Quillaja saponaria Saponins with Potential to Enhance the Effectiveness of Disinfection Processes in the Beverage Industry
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Antimicrobial Compounds
2.3. Minimal Inhibitory Concentration (MIC)
2.4. Pretreatment of Planktonic Bacterial Cells with Saponin Extract
2.5. Biofilm Eradication
2.6. Statistics
3. Results and Discussion
3.1. Minimal Inhibitory Concentration
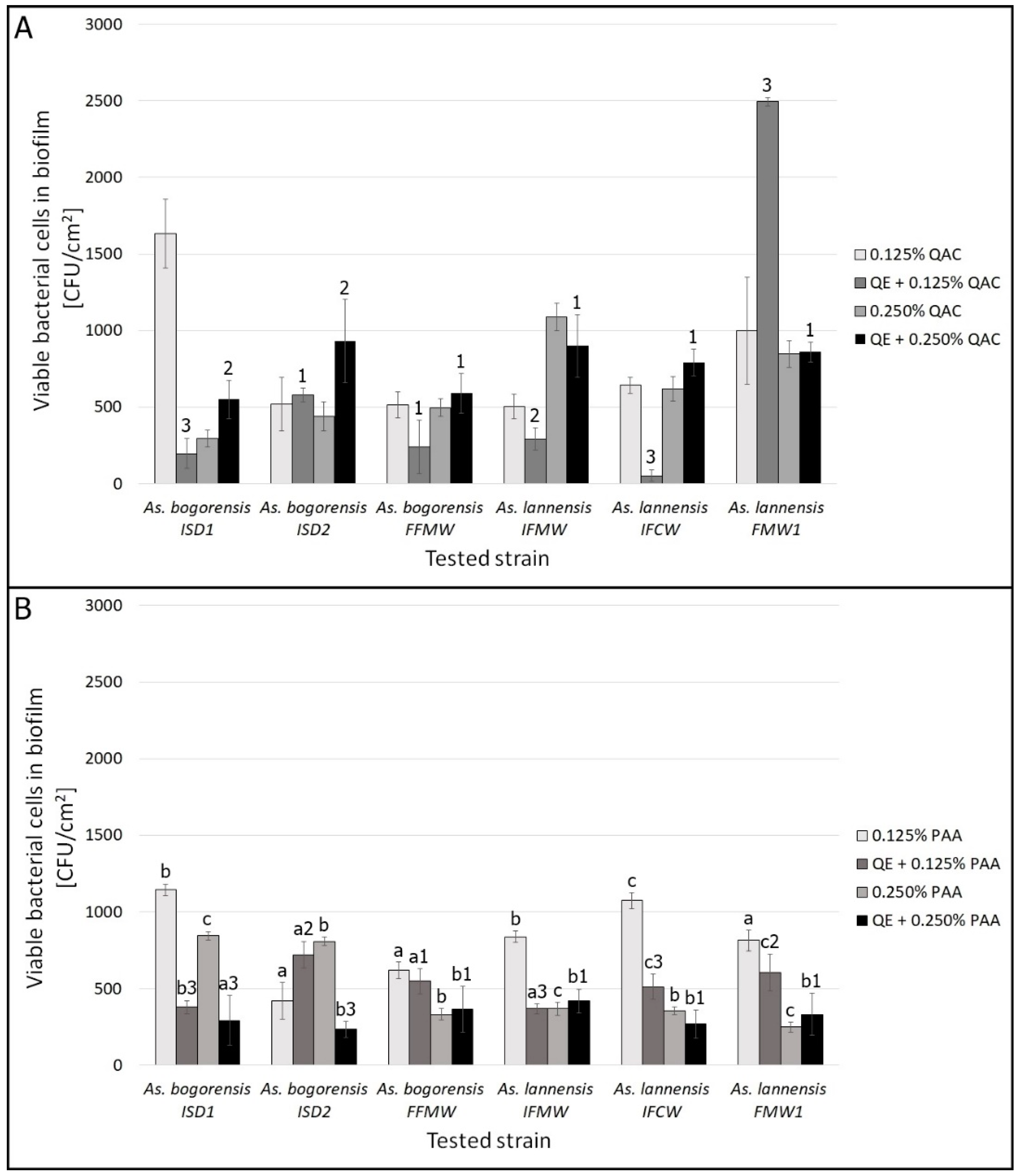
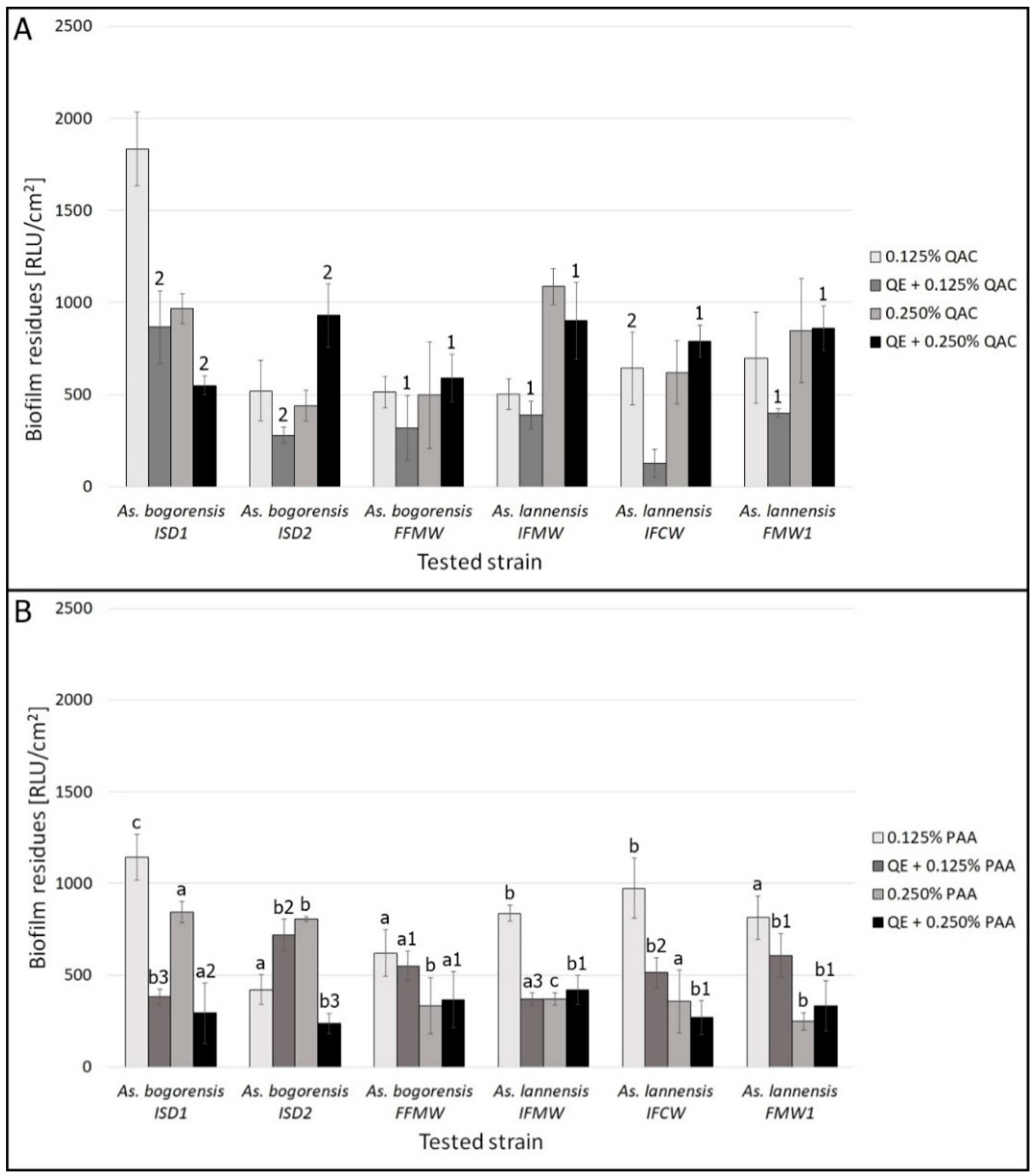
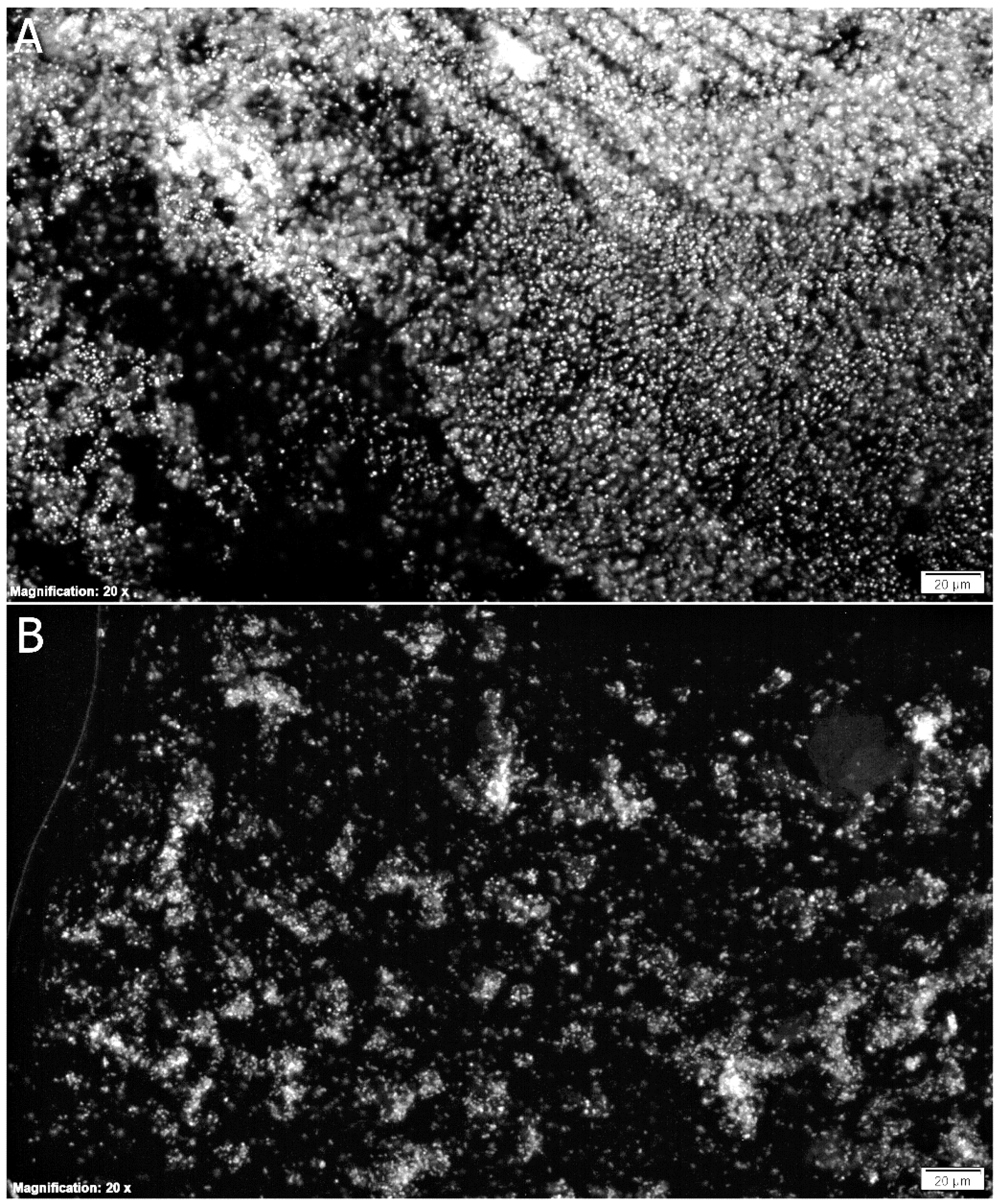
3.2. Biofilm Eradication
4. Conclusions
5. Patents
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antolak, H.; Kręgiel, D.; Czyżowska, A. Adhesion of Asaia bogorensis to glass and polystyrene in the presence of cranberry juice. J. Food Prot. 2015, 78, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Horsáková, I.; Voldřich, M.; Čeřovskỳ, M.; Sedláčková, P.; Šicenrová, P.; Ulbrich, P. Asaia sp. as a bacterium decaying the packaged still fruit beverages. Czech J. Food Sci. 2009, 27, 362–365. [Google Scholar] [CrossRef]
- Kręgiel, D.; Rygała, A.; Libudzisz, Z.; Walczak, P.; Ołtuszak-Walczak, E. Asaia lannensis—The spoilage acetic acid bacteria isolated from strawberry-flavored bottled water in Poland. Food Control 2012, 26, 147–150. [Google Scholar] [CrossRef]
- Moore, J.E.; McCalmont, M.; Xu, J.; Millar, B.C.; Heaney, N. Asaia sp., an unusual spoilage organism of fruit-flavoured bottled water. Appl. Environ. Microbiol. 2002, 68, 4130–4131. [Google Scholar] [CrossRef] [PubMed]
- Chouaia, B.; Gaiarsa, S.; Crotti, E.; Comandatore, F.; Degli Esposti, M.; Ricci, I.; Alma, A.; Favia, G.; Bandi, C.; Daffonchio, D. Acetic acid bacteria genomes reveal functional traits for adaptation to life in insect guts. Genome Biol. Evol. 2014, 6, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Mateo, E.; Torija, M.J.; Mas, A.; Bartowsky, E.J. Acetic acid bacteria isolated from grapes of South Australian vineyards. Int. J. Food Microbiol. 