An Environmentally Friendly Class of Fluoropolyether: α,ω-Dialkoxyfluoropolyethers
Abstract
:1. Introduction
2. Syntheses of α,ω-Dialkoxyfluoropolyethers
2.1. Oxypolymerization of Perfluoroolefin
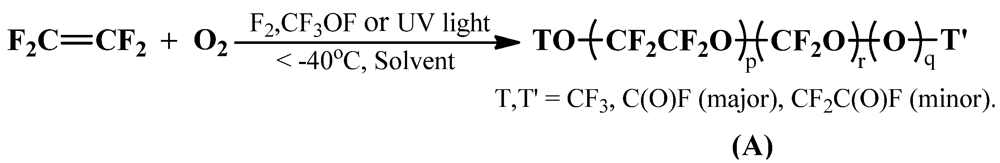
2.2. Deperoxidation of P-PFPEs
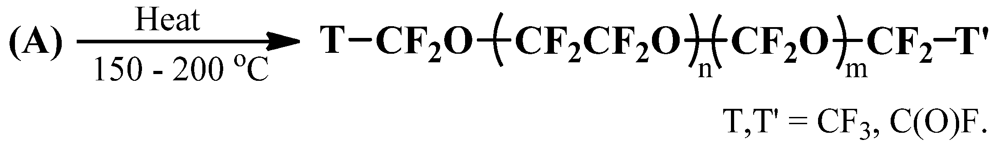
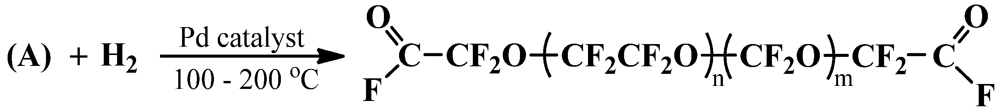
2.3. Preparation of DA-FPEs by DAF-PFPE Alkylation

2.3.1. Alkylation of PFPE Dialkoxides

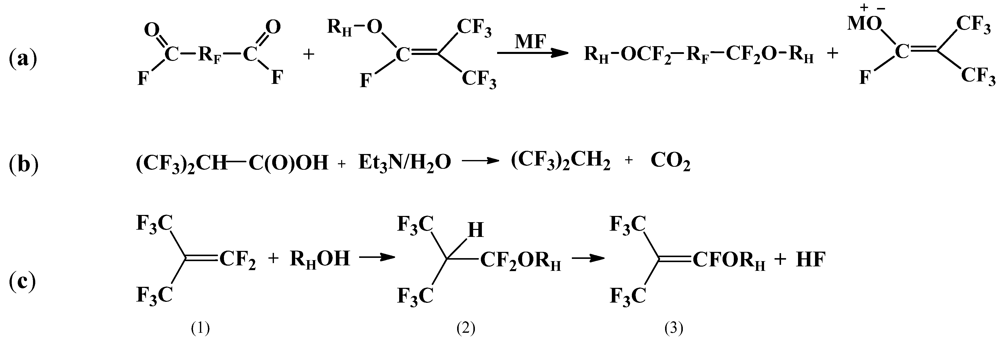
2.3.2. Catalytic Alkylation of Perfluoro(mono)acyl Fluorides and DAF-PFPE

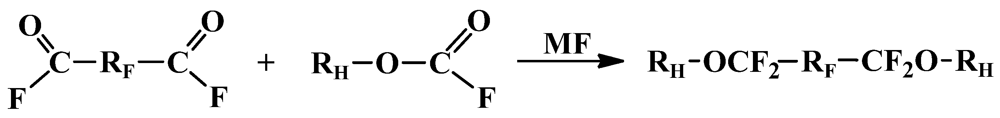
| Acylfluoride | Fluoroformate | HFE | Conv.% | Sel.% |
|---|---|---|---|---|
| z-DAF a | CH3OC(O)F | CH3O-(Z-PFPE)-OCH3 | 90 | 100 |
| z-DAF a | CH3CH2OC(O)F | CH3CH2O-(Z-PFPE)-OCH2CH3 | 96 | 100 |
| z-DAF a | CH2=CHCH2O(O)F | CH2=CHCH2O-(Z-PFPE)-OCH2CH=CH2 | 90 | 100 |

