Development of Multiphoton Ionization Time-of-Flight Mass Spectrometry for the Detection of Small Emulsion Droplets
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Sample Preparation
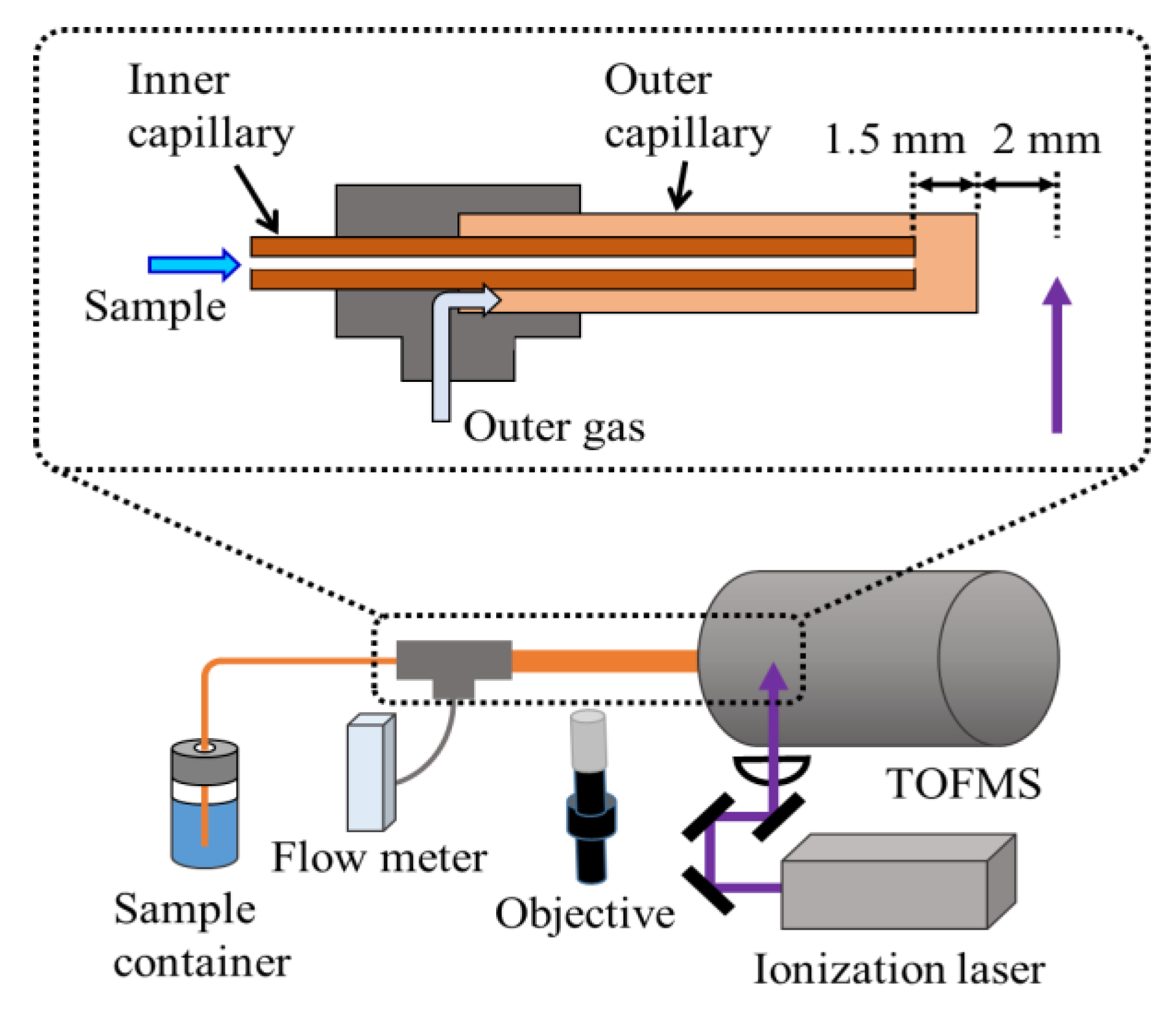
2.2. Apparatus
3. Results and Discussion
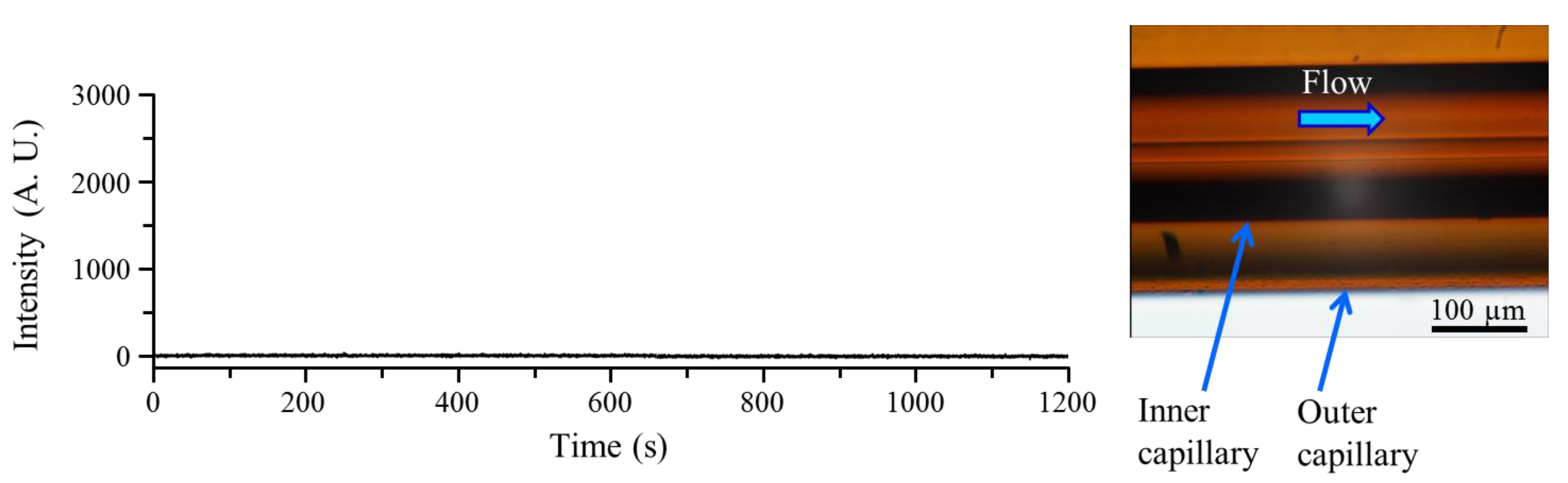
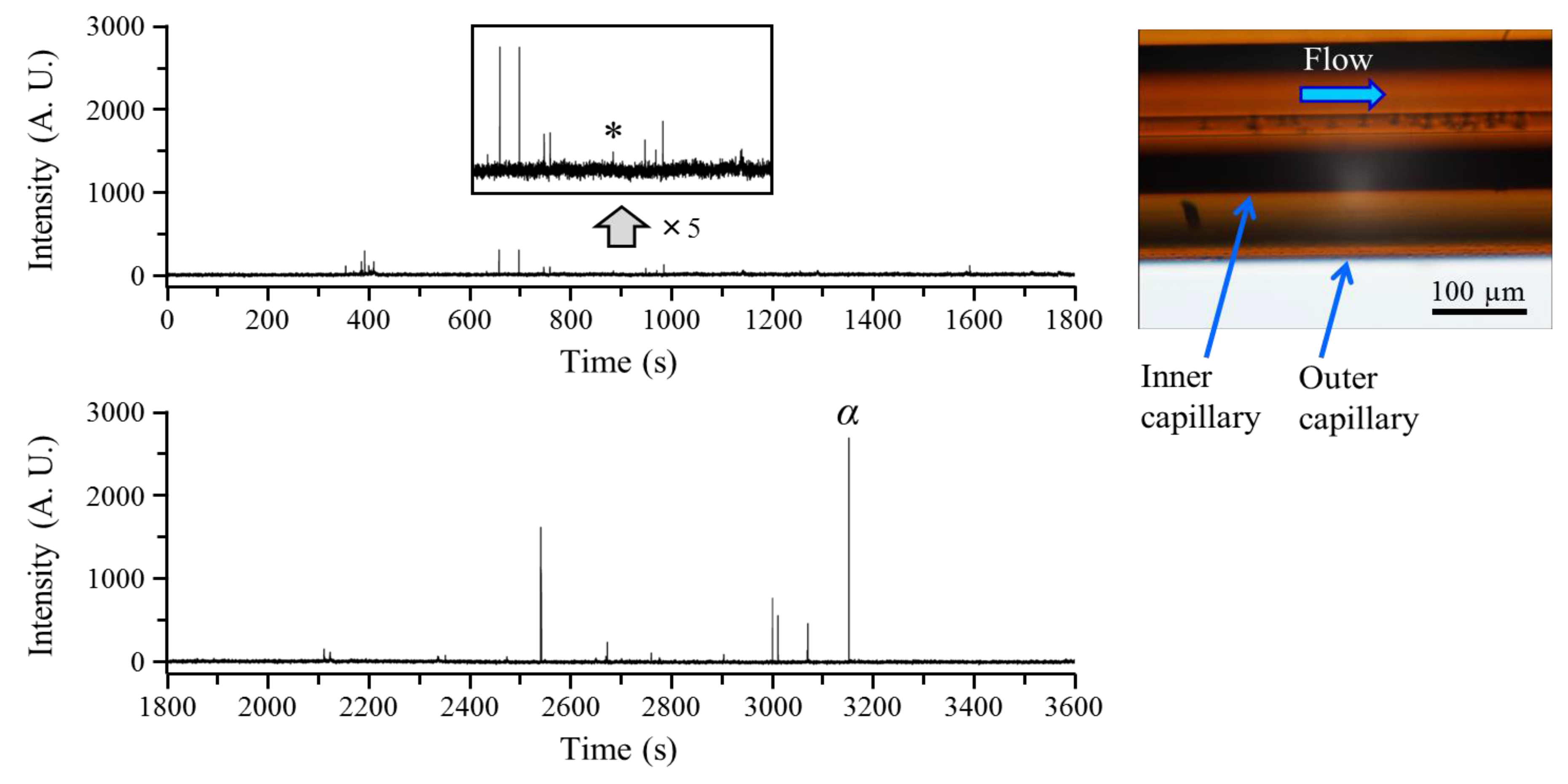
3.1. Microscopic Observation and Time Profiles
3.2. The Minimum Droplet Size that Can be Detected as a Spike
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tadros, T.F. Emulsion Science and Technology; Tadros, T.F., Ed.; Wiley-VCH: Weinheim, Germany, 2009; Chapter 1. [Google Scholar]
- Mitsunobu, M.; Kobayashi, S.; Takeyasu, N.; Kaneta, T. Temperature-induced coalescence of droplets manipulated by optical trapping in an oil-in-water emulsion. Anal. Sci. 2017, 33, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-H.; Urban, P.L. Fusion of microlitre water-in-oil droplets for simple, fast and green chemical assays. Analyst 2015, 140, 5145–5151. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, C.; Westberg, M.; Breitenbach, T.; Bregnhøj, M.; Ogilby, P.R. Monitoring interfacial lipid oxidation in oil-in-water emulsions using spatially resolved optical techniques. Anal. Chem. 2017, 89, 6239–6247. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Yamaguchi, A.; Ogawa, A.; Kido, H.; Nakamura, S.; Takada, M.; Kawakami, S.; Kuroda, N.; Nakashima, K. Luminol chemiluminescence profile of o/w emulsions during thermal oxidation. Anal. Sci. 2017, 33, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Nagatani, H.; Osakai, T. Determination of the electrostatic potential of oil-in-water emulsion droplets by combined use of two membrane