2014, 178, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Antolak, H.; Kręgiel, D. Bakterie kwasu octowego Asaia sp. i ich unikalne zdolności adaptacyjne. Postep. Mikrobiol. 2016, 55, 392–397. (In Polish) [Google Scholar]
- Kręgiel, D. Attachment of Asaia lannensis to materials commonly used in beverage industry. Food Control 2013, 32, 537–542. [Google Scholar] [CrossRef]
- Kręgiel, D.; James, S.A.; Rygała, A.; Berłowska, J.; Antolak, H.; Pawlikowska, E. Consortia formed by yeasts and acetic acid bacteria Asaia spp. in soft drinks. Antonie Leeuwenhoek 2017. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. Mater. Int. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Taraszkiewicz, A.; Fila, G.; Grinholc, M.; Nakonieczna, J. Innovative strategies to overcome biofilm resistance. BioMed Res. Int. 2013, 2013, 150653. [Google Scholar] [CrossRef] [PubMed]
- Kruk, T.; Szczepanowicz, K.; Kręgiel, D.; Szyk-Warszyńska, L.; Warszyński, P. Nanostructured multilayer polyelectrolyte films with silver nanoparticles as antibacterial coatings. Colloids Surf. B. Biointerfaces 2016, 137, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kręgiel, D. Advances in biofilm control for food and beverage industry using organo-silane technology: A review. Food Control 2014, 40, 32–40. [Google Scholar] [CrossRef]
- Kręgiel, D.; Berłowska, J.; Mizerska, U.; Fortuniak, W.; Chojnowski, W.; Ambroziak, W. Chemical modification of polyvinyl chloride and silicone elastomer in inhibiting adhesion of Aeromonas hydrophila. World J. Microbiol. Biotechnol. 2013, 29, 1197. [Google Scholar] [CrossRef] [PubMed]
