Optimization of Subcritical Water Extraction of Resveratrol from Grape Seeds by Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Subcritical Water Extraction Procedure
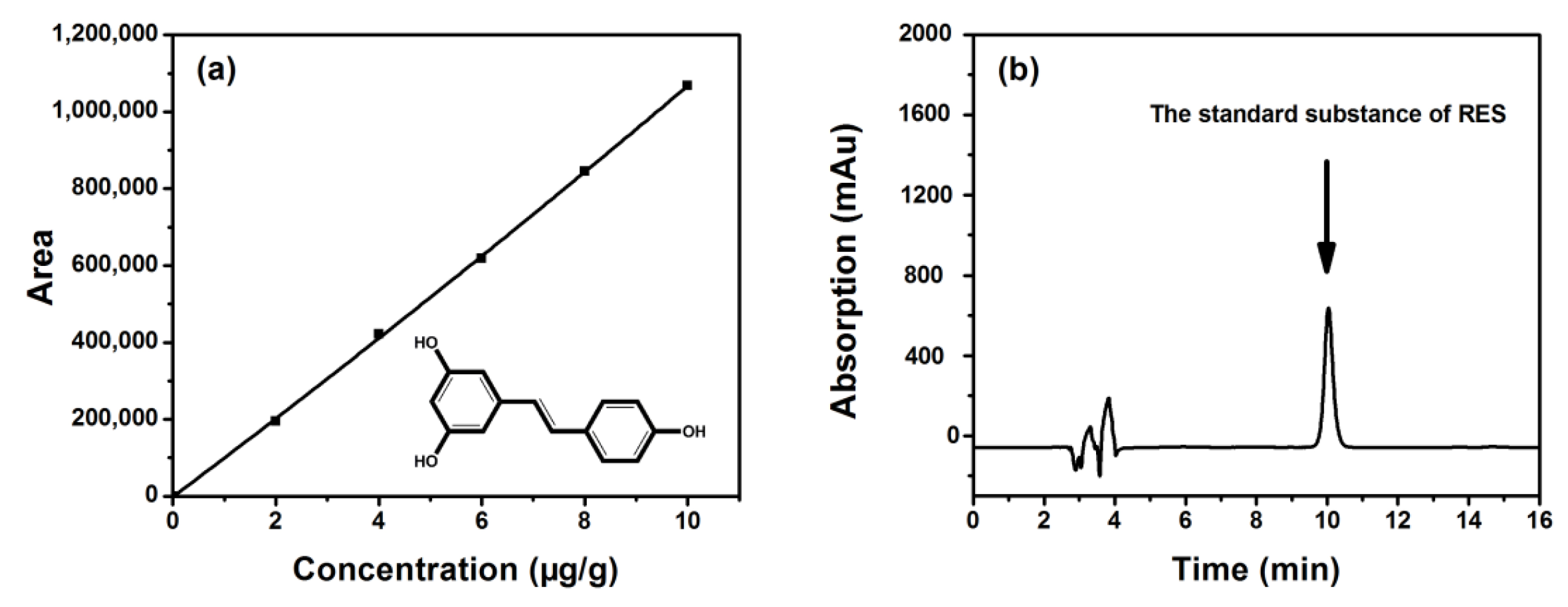
2.3. HPLC Analyses
2.4. The Calculation of Yield of RES
2.5. Single Factor Experimental Design
2.6. Experimental Design of RSM
2.7. Spike Recovery Experiment
2.8. Comparison of Different Extraction Methods
2.8.1. Reflux Extraction (RE)
2.8.2. Ultrasonic Extraction (UE)
2.8.3. Microwave-Assisted Extraction (ME)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Single-Factor Experimental Analysis
3.2. Fitting the Model
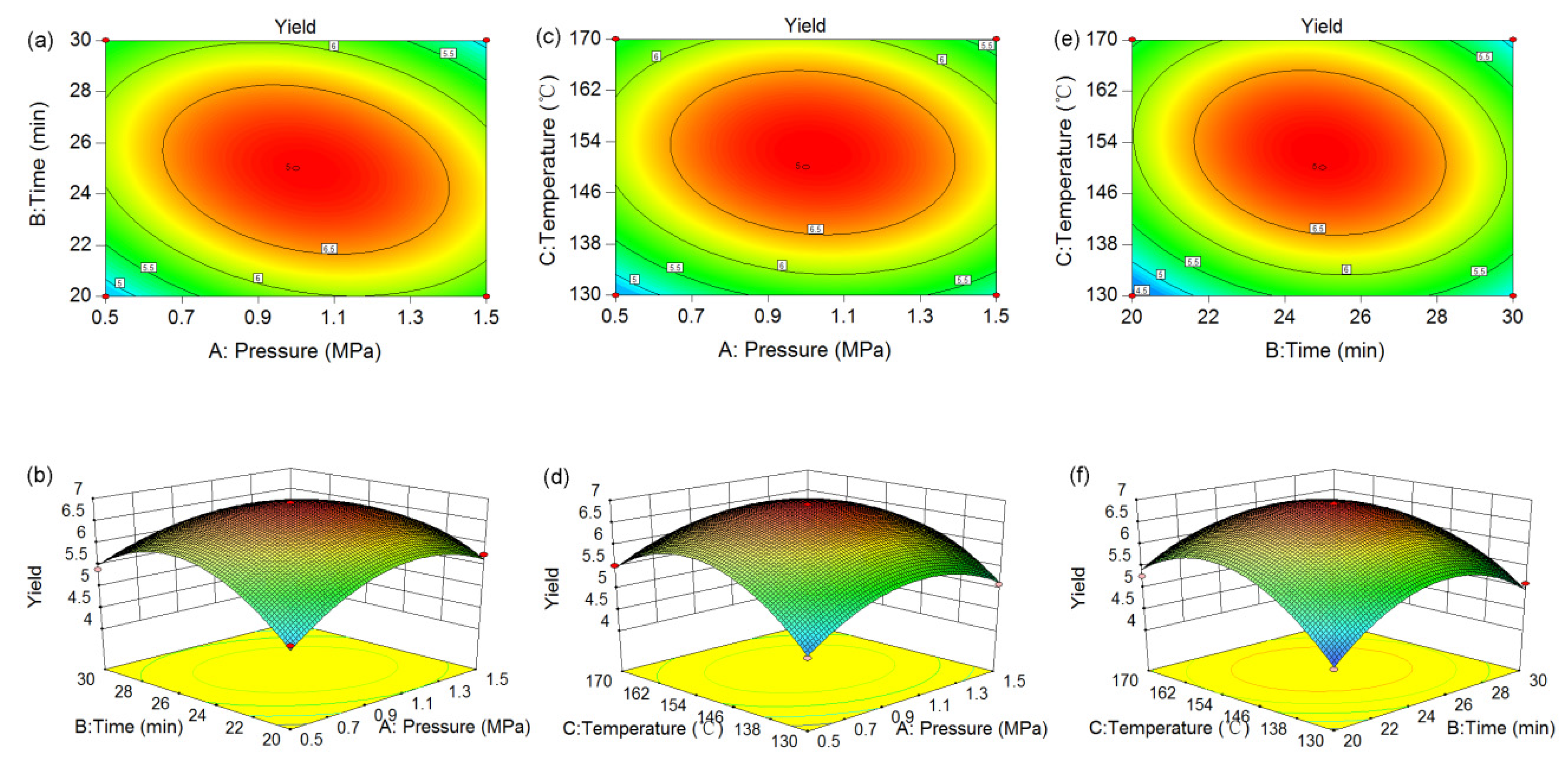
3.3. Modeling of the Extraction Process and Test of Significance
3.4. Optimization of Subcritical Water Extraction
3.5. Analysis of Normal Probability Plot
3.6. Spike Recovery Experiment
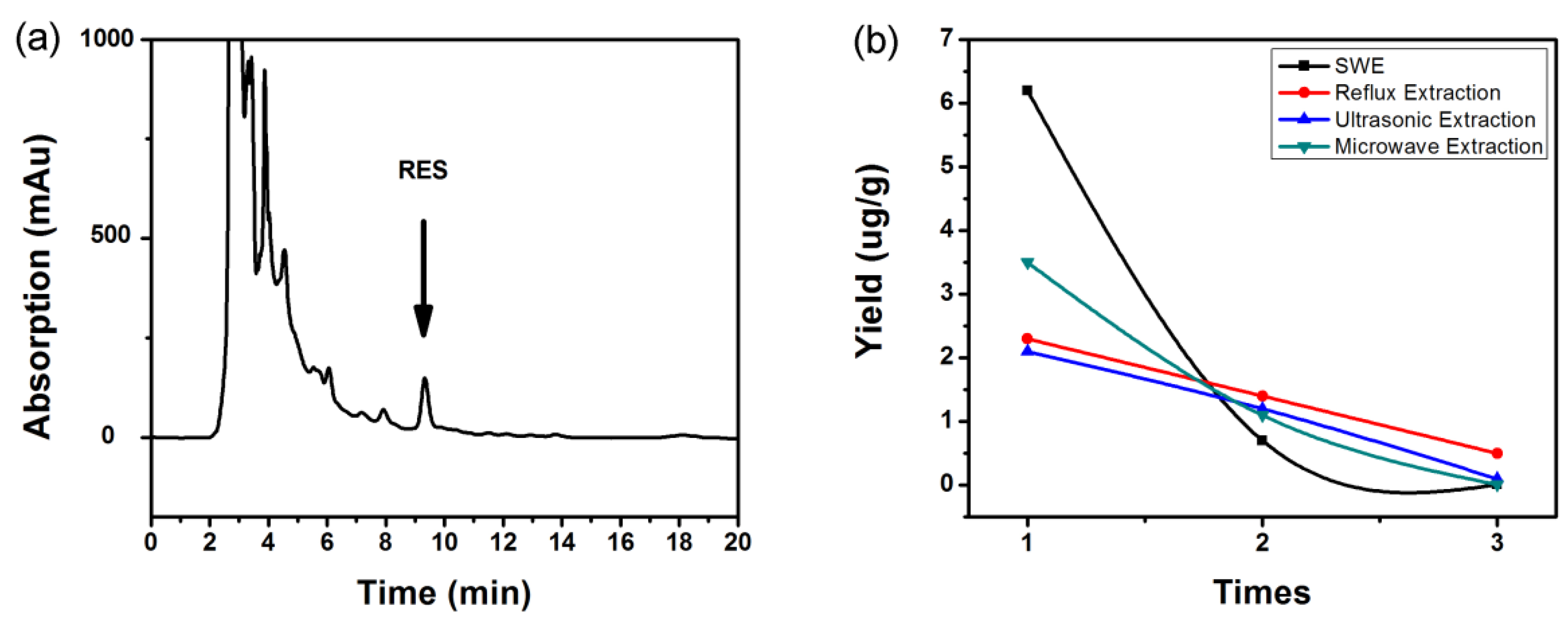
3.7. Comparison of SWE with Other Extraction Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marques, J.L.; Della Porta, G.; Reverchon, E.; Renuncio, J.A.R.; Mainar, A.M. Supercritical antisolvent extraction of antioxidants from grape seeds after vinification. J. Supercrit. Fluids. 2013, 82, 238–243. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Available online: http://www.oiv.int/ (accessed on 19 September 2016).
- Yilmaz, E.; Ozvural, E.; Vural, H. Extraction and identification of proanthocyanidins from grape seed (Vitis vinifera) using supercritical carbon dioxide. J. Supercrit. Fluids 2011, 55, 924–928. [Google Scholar] [CrossRef]
- Kurabachew, S.D.; Alessandro, A.C.; Hatem, B.M.; Patrizia, P.; Luca, F. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and modeling. Food Bioprod. Process. 2015, 94, 29–38. [Google Scholar]
- Ben, M.H.; Duba, K.S.; Fiori, L.; Abdelgawed, H.; Tlili, I.; Tounekti, T.; Zrig, A. Bioactive compounds and antioxidant activities of different grape (Vitis vinifera L.) seed oils extracted by supercritical CO2 and organic solvent. LWT Food Sci. Technol. 2016, 74, 557–562. [Google Scholar]