potential-sensitive dyes. Anal. Sci. 2017, 33, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Ren, Y.; Jia, Y.; Deng, X.; Tang, Z.; Tao, Y.; Jiang, H. A simple microfluidic method for one-step encapsulation of reagents with varying concentrations in double emulsion drops for nanoliter-scale reactions and analyses. Anal. Methods 2017, 9, 2511–2516. [Google Scholar] [CrossRef]
- Pal, N.; Verma, S.D.; Singh, M.K.; Sen, S. Fluorescence correlation spectroscopy: An efficient tool for measuring size, size-distribution and polydispersity of microemulsion droplets in solution. Anal. Chem. 2011, 83, 7736–7744. [Google Scholar] [CrossRef] [PubMed]
- Vélez, G.; Fernández, M.A.; Muñoz, J.; Williams, P.A.; English, R.J. Role of hydrocolloids in the creaming of oil in water emulsions. J. Agric. Food Chem. 2003, 51, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, C.; Xu, J.; Hao, J.; Sun, D. Highly stable concentrated nanoemulsions by the phase inversion composition method at elevated temperature. Langmuir 2012, 28, 14547–14552. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Li, X.; Mize, T.H.; Sharpe, T.D.; Graziani, E.I.; Abell, C.; Huck, W.T.S. Sensitive, high throughput detection of proteins in individual, surfactant-stabilized picoliter droplets using nanoelectrospray ionization mass spectrometry. Anal. Chem. 2013, 85, 3812–3816. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, H.; Tsuda, Y.; Uchimura, T. Laser ionization/time-of-flight mass spectrometry for the direct analysis of emulsions. Anal. Methods 2014, 6, 5615–5619. [Google Scholar] [CrossRef]
- Boesl, U. Time-of-flight mass spectrometry: Introduction to the basics. Mass Spectrom. Rev. 2017, 36, 86–109. [Google Scholar] [CrossRef] [PubMed]