- Antolak, H.; Czyżowska, A.; Kręgiel, D. Black currant (Ribes nigrum L.) and bilberry (Vaccinium myrtillus L.) fruit juices inhibit adhesion of Asaia spp. BioMed Res. Int. 2016, 2016, 3671306. [Google Scholar] [CrossRef]
- Antolak, H.; Czyżowska, A.; Sakač, M.; Mišan, A.; Đuragić, O.; Kręgiel, D. Phenolic compounds contained in little-known wild fruits as antiadhesive agents against the beverage-spoiling bacteria Asaia spp. Molecules 2017, 22, 1256. [Google Scholar] [CrossRef] [PubMed]
- Antolak, H.; Oracz, J.; Otlewska, A.; Żyżelewicz, D.; Kręgiel, D. Identification of carotenoids and isoprenoid quinones from Asaia lannensis and Asaia bogorensis. Molecules 2017, 22, 1608. [Google Scholar] [CrossRef] [PubMed]
- Kręgiel, D.; Berłowska, J.; Witońska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-based, biological-active surfactants from plants. In Application and Characterization of Surfactants, 1st ed.; Najjar, E., Ed.; InTech: Rijeka, Croatia, 2017; pp. 183–205. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. 61st JECFA. Quillaja Extracts. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/detail/en/c/476/ http://www.fao.org/fileadmin/tem‐plates/agns/pdf/jecfa/cta/61/QUILLAIA.pdf (accessed on 20 December 2017).
- European Food Safety Authority. Call for Food Additives Usage Level and/or Concentration Data in Food and Beverages Intended for Human Consumption. Available online: https://www.efsa.europa.eu/en/data/call/160524 (accessed 20 December 2017).
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32008R1333&from=EN (accessed 20 December 2017).