- Locatelli, M.; Travaglia, F.; Coisson, J.D.; Bordiga, M.; Arlorio, M. Phenolic composition of Nebbiolo grape (Vitis vinifera L.) from Piedmont: Characterization during ripening of grapes selected in different geographic areas and comparison with Uva Rara and Vespolina cv. Eur. Food Res. Technol. 2016, 242, 1057–1068. [Google Scholar] [CrossRef]
- Gabriel, D.P.; Inigo, A.; Maria, R. Grape seed extract: Additive and functional ingredient. Agro Food Ind. Hi-Tech 2013, 24, 41–43. [Google Scholar]
- Jin, H.F.; Liu, M.C.; Zhang, X.; Pan, J.J.; Han, J.Z.; Wang, Y.D.; Lei, H.X.; Ding, Y.C.; Yuan, Y.H. Grape seed procyanidin extract attenuates hypoxic pulmonary hypertension by inhibiting oxidative stress and pulmonary arterial smooth muscle cells proliferation. J. Nutr. Biochem. 2016, 36, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.C.; Calmasini, F.B.; de Oliveira, M.G.; Silva, F.H.; da Silva Carmem, P.V.; Andre, D.M.; Leonardo, F.C.; Delbin, M.A.; Antunes, E. Chronic treatment with resveratrol improves overactive bladder in obese mice via antioxidant activity. Eur. J. Pharmacol. 2016, 788, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.B.; Kevin, J.P.; Nathan, L.P.; Hamish, A.J.; Carles, L.; Avash, K.; Vinayakumar, V.P.; Joanne, S.A.; Guillermo, L.L.; Kaitlyn, L.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar]
- Mikami, S.; Ota, I.; Masui, T.; Asako, I.H.; Shobatake, R.; Okamoto, H.; Takasawa, S.; Kitahara, T. Effect of resveratrol on cancer progression through the REG III expression pathway in head and neck cancer cells. Int. J. Oncol. 2016, 49, 1553–1560. [Google Scholar] [PubMed]
- Chen, F.L.; Zhang, X.L.; Du, X.Q.; Yang, L.; Zu, Y.G.; Yang, F.J. A new approach for obtaining trans-resveratrol from tree peony seed oil extracted residues using ionic liquid-based enzymatic hydrolysis in situ extraction. Sep. Purif. Technol. 2016, 107, 294–305. [Google Scholar] [CrossRef]