- Lubman, D.M.; Kronick, M.N. Mass spectrometry of aromatic molecules with resonance-enhanced multiphoton ionization. Anal. Chem. 1982, 54, 660–665. [Google Scholar] [CrossRef]
- Lin, J.L.; Huang, C.J.; Lin, C.H.; Tzeng, W.B. Resonant two-photon ionization and mass-analyzed threshold ionization spectroscopy of the selected rotamers of m-methoxyaniline and o-methoxyaniline. J. Mol. Spectrosc. 2007, 244, 1–8. [Google Scholar] [CrossRef]
- Matsumoto, J.; Misawa, K.; Ishiuchi, S.; Fujii, M. In situ, fast-response, molecular-selective methods for measuring emission factors of volatile organic compounds (VOCs) into the atmosphere. Chem. Lett. 2009, 38, 74–75. [Google Scholar] [CrossRef]
- Imasaka, T. Ultraviolet femtosecond laser ionization mass spectrometry. Chem. Rec. 2008, 8, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Gullett, B.K.; Oudejans, L.; Tabor, D.; Touati, A.; Ryan, S. Near-real-time combustion monitoring for PCDD/PCDF indicators by GC-REMPI-TOFMS. Environ. Sci. Technol. 2012, 46, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Hamachi, A.; Imasaka, T.; Nakamura, H.; Li, A.; Imasaka, T. Determination of nerve agent metabolites by ultraviolet femtosecond laser ionization mass spectrometry. Anal. Chem. 2017, 89, 5030–5035. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, T.; Shimizu, S.; Miura, A.; Fukuyama, A.; Uchimura, T. Rapid evaluation of the bioremediation of fuel oil in soil by gas chromatography–laser ionization time-of-flight mass spectrometry. Anal. Lett. 2017, 50, 2859–2868. [Google Scholar] [CrossRef]
- Li, L.; Lubman, D.M. Analytical jet spectroscopy of tyrosine and its analogs using a pulsed laser desorption volatilization method. Appl. Spectrosc. 1988, 42, 418–424. [Google Scholar] [CrossRef]