- Berłowska, J.; Dudkiewicz, M.; Kręgiel, D.; Czyżowska, A.; Witońska, I. Cell lysis induced by membrane-damaging detergent saponins from Quillaja saponaria. Enzyme Microb. Technol. 2015, 75–76, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Berłowska, J.; Dudkiewicz-Kołodziejska, M.; Pawlikowska, E.; Pielech-Przybylska, K.; Balcerek, M.; Czyżowska, A.; Kręgiel, D. Utilization of post-fermentation yeasts for yeast extract production by autolysis: The effect of yeast strain and saponin from Quillaja saponaria. J. Inst. Brew. 2017, 123, 396–401. [Google Scholar] [CrossRef]
- Koziróg, A.; Kręgiel, D.; Brycki, B. Action of monomeric/gemini surfactants on free cells and biofilm of Asaia lannensis. Molecules 2017, 22, 2036. [Google Scholar] [CrossRef] [PubMed]
- Spoering, A.L.; Lewis, K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 2001, 183, 6746–6751. [Google Scholar] [CrossRef] [PubMed]
- Briñez, W.J.; Roig-Sagués, A.X.; Herrero, M.M.H.; López-Pedemonte, T.; Guamis, B. Bactericidal efficacy of peracetic acid in combination with hydrogen peroxide against pathogenic and non pathogenic strains of Staphylococcus spp., Listeria spp. and Escherichia coli. Food Control 2006, 17, 516–521. [Google Scholar] [CrossRef]
- Rasimus, S.; Kolari, M.; Rita, H.; Hoornstra, D.; Salkinoja-Salonen, M. Biofilm-forming bacteria with varying tolerance to peracetic acid from a paper machine. J. Ind. Microbiol. Biotechnol. 2011, 38, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Chaidez, C.; Lopez, J.; Castro-del Campo, N. Quaternary ammonium compounds: An alternative disinfection method for fresh produce wash water. J. Water Health 2007, 5, 329–333. [Google Scholar] [PubMed]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Ortega Morente, E.; Fernández-Fuentes, M.A.; Grande Burgos, M.J.; Abriouel, H.; Pérez Pulido, R.; Gálvez, A. Biocide tolerance in bacteria. Int. J. Food Microbiol. 2013, 162, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Gadea, R.; Glibota, N.; Pulido, R.P.; Gálvez, A.; Ortega, W. Effects of exposure to biocides on susceptibility to essential oils and chemical preservatives in bacteria from organic foods. Food Control 2017, 80, 176–182. [Google Scholar] [CrossRef]