3. Chemical Physical Properties of α,ω-Dialkoxyperfluoropolyethers
| Sample | Structure | MS | Purity (%, by GC) |
|---|---|---|---|
| DM01 | CH3OCF2CF2OCH3 | 162 | 99.8 |
| DM02 | CH3OCF2CF2OCF2CF2OCH3 | 278 | 99.9 |
| DM03 | CH3OCF2CF2OCF2CF2OCF2CF2OCH3 | 394 | 99.0 |
| DM13 | CH3OCF2CF2OCF2OCF2CF2OCF2CF2OCH3 | 460 | 96.8 |
3.1. Boiling Point

3.2. Density
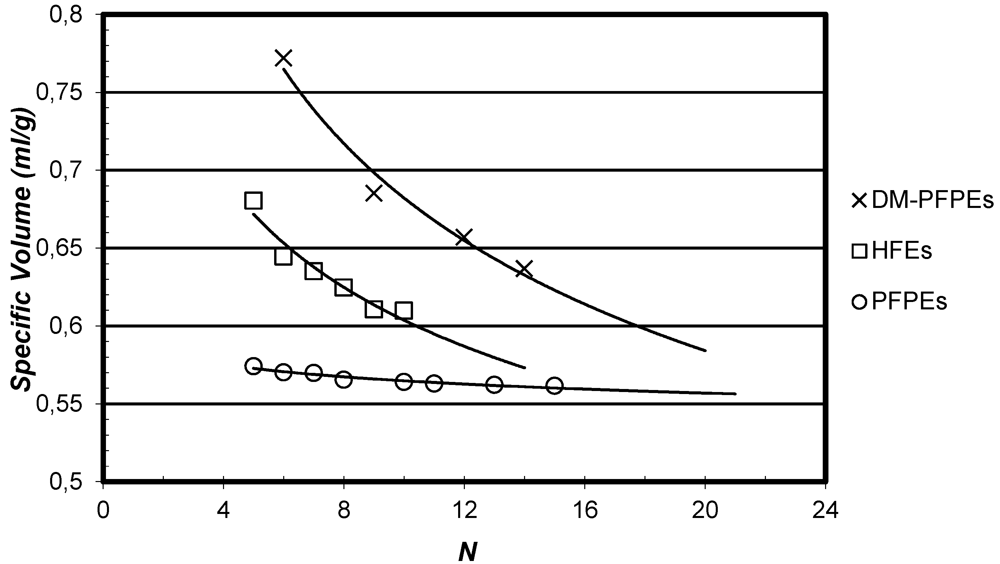
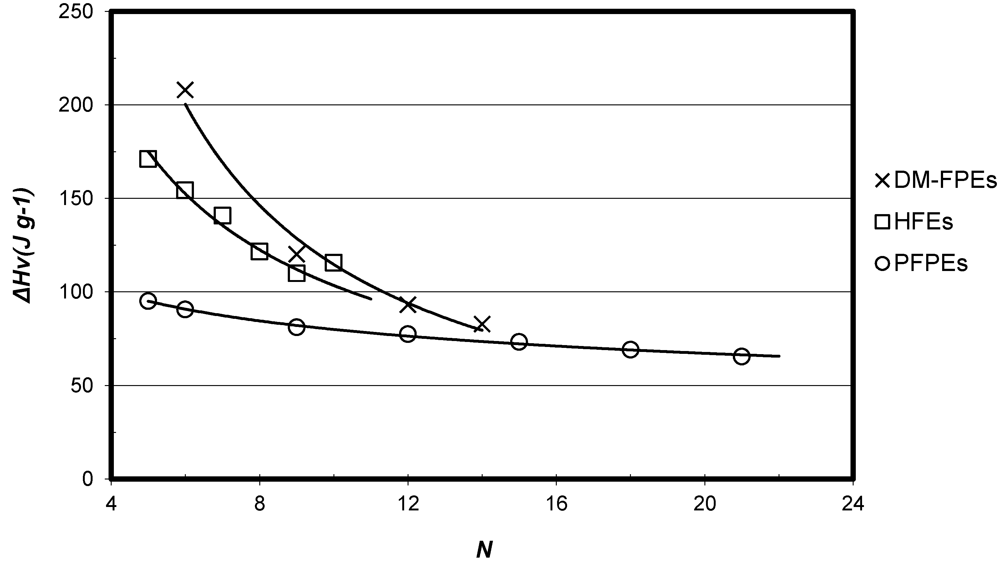
3.3. Vapor Pressure and Vaporization Enthalpy

