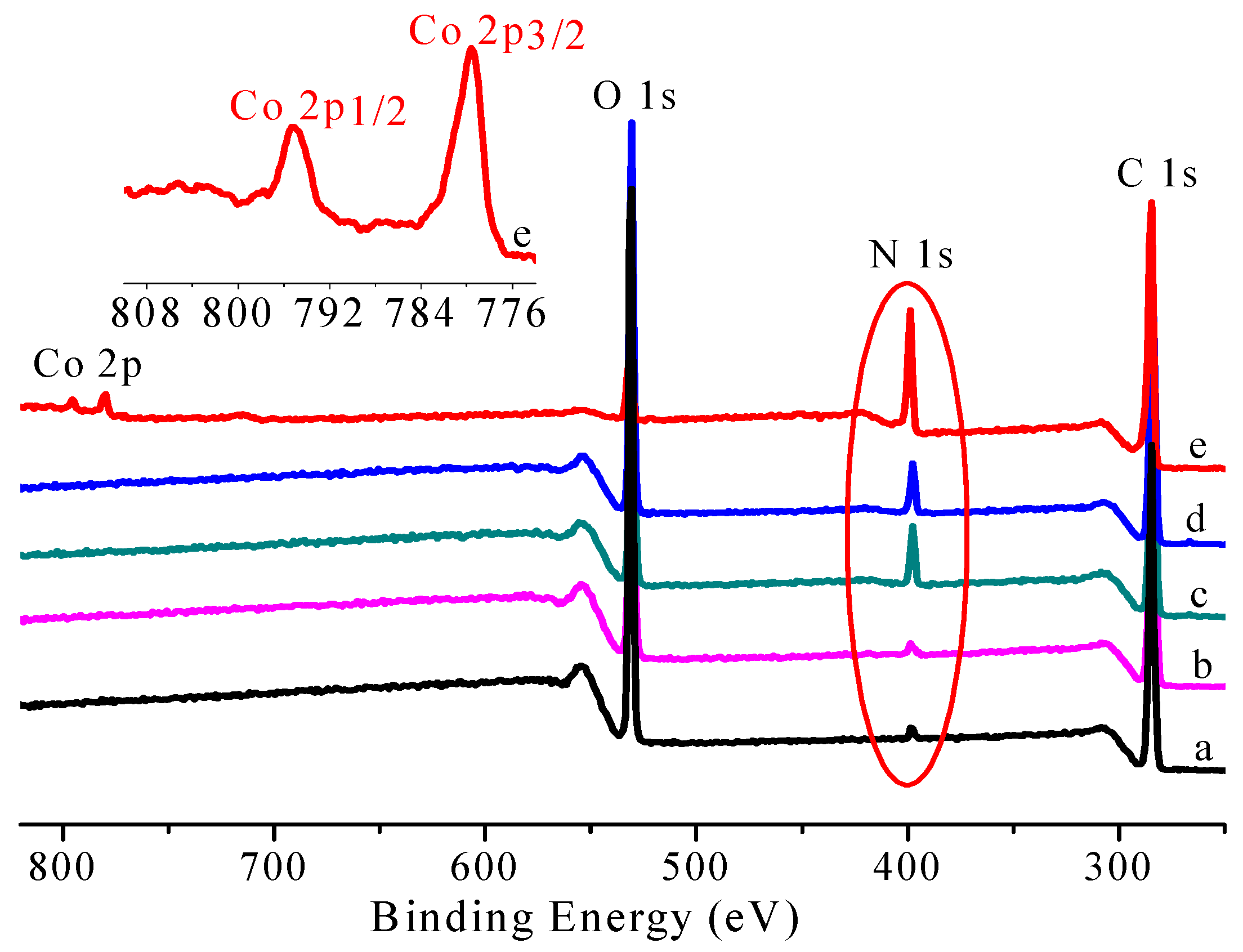




| C (mol %) | O (mol %) | N (mol %) | Co (mol %) | |
|---|---|---|---|---|
| BC | 63.13 | 36.87 | - | - |
| Oxidized BC | 65.51 | 34.49 | - | - |
| TP-attached BC | 63.55 | 29.61 | 6.84 | - |
| Glutaraldehyde-activated BC | 64.53 | 28.56 | 6.91 | - |
| CoPc@TP-BC | 72.44 | 7.54 | 19 | 1.01 |
| Sample | Slope | Correlation Ratio |
|---|---|---|
| BC | 0.0563 | 0.9668 |
| CoPc@BC | 0.1723 | 0.9946 |
| CoPc@DT-BC | 0.2453 | 0.9921 |
| CoPc@TT-BC | 0.3466 | 0.9955 |
| CoPc@TP-BC | 0.3852 | 0.9942 |
| CoPc@PH-BC | 0.2240 | 0.9985 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Q.; Chen, S.; Xie, W. Preparation of Phthalocyanine Immobilized Bacterial Cellulose Nanocomposites for Decoloration of Dye Wastewater: Key Role of Spacers. Appl. Sci. 2018, 8, 1021. https://doi.org/10.3390/app8071021
Teng Q, Chen S, Xie W. Preparation of Phthalocyanine Immobilized Bacterial Cellulose Nanocomposites for Decoloration of Dye Wastewater: Key Role of Spacers. Applied Sciences. 2018; 8(7):1021. https://doi.org/10.3390/app8071021
Chicago/Turabian StyleTeng, Qiaoling, Shiliang Chen, and Wenjie Xie. 2018. "Preparation of Phthalocyanine Immobilized Bacterial Cellulose Nanocomposites for Decoloration of Dye Wastewater: Key Role of Spacers" Applied Sciences 8, no. 7: 1021. https://doi.org/10.3390/app8071021
APA StyleTeng, Q., Chen, S., & Xie, W. (2018). Preparation of Phthalocyanine Immobilized Bacterial Cellulose Nanocomposites for Decoloration of Dye Wastewater: Key Role of Spacers. Applied Sciences, 8(7), 1021. https://doi.org/10.3390/app8071021