- Bridier, A.; Dubois-Brissonnet, F.; Greub, G.; Thomas, V.; Briandet, R. Dynamics of biocide action in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2011, 55, 2648–2654. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Diamanti, A.; Carbone, M.; Bauer, E.M.; Palleschi, G. New cleaning strategies based on carbon nanomaterials applied to the deteriorated marble surfaces: A comparative study with enzyme based treatments. Appl. Surf. Sci. 2012, 258, 5965–5980. [Google Scholar] [CrossRef]
- Von Rybinski, W. Physical aspects of cleaning processes. In Handbook for Cleaning/Decontamination of Surfaces, 1st ed.; Johansson, I., Somasundaran, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–55. ISBN 978-0-444-51664-0. [Google Scholar]
- Anderson, G.G.; Kenney, T.F.; Macleod, D.L.; Henig, N.R.; O’Toole, G.A. Eradication of Pseudomonas aeruginosa biofilms on cultured airway cells by a fosfomycin/tobramycin antibiotic combination. Pathog. Dis. 2013, 67, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Monzón, M.; Oteiza, C.; Leiva, J.; Amorena, B. Synergy of different antibiotic combinations in biofilms of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2001, 48, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Padalia, H.; Moteriya, P.; Baravalia, Y.; Chanda, S. Antimicrobial and Synergistic effects of Some Essential Oils to Fight against Microbial Pathogens—A Review. Available online: http://www.microbiology5.org/microbiology5/book/34-45.pdf (accessed 20 December 2017).
- Pelah, D.; Abramovich, Z.; Markus, A.; Wiesman, Z. The use of commercial saponin from Quillaja saponaria bark as a natural larvicidal agent against Aedes aegypti and Culex pipiens. J. Ethnopharmacol. 2002, 81, 407–409. [Google Scholar] [CrossRef]