3.4. Refractive Index

4. Atmospheric Chemistry of DA-FPEs
4.1. Atmospheric Lifetimes and Decomposition Mechanism of α,ω-Dialkoxyperfluoropolyethers
4.2. Global Warming Potential
| IF(W·m−2·ppb−1) | Lifetime(y) | GWP 100 yearsTime horizon | Reference | |
|---|---|---|---|---|
| CFCl3 (CFC11) | 0.25 | 45 | 4750 | IPCC/TEAP |
| CF2HOCF3 (HFE125) | 0.44 | 136 | 14900 | IPCC/TEAP |
| CH3OCF3 (HFE143a) | 0.27 | 4.3 | 756 | IPCC/TEAP |
| C4F9OCH3(HFE-7100) | 0.31 | 5 | 390 | [50] |
| HCF2OCF2H | 0.40 | 11.3 | 3699 | [8,9] |
| HCF2OCF2OCF2H | 0.66 | 12.1 | 2700 | [50] |
| HCF2OCF2CF2OCF2H | 0.87 | 6.2 | 1500 | [50] |
| HCF2OCF2OCF2CF2OCF2H | 1.37 | 6.3 | 1840 | [8,9] |
| CH3OCH3 | 0.020 | 0.015 | 0.3 | [41,42] |
| DM01 | 0.32 | 2 | 230 | [1] |
| DM02 | 0.61 | 2 | 270 | [1] |
| DM03 | 0.83 | 2 | 250 | [1] |