- Elsila, J.E.; de Leon, N.P.; Zare, R.N. Factors affecting quantitative analysis in laser desorption/laser ionization mass spectrometry. Anal. Chem. 2004, 76, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Haefliger, O.P.; Zenobi, R. Laser mass spectrometric analysis of polycyclic aromatic hydrocarbons with wide wavelength range laser multiphoton ionization spectroscopy. Anal. Chem. 1998, 70, 2660–2665. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, S.; Uchimura, T. Pyrolysis-gas chromatography/multiphoton ionization/time-of-flight mass spectrometry for the rapid and selective analysis of polycyclic aromatic hydrocarbons in aerosol particulate matter. Anal. Sci. 2014, 30, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.; De Camillis, S.; Alexander, G.; Hamilton, K.; Kelly, T.J.; Costello, J.T.; Zepf, M.; Williams, I.D.; Greenwood, J.B. Detection limits of organic compounds achievable with intense, short-pulse lasers. Analyst 2015, 140, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Passig, J.; Schade, J.; Oster, M.; Fuchs, M.; Ehlert, S.; Jäger, C.; Sklorz, M.; Zimmermann, R. Aerosol mass spectrometer for simultaneous detection of polyaromatic hydrocarbons and inorganic components from individual particles. Anal. Chem. 2017, 89, 6341–6345. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, H.; Tsuda, Y.; Uchimura, T. Laser ionization time-of-flight mass spectrometry for the evaluation of a local microenvironment in an emulsion. Anal. Methods 2016, 8, 270–274. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ishigami, H.; Uchimura, T. Online monitoring of a styrene monomer and a dimer in an emulsion via laser ionization time-of-flight mass spectrometry. Anal. Sci. 2017, 33, 731–733. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsuda, Y.; Uchimura, T. Evaluating the aging of multiple emulsions using resonance-enhanced multiphoton ionization time-of-flight mass spectrometry. Anal. Sci. 2016, 32, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, H.; Uchimura, T. A quantitative analysis of an oil component in an emulsion by multiphoton ionization mass spectrometry. Anal. Sci. 2017, 33, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Shimo, Y.; Uchimura, T. Time-profile measurement of an emulsion using multiphoton ionization time-of-flight mass spectrometry in combination with a microscope. Anal. Sci. 2016, 32, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, C.; Sugimura, Y.; Uchimura, T. Development of Multiphoton Ionization Time-of-Flight Mass Spectrometry for the Detection of Small Emulsion Droplets. Appl. Sci. 2018, 8, 413. https://doi.org/10.3390/app8030413
Fujita C, Sugimura Y, Uchimura T. Development of Multiphoton Ionization Time-of-Flight Mass Spectrometry for the Detection of Small Emulsion Droplets. Applied Sciences. 2018; 8(3):413. https://doi.org/10.3390/app8030413
Chicago/Turabian StyleFujita, Chiaki, Yoshiki Sugimura, and Tomohiro Uchimura. 2018. "Development of Multiphoton Ionization Time-of-Flight Mass Spectrometry for the Detection of Small Emulsion Droplets" Applied Sciences 8, no. 3: 413. https://doi.org/10.3390/app8030413
APA StyleFujita, C., Sugimura, Y., & Uchimura, T. (2018). Development of Multiphoton Ionization Time-of-Flight Mass Spectrometry for the Detection of Small Emulsion Droplets. Applied Sciences, 8(3), 413. https://doi.org/10.3390/app8030413