- Toniolo, P. Peracetic Acid: A good choice for wastewater reuse. In Wastewater and Biosolids Treatment and Reuse: Bridging Modeling and Experimental Studies; Santoro, D., Ed.; ECI Symposium Series; ECI: New York, NY, USA, 2014; Available online: http://dc.engconfintl.org/wbtr_i/23 (accessed 20 December 2017).
- Lai, Y.S.; Ontiveros-Valencia, A.; Ilhan, Z.E.; Zhou, Y.; Miranda, E.; Maldonado, J.; Krajmalnik-Brown, R.; Rittmann, B.E. Enhancing biodegradation of C16-alkyl quaternary ammonium compounds using an oxygen-based membrane biofilm reactor. Water Res. 2017, 123, 825–833. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antolak, H.; Mizerska, U.; Berłowska, J.; Otlewska, A.; Kręgiel, D. Quillaja saponaria Saponins with Potential to Enhance the Effectiveness of Disinfection Processes in the Beverage Industry. Appl. Sci. 2018, 8, 368. https://doi.org/10.3390/app8030368
Antolak H, Mizerska U, Berłowska J, Otlewska A, Kręgiel D. Quillaja saponaria Saponins with Potential to Enhance the Effectiveness of Disinfection Processes in the Beverage Industry. Applied Sciences. 2018; 8(3):368. https://doi.org/10.3390/app8030368
Chicago/Turabian StyleAntolak, Hubert, Urszula Mizerska, Joanna Berłowska, Anna Otlewska, and Dorota Kręgiel. 2018. "Quillaja saponaria Saponins with Potential to Enhance the Effectiveness of Disinfection Processes in the Beverage Industry" Applied Sciences 8, no. 3: 368. https://doi.org/10.3390/app8030368
APA StyleAntolak, H., Mizerska, U., Berłowska, J., Otlewska, A., & Kręgiel, D. (2018). Quillaja saponaria Saponins with Potential to Enhance the Effectiveness of Disinfection Processes in the Beverage Industry. Applied Sciences, 8(3), 368. https://doi.org/10.3390/app8030368