Acknowledgements
References
- Andersen, M.P.S.; Hurley, M.D.; Wallington, T.J.; Blandini, F.N.; Jensen, R.; Librando, V.; Hjorth, J.; Marchionni, G.; Avataneo, M.; Visca, M.; et al. Atmospheric chemistry of CH3O(CF2CF2O)nCH3 (n = 1–3): Kinetics and mechanism of oxidation initiated by Cl atoms and OH radicals, IR spectra, and global warming potentials. Phys.Chem. A 2004, 108, 1964–1972. [Google Scholar]
- WMO (World Meteorological Organization), Scientific Assessment of Ozone Depletion: 2002, Global Ozone Research and Monitoring Project; Report No. 47. Geneva, Switzerland, 2003.
- Wallington, T.J.; Schneider, W.F.; Sehested, J.; Bilde, M.; Platz, J.; Nielsen, O.J.; Molina, M.J. Atmospheric chemistry of HFE-7100 (C4F9OCH3): Kinetics of its reaction with OH radicals, UV spectra and kinetic data for C4F9OCH2• and C4F9OCH2O2• radicals, and the atmospheric fate of C4F9OCH2O• radicals. J. Phys. Chem.A 1997, 101, 8264–8274. [Google Scholar]
- Marchionni, G.; Avataneo, M.; de Patto, U.; Maccone, P.; Pezzin, G. Physical properties of four alpha-omega-dimethoxyfluoropolyethers. J. Fluor. Chem. 2005, 126, 465–473. [Google Scholar]
- Marchionni, G.; de Patto, U.; Avataneo, M. Liquid-liquid extraction of polar organic substances from their aqueous solutions with fluorinated extracting liquids. European Patent Application EP 1346757 A, 24 September 2003. [Google Scholar]
- Marchionni, G.; Visca, M. Perfluoropolyethers (PFPEs) having at least an alkylether end group and respective preparation process. European Patent Application. EP 1275678, 23 April 2003. [Google Scholar]
- Marchionni, G.; Petricci, S.; Guarda, P.A.; Spataro, G.; Pezzin, G. The comparison of thermal stability of some hydrofluoroethers and hydrofluoropolyethers. J. Fluor. Chem. 2004, 125, 1081–1086. [Google Scholar] [CrossRef]
- Marchionni, G.; Maccone, P.; Pezzin, G. Thermodynamic and other physical properties of several hydrofluoro-compounds. J. Fluor. Chem. 2002, 118, 149–155. [Google Scholar] [CrossRef]
- Malavasi, M.; Sianesi, D. Novelties and prospects in the synthesis of perfluoropolyethers by oxidative polymerization of fluoroolefins. J. Fluor. Chem. 1999, 95, 19–25. [Google Scholar] [CrossRef]
- Guarda, P.A.; Barchiesi, E.; Fontana, G.; Petricci, S.; Pianca, M.; marchionni, G. Peroxidic perfluoropolyether from tetrafluoroethylene oxidation: Micro structural analysis by NMR spectroscopy and mechanistic considerations. J. Fluor. Chem. 2005, 126, 141–153. [Google Scholar] [CrossRef]
- Sianesi, D.; Marchionni, G.; de Pasquale, R.J. Perfluoropolyethers (PFPEs) from perfluoroolefin photooxidation: Fomblin® and Galden® fluids. In Organofluorine Chemistry Principles and Commercial Applications; Bank, R.E., Smart, R.E., Tatlow, J.C., Eds.; Plenum Publishing Corporation: New York, NY, USA, 1994; pp. 431–457. [Google Scholar]
- Sansotera, M.; Navarrini, W.; Gola, M.; Bianchi, L.C.; Wormald, P.; Famulari, A.; Avataneo, M. Peroxidic perfluoropolyether for the covalent binding of perfluoropolyether chains on carbon black surface. J. Fluor. Chem. 2011, 132, 1254–1261. [Google Scholar] [CrossRef]
- Navarrini, W.; Venturini, F.; Sansotera, M.; Ursini, M.; Metrangolo, P.; Resnati, G.; Galimberti, E.; Barchiesi, E.; Dardani, P. The use of perfluoroalkyl hypofluorites for an efficient synthesis of perfluorinated ethers characterized by low Ostwald coefficient. J. Fluor. Chem. 2008, 129, 680–685. [Google Scholar] [CrossRef]
- Sianesi, D.; Caporiccio, G. Sulphonic derivatives having the structure of polyoxapoly fluoroalkanes. U.S. Patent, 3,847,978, 23 December 1974. [Google Scholar]
- Prakash, G.K.S.; Hu, J.; Olah, G.A. Alkylation of in situ generated fluorinated alkoxides: Novel synthesis of partially fluorinated ethers. Arkivoc SD-369C 2003, 104–119. [Google Scholar]
- Evans, F.W.; Litt, M.H.; Weidler-Kubanee, A.M.; Avonda, F.P. Formation of adducts between fluorinated ketones and metal fluorides. J. Org. Chem. 1968, 33, 1837–1839. [Google Scholar]
- Parker, A.J. The effects of solvation on the properties of anions in dipolar aprotic solvents. Q. Rev. Chem. Soc. 1962, 16, 163–187. [Google Scholar] [CrossRef]
- Lerman, O.; Rozen, S. Novel method for introduction of the perfluoroethoxy group using elemental fluorine. Synthesis and chemistry of fluoroxypentafluoroethane. J. Org. Chem. 1980, 45, 4122–4125. [Google Scholar] [CrossRef]
- Navarrini, W.; Tortelli, V.; Russo, A.; Corti, S. Organic hypofluorites and their new role in industrial fluorine chemistry. J. Fluor. Chem. 1999, 95, 27–39. [Google Scholar] [CrossRef]
- Feiring, E.A.; Wonchoba, R.E.; Rozen, S. Synthesis of partially fluorinated monomers and polymers for ion-exchange resins. J. Fluor. Chem. 1999, 93, 93–101. [Google Scholar] [CrossRef]
- Croix, L.S.; Szur, A.J. Ether compounds as inhalant anesthetics. U.S. Patent, 3,962,460, 8 June 1976. [Google Scholar]
- Paquette, L.A. Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 1995; pp. 2132–2135. [Google Scholar]
- Suter, C.M. The Organic Chemistry of Sulfur; Wiley & Sons: Hoboken, NJ, USA, 1944; pp. 48–74. [Google Scholar]
- Marchionni, G.; Mario, V. PFPEs having at least an alkylether end group and respective preparation process. U.S. Patent 7,488,852, 10 February 2009. [Google Scholar]
- Lamanna, W.M.; Flynn, R.M.; Vitcak, D.R.; Qiu, Z.-M. Catalytic process for making hydrofluoroethers. PCT WO 9947480. 23 September 1999. [Google Scholar]
- Behr, F.E.; Cheburkov, Y. Process for preparing hydrofluoroethers. U.S. Patent, 6,023,002, 8 February 2000. [Google Scholar]
- Lamanna, W.M.; Flynn, R.M.; Vitcak, D.R.; Qiu, Z.-M. Catalytic process for making hydrofluoroethers. U.S. Patent 6,046,368, 4 April 2000. [Google Scholar]
- Galimberti, M.; Fontana, G.; Resnati, G.; Navarrini, W. New catalytic alkylation of in situ generated perfluoro-alkyloxy-anions and perfluoro-carbanions. J. Fluor. Chem. 2005, 126, 1578–1586. [Google Scholar] [CrossRef]
- Navarrini, W.; Galimberti, M.; Fontana, G. Process for preparing hydrofluoroethers. European patent application EP 1462434A, 16 application 2004. [Google Scholar]
- Klauke, E. Braden, R. Fluoroformates. GB 1216639. 23 December 1970. [Google Scholