Aqueous Microwave-Assisted Solid-Phase Synthesis Using Boc-Amino Acid Nanoparticles
Abstract
:1. Introduction
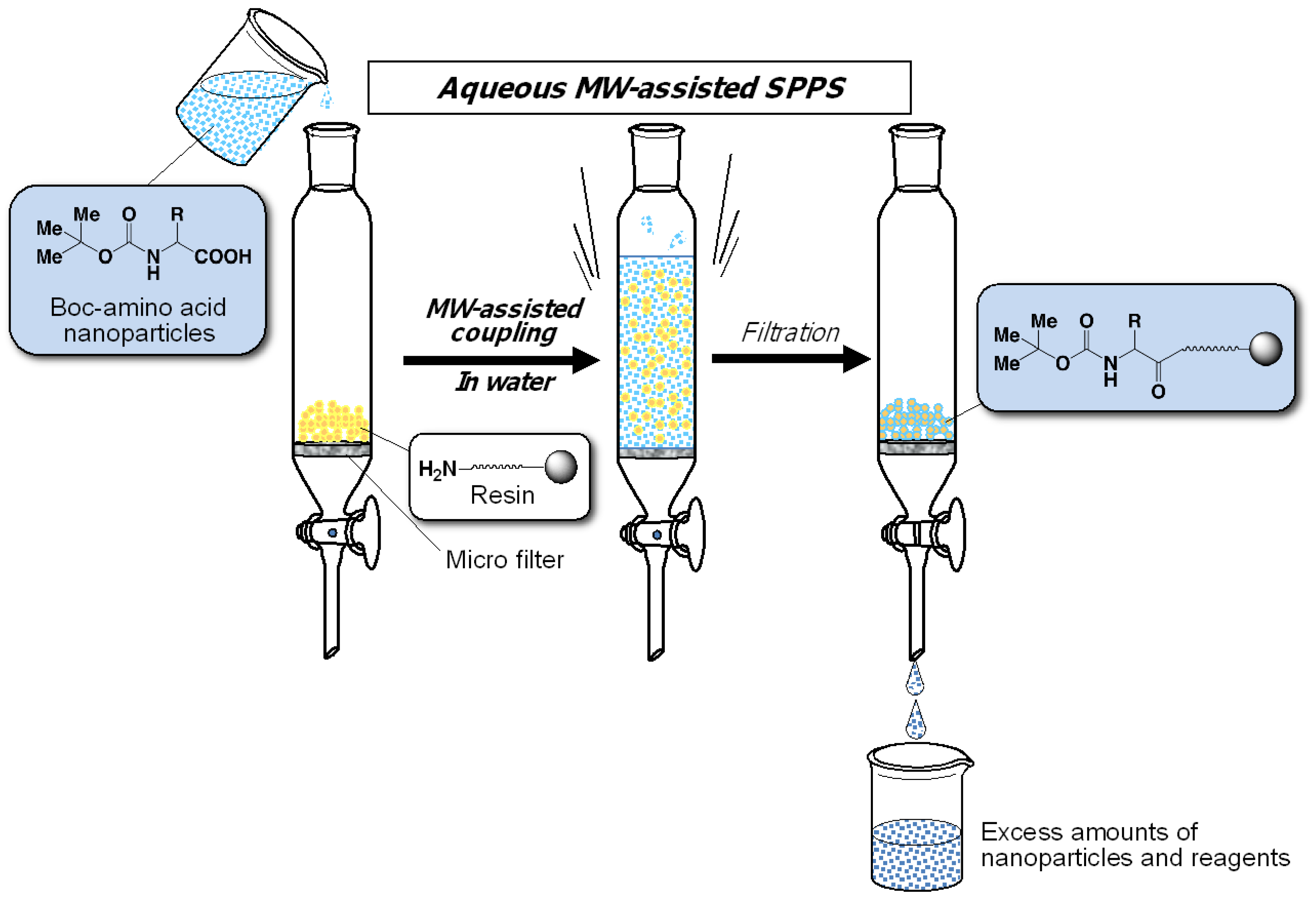
2. Experimental Section