- Lin, J.A.; Kuo, C.H.; Chen, B.Y.; Li, Y.; Liu, Y.C.; Chen, J.H.; Shieh, C.J. A novel enzyme-assisted ultrasonic approach for highly efficient extraction of resveratrol from Polygonum cuspidatum. Ultrason Sonochem. 2016, 32, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Mantell, C.; Rodríguez, M.; de la Ossa, E.M. Ascreening analysis o fthe high-pressure extraction of anthocyanins from red grape pomace with carbondioxide+co-solvent. Eng. Life Sci. 2003, 3, 38–42. [Google Scholar] [CrossRef]
- Lourdes, C.; Mantell, C.; Rodriguez, M.; Ana, B. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar]
- Erkan, K.; Giuseppe, M.; Levent, B.; Nevzat, A. Extraction of Bioactive Compounds from Milled Grape Canes (Vitis vinifera) Using a Pressurized Low-Polarity Water Extractor. Food Bioprocess Technol. 2012, 5, 359–371. [Google Scholar]
- Lu, W.; Chen, X.W.; Wang, J.M.; Yang, X.Q.; Qi, J.R. Enzyme-assisted subcritical water extraction and characterization of soy protein from heat-denatured meal. J. Food Eng. 2016, 169, 250–258. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lu, Y.C.; Chen, J.; Li, J.C.; Liu, S.H. Subcritical water extraction of alkaloids in Saphora flavescens Ait. and determination by capillary electrophoresis with field-amplified samples stacking. J. Pharm. Biomed. 2012, 58, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Kubatova, A.; Lagadec, A.J.; Miller, D.J.; Hawthorne, S.B. Selective extraction of oxygenates from savory and peppermint using subcritical water. J. Flavour Fragr. 2001, 16, 64–73. [Google Scholar] [CrossRef]
- Vladic, J.; Canli, O.; Pavlic, B.; Zekovic, Z.; Vidovic, S.; Kaplan, M. Optimization of Satureja montana subcritical water extraction process and chemical characterization of volatile fraction of extracts. J. Supercrit. Fluids 2017, 120, 86–94. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.P.; Wang, X.; Cui, H.; Lu, F. Statistical analysis of process parameters to eliminate hot cracking of fiber laser welded aluminum alloy. Opt. Laser Technol. 2015, 66, 15–21. [Google Scholar] [CrossRef]