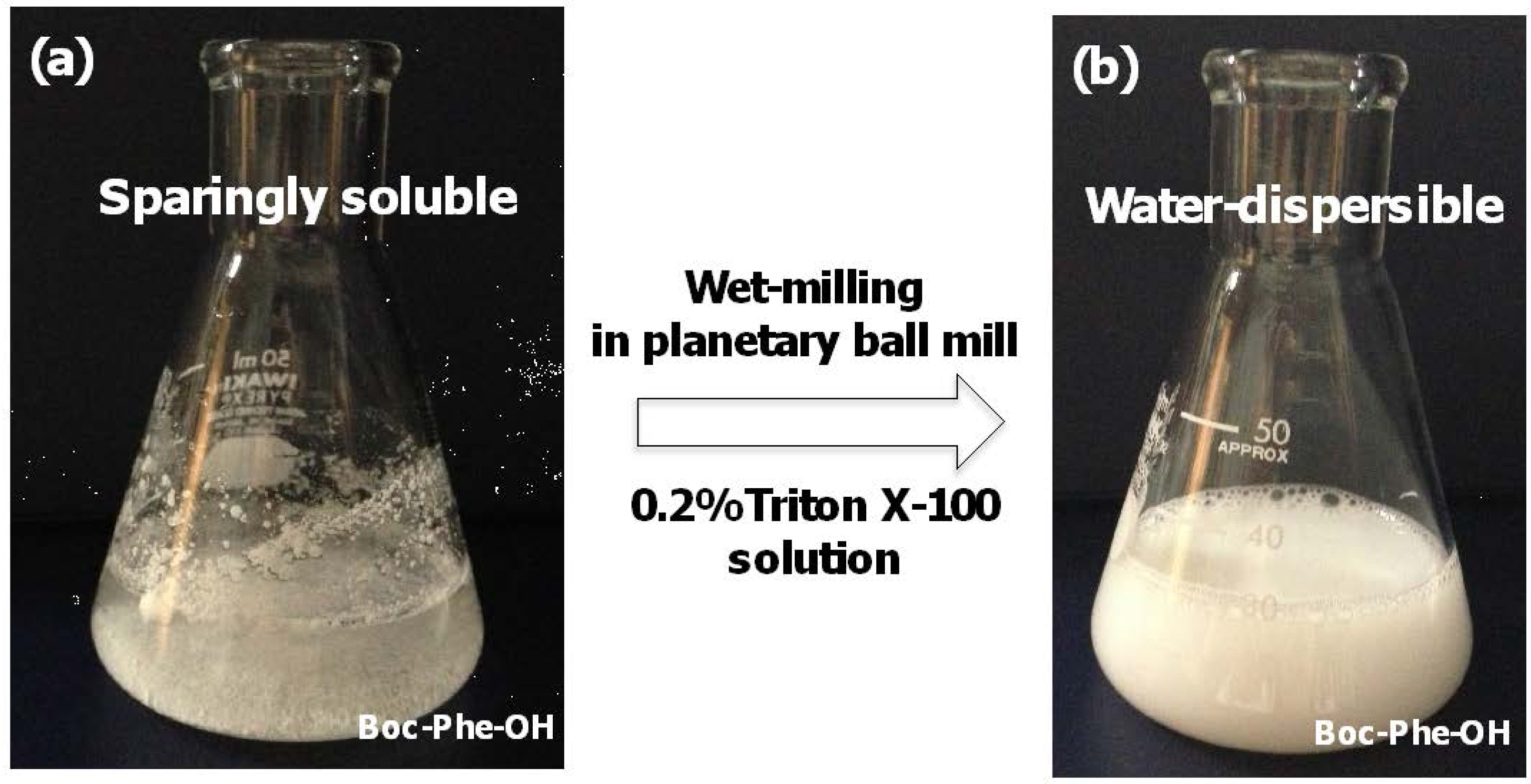
2.1. Preparation of Water-Dispersible Boc-Amino Acid Nanoparticles
- Boc-Leu-OH nanoparticles: particle size (mass mediam diameter) = 940.0 ± 83.1 nm.
- Boc-Tyr(tBu)-OH nanoparticles: particle size (mass mediam diameter) = 281.5 ± 2.7 nm.
- Boc-Tyr(BrZ)-OH nanoparticles: particle size (mass mediam diameter) = 394.2 ± 10.1 nm.
2.2. Aqueous MW Assisted Coupling Reaction Study using Water-Soluble Coupling Reagents
| Entry | Reagent | Additive | Time/min | Kaiser test a |
|---|---|---|---|---|
| 1 | WSCI | sulfo-HOSu | 10 | − |
| 2 | WSCI | sulfo-HOSu | 3 | − |
| 3 | WSCI | sulfo-HOSu | 1 | + |
| 4 | DMTMM | – | 10 | − |
| 5 | DMTMM | – | 3 | − |
| 6 | DMTMM | – | 1 | − |
2.3. General Procedure for Aqueous MW Assisted Solid-Phase Peptide Synthesis
| Step | Reagents | Time |
|---|---|---|
| Washing | Water | 3 min × 10 |
| Coupling reaction | Water-dispersible Boc-amino acids (3 equivalent) DMTMM, NMM, MW; 70 °C; 70 W | 3 min |
| Washing | 0.2% Triton X-100 solution | 3 min × 5 |
| Washing | EtOH | 3 min × 3 |
| Deprotection | TFA | 10 min × 2 |
| Washing | 0.5 mol/L NaHCO3 solution | 3 min × 3 |
3. Results and Discussion
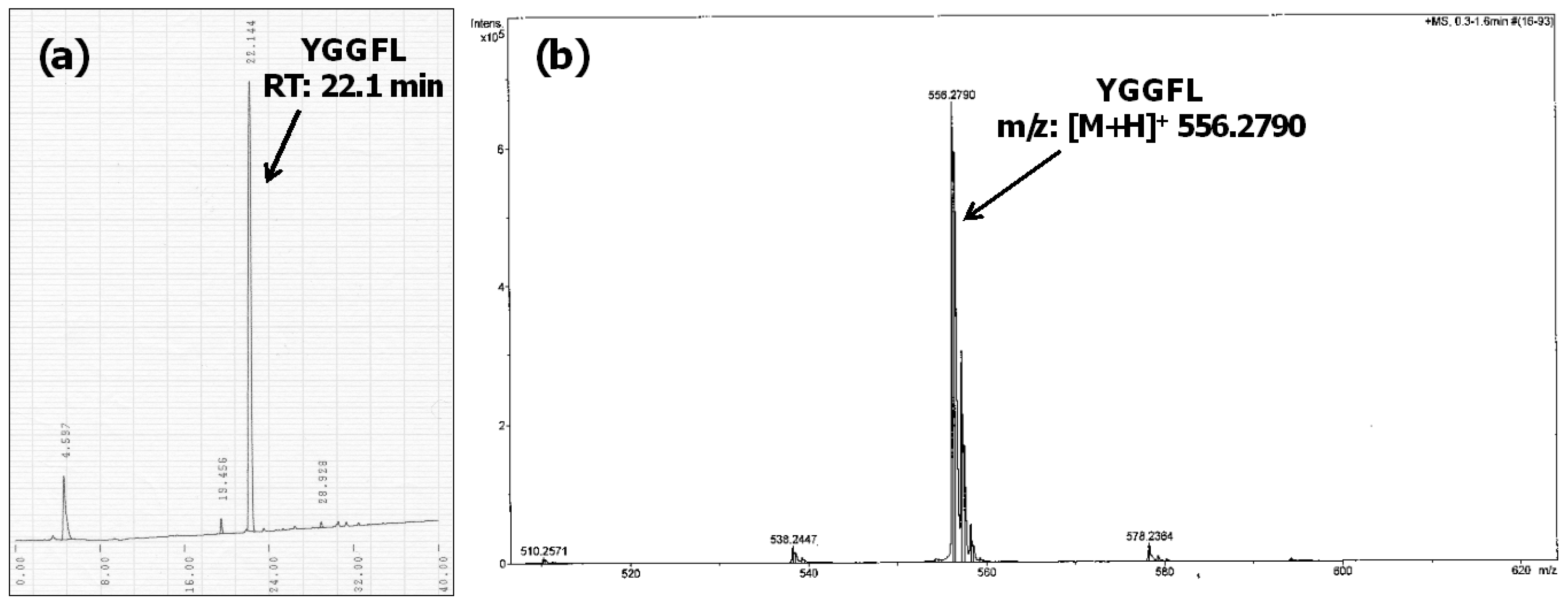
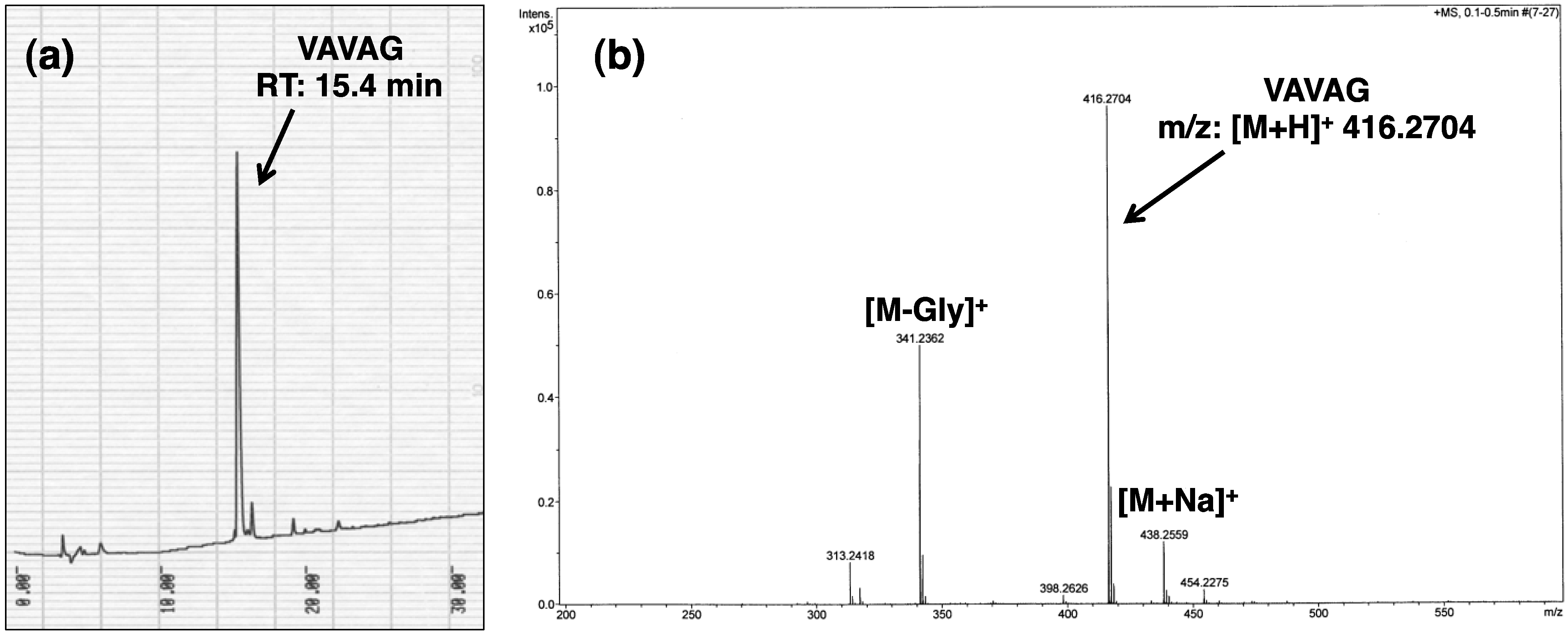
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Polshettiwar, V.; Varma, R.S. Environmentally Benign Chemical Synthesis via Mechanochemical Mixing and Mirowave Irradiation. In Eco-Friendly Synthesis of Fine Chemicals; Ballin, R., Ed.; RSC publishing: Cambridge, UK, 2009; pp. 275–292. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Winterton, N. Twelve more green chemistry principles. Green Chem. 2001, 3, G73–G75. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Hojo, K.; Maeda, M.; Kawasaki, K. A new water-soluble N-protecting group, 2-[phenyl(methyl)sulfonio]ethyloxycarbonyl tetrafluoroborate, and its application to solid phase peptide synthesis in water. J. Pept. Sci. 2001, 7, 615–618. [Google Scholar]
- Hojo, K.; Maeda, M.; Kawasaki, K. A water-soluble N-protecting group, 2-[phenyl(methyl)sulfonio]ethoxycarbonyl tetrafluoroborate, and its application to peptide synthesis. Tetrahedron 2004, 60, 1875–1866. [Google Scholar]
- Hojo, K.; Maeda, M.; Kawasaki, K. 2-(4-Sulfophenyl)ethoxycarbonyl group: A new water-soluble N-protecting group and its application to solid-phase peptide synthesis in water. Tetrahedron Lett. 2004, 45, 9293–9295. [Google Scholar] [CrossRef]
- Hojo, K.; Ichikawa, H.; Maeda, M.; Kida, S.; Fukumori, Y.; Kawasaki, K. Solid-phase peptide synthesis using nanoparticulate amino acids in water. J. Pept. Sci. 2007, 13, 493–497. [Google Scholar] [CrossRef]
- Hojo, K.; Ichikawa, H.; Fukumori, Y.; Kawasaki, K. Development of a method for solid-phase peptide synthesis in water. Int. J. Pept. Res. Ther. 2008, 14, 373–380. [Google Scholar] [CrossRef]
- Hojo, K.; Hara, A.; Kitai, H.; Onishi, M.; Ichikawa, H.; Fukumori, Y.; Kawasaki, K. Development of a method for environmentally friendly chemical peptide synthesis in water using water-dispersible amino acid nanoparticles. Chem. Cent. J. 2011, 5, 49. [Google Scholar] [CrossRef]
- Hojo, K.; Ichikawa, H.; Onishi, M.; Fukumori, Y.; Kawasaki, K. Peptide synthesis “in water” by a solution-phase method using water-dispersible nanoparticle Boc-amino acids. J. Pept. Sci. 2011, 17, 487–492. [Google Scholar] [CrossRef]
- McKay, F.C.; Albertson, W.F. New Amine-masking groups for peptide synthesis. J. Am. Chem. Soc. 1957, 79, 4686–4690. [Google Scholar] [CrossRef]
- Carpino, L.A.; Han, G. The 9-fluorenylmethoxycarbonyl function, a new base-sensitive. Amino-protecting group. J. Am. Chem. Soc. 1970, 92, 5748–5749. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A. Microwaves in Organic and Medicinal Chemistry; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Olivos, H.J.; Alluri, P.G.; Reddy, M.M.; Salony, D.; Kodadek, T. Microwave-assisted solid-phase syntheisis of peptides. Org. Lett. 2002, 4, 4057–4059. [Google Scholar] [CrossRef]
- Erdélyi, M.; Gogoll, A. Rapid microwave-assisted solid-phase peptide synthesis. Synthesis 2002, 11, 1592–1596. [Google Scholar]
- Yu, H.M.; Chen, S.T.; Wang, J.T. Enhanced coupling efficiency in solid-phase peptide synthesis by microwave irradiation. J. Org. Chem. 1992, 57, 4781–4784. [Google Scholar] [CrossRef]
- Collins, J.M.; Collins, M.J. Novel method for enhanced solid-phase peptide synthesis using microwave energy. Biopolymers 2003, 71, 361–366. [Google Scholar]
- Murray, J.K.; Gellman, D.H. Application of microwave irradiation to the synthesis of 14-helical β-peptides. Org. Lett. 2005, 7, 1517–1520. [Google Scholar] [CrossRef]
- Bacsa, B.; Horvati, K.; Bosze, S.; Andreae, F.; Kappe, C.O. Solid-phase synthesis of difficult peptide sequences at elevated temperatures: A critical comparison of microwave and conventional heating technologies. J. Org. Chem. 2008, 73, 7532–7542. [Google Scholar] [CrossRef]
- Galanis, A.S.; Albericio, F.; Grøtli, M. Solid-phase peptide synthesis in water using microwave assisted heating. Org. Lett. 2009, 20, 4488–4491. [Google Scholar] [CrossRef]
- Hojo, K.; Ichikawa, H.; Hara, A.; Onishi, M.; Kawasaki, K.; Fukumori, Y. Aqueous microwave-assisted solid-phase peptide synthesis using Fmoc strategy: In-water synthesis of “difficult sequences”. Protein Pept. Lett. 2012, 19, 1231–1236. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Bayer, E. Towards the chemical synthesis of proteins. Angew. Chem. Int. Ed. 1991, 30, 113–129. [Google Scholar] [CrossRef]
- Rink, H. Solid-phase synthesis of protected peptide fragments using a trialkoxy-diphenyl-methylester resin. Tetrahedron Lett. 1987, 28, 3787–3790. [Google Scholar] [CrossRef]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Discov. 2004, 3, 785–795. [Google Scholar] [CrossRef]
- Liversidge, G.G.; Conzentino, P. Drug particle size reduction for decreasing gastric irritancy and enhancing absorption of naproxen in rats. Int. J. Pharm. Sci. 1995, 125, 309–313. [Google Scholar] [CrossRef]
- Liversidge, G.G.; Cundy, K.C. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. Sci. 1995, 125, 91–97. [Google Scholar] [CrossRef]
- Sheehan, J.C.; Hlavka, J.J. The use of water-soluble and basic carbodiimides in peptide synthesis. J. Org. Chem. 1956, 21, 439–441. [Google Scholar] [CrossRef]
- Kaminski, Z.J.; Paneth, P.; Rudzinski, J.A. Study on the activation of carboxylic acids by means of 2-chloro-4,6-dimethoxy-1,3,5-triazine and 2-chloro-4,6-diphenoxy-1.3.5-triazine. J. Org. Chem. 1998, 63, 4248–4225. [Google Scholar] [CrossRef]
- Kunishima, M.; Kawachi, C.; Morita, J.; Terao, K.; Iawasaki, F.; Tani, S. 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride: An efficient condensing agent leading to the formulation of amide and esters. Tetrahedron 1996, 55, 13159–13179. [Google Scholar]
- Staros, J.V.; Wright, R.W.; Swingle, D.M. Enhancement by N-hydroxy-sulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 1986, 156, 220–222. [Google Scholar] [CrossRef]
- Sheppard, R.C.; Williams, B.J. Acid-labile resin linkage agent for use in solid phase peptide synthesis. Int. J. Pept. Protein Res. 1982, 20, 451–454. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Devaky, K.S. Solid phase synthesis of hydrophobic difficult sequence peptides on BDDMA-PS support. J. Pept. Sci. 2001, 7, 641–649. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hojo, K.; Shinozaki, N.; Nozawa, Y.; Fukumori, Y.; Ichikawa, H. Aqueous Microwave-Assisted Solid-Phase Synthesis Using Boc-Amino Acid Nanoparticles. Appl. Sci. 2013, 3, 614-623. https://doi.org/10.3390/app3030614
Hojo K, Shinozaki N, Nozawa Y, Fukumori Y, Ichikawa H. Aqueous Microwave-Assisted Solid-Phase Synthesis Using Boc-Amino Acid Nanoparticles. Applied Sciences. 2013; 3(3):614-623. https://doi.org/10.3390/app3030614
Chicago/Turabian StyleHojo, Keiko, Natsuki Shinozaki, Yoshimi Nozawa, Yoshinobu Fukumori, and Hideki Ichikawa. 2013. "Aqueous Microwave-Assisted Solid-Phase Synthesis Using Boc-Amino Acid Nanoparticles" Applied Sciences 3, no. 3: 614-623. https://doi.org/10.3390/app3030614
APA StyleHojo, K., Shinozaki, N., Nozawa, Y., Fukumori, Y., & Ichikawa, H. (2013). Aqueous Microwave-Assisted Solid-Phase Synthesis Using Boc-Amino Acid Nanoparticles. Applied Sciences, 3(3), 614-623. https://doi.org/10.3390/app3030614