- Abdussamet, S.; Bayram, S.; Irfan, K. Multi-objective optimization of a honeycomb heat sink using Response Surface Method. Int. J. Heat. Mass Trans. 2016, 101, 295–302. [Google Scholar]
- Geana, E.I.; Dinca, O.R.; Lonete, R.E.; Artem, V.; Niculescu, V.C. Monitoring trans-Resveratrol in Grape Berry Skins during Ripening and in Corresponding Wines by HPLC. Food Technol. Biotechnol. 2015, 53, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Chainukool, S.; Goto, M.; Hannongbua, S.; Shotipruk, A. Subcritical Water Extraction of Resveratrol from Barks of Shorea roxburghii G. Don. Sep. Sci. Technol. 2014, 49, 2073–2078. [Google Scholar] [CrossRef]
- Rodriguez-Cabo, T.; Rodriguez, I.; Ramil, M.; Cela, R. Comprehensive evaluation of the photo-transformation routes of trans-resveratrol. J. Chromatogr. A 2015, 1410, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.G.; Wang, W.Y.; Liu, X.; Yuan, F.; Gao, Y.X. Antioxidative phenolics obtained from spent coffee grounds (Coffea arabica L.) by subcritical water extraction. Ind. Crop Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Selin, S.; Rüya, S. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason Sonochem. 2013, 20, 595–602. [Google Scholar]
- Nermeen, A.E.; Hassan, A.H.; Ebrahim, G.M.; Abou, E. Response Surface Methodology as a Tool for Optimizing the Production of Antimicrobial Agents from Bacillus licheniformis SN2. Curr. Res. Bacteriol. 2009, 39, 1–14. [Google Scholar]
- Li, P.; Zhou, L.; Mou, Y.; Mao, Z. Extraction optimization of polysaccharide from Zanthoxylum bungeanum using RSM and its antioxidant activity. Int. J. Biol. Macromol. 2015, 72, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.J.; Cheigh, C.I.; Chung, M.S. Optimization of Subcritical Water Extraction of Flavanols from Green Tea Leaves. J. Agric. Food Chem. 2014, 62, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, P.; Yao, W.J.; Wang, J.; Wang, L.Y.; Deng, L.H.; He, J.; Zhang, G.F.; Lei, J.D. Subcritical water extraction of betulinic acid from birch bark. Ind. Crop Prod. 2015, 74, 557–565. [Google Scholar] [CrossRef]
- Gu, T.; Chen, Z.; Jiang, X.; Zhou, L.; Liao, Y.; Duan, M.; Wang, H.; Pu, Q. Synthesis and inhibition of N-alkyl-2-(4-hydroxybut-2-ynyl) pyridinium bromide for mild steel in acid solution: Box–Behnken design optimization and mechanism probe. Corros. Sci. 2015, 90, 118–132. [Google Scholar] [CrossRef]
- Kirmizakis, P.; Tsamoutsoglou, C.; Kaya, B.; Kalderis, D. Subcritical water treatment of landfill leachate: Application of response surface methodology. J. Environ. Manag. 2014, 146, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, N.; Tixier, A.S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefin. 2014, 8, 530–544. [Google Scholar] [CrossRef]


| Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| X1: Extraction pressure (MPa) | 0.5 | 1.0 | 1.5 |
| X2: Extraction time (min) | 20 | 25 | 30 |
| X3: Extraction temperature (°C) | 130 | 150 | 170 |
| Run | Parameters and Levels | RES Yields (μg/g Material) | ||
|---|---|---|---|---|
| A: Pressure (MPa) | B: Time (min) | C: Temperature (°C) | ||
| 1 | 0.5 | 25 | 130 | 4.59 ± 0.02 |
| 2 | 0.5 | 30 | 150 | 5.39 ± 0.01 |
| 3 | 1.0 | 30 | 170 | 4.91 ± 0.04 |
| 4 | 1.5 | 25 | 130 | 5.09 ± 0.03 |
| 5 | 1.5 | 25 | 170 | 5.30 ± 0.06 |
| 6 | 1.5 | 20 | 150 | 5.74 ± 0.08 |
| 7 | 1.0 | 20 | 170 | 5.27 ± 0.02 |
| 8 | 1.5 | 30 | 150 | 4.77 ± 0.03 |
| 9 | 1.0 | 30 | 130 | 5.10 ± 0.06 |
| 10 | 1.0 | 20 | 130 | 4.34 ± 0.01 |
| 11 | 0.5 | 25 | 170 | 5.53 ± 0.04 |
| 12 | 1.0 | 25 | 150 | 6.90 ± 0.03 |
| 13 | 1.0 | 25 | 150 | 6.90 ± 0.03 |
| 14 | 0.5 | 20 | 150 | 4.81 ± 0.04 |
| 15 | 1.0 | 25 | 150 | 6.90 ± 0.03 |
| 16 | 1.0 | 25 | 150 | 6.90 ± 0.03 |
| 17 | 1.0 | 25 | 150 | 6.90 ± 0.03 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 13.53 | 9 | 1.50 | 107.09 | <0.0001 |
| X1 | 0.043 | 1 | 0.043 | 3.06 | 0.1235 |
| X2 | 0.12 | 1 | 0.12 | 14.64 | 0.9312 |
| X3 | 0.45 | 1 | 0.45 | 32.30 | 0.0007 |
| X1X2 | 0.60 | 1 | 0.60 | 42.83 | 0.0003 |
| X1X3 | 0.13 | 1 | 0.13 | 9.39 | 0.0182 |
| X2X3 | 0.32 | 1 | 0.32 | 22.52 | 0.0021 |
| X12 | 2.37 | 1 | 2.37 | 168.79 | <0.0001 |
| X22 | 3.97 | 1 | 3.97 | 238.09 | <0.0001 |
| X32 | 4.41 | 1 | 4.41 | 313.87 | <0.0001 |
| Residual | 0.098 | 7 | 0.014 | - | - |
| Lack of fit | 0.098 | 3 | 0.033 | - | - |
| Pure error | 0.000 | 4 | 0.000 | - | - |
| Total | 13.63 | 16 | - | - | - |
| No. | Added (μg/g) | Detection Value (μg/g) | Rate of Recovery (%) | Average Absolute Recovery (%) | RSD |
|---|---|---|---|---|---|
| 1 | 9.90 | 87.00 | |||
| 2 | 3.45 | 9.78 | 83.60 | 86.47 | 0.03 |
| 3 | 9.96 | 88.80 | |||
| 1 | 13.10 | 89.90 | |||
| 2 | 6.90 | 12.94 | 87.54 | 87.78 | 0.02 |
| 3 | 12.83 | 85.90 | |||
| 1 | 19.73 | 93.00 | |||
| 2 | 13.80 | 19.61 | 92.07 | 91.98 | 0.01 |
| 3 | 19.44 | 90.87 |
| Method | Solvent Type | Organic Solvent Consumption (%) | Extraction Time | RES Yield (μg/g Material) |
|---|---|---|---|---|
| SWE | Ultrapure water | 0 | 25 min | 6.90 ± 0.03 |
| RE | Ethyl acetate | 60~90 | 5~12 h | 4.16 ± 0.2 |
| UE | Methanol | 30~75 | >10 min | 3.42 ± 0.26 |
| ME | Acetone | 30~75 | >10 min | 4.66 ± 0.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Wang, Y.; Ma, Y.; Zhu, P.; He, J.; Lei, J. Optimization of Subcritical Water Extraction of Resveratrol from Grape Seeds by Response Surface Methodology. Appl. Sci. 2017, 7, 321. https://doi.org/10.3390/app7040321
Tian Y, Wang Y, Ma Y, Zhu P, He J, Lei J. Optimization of Subcritical Water Extraction of Resveratrol from Grape Seeds by Response Surface Methodology. Applied Sciences. 2017; 7(4):321. https://doi.org/10.3390/app7040321
Chicago/Turabian StyleTian, Yajie, Yingsa Wang, Yunyun Ma, Pengbo Zhu, Jing He, and Jiandu Lei. 2017. "Optimization of Subcritical Water Extraction of Resveratrol from Grape Seeds by Response Surface Methodology" Applied Sciences 7, no. 4: 321. https://doi.org/10.3390/app7040321
APA StyleTian, Y., Wang, Y., Ma, Y., Zhu, P., He, J., & Lei, J. (2017). Optimization of Subcritical Water Extraction of Resveratrol from Grape Seeds by Response Surface Methodology. Applied Sciences, 7(4), 321. https://doi.org/10.3390/app7040321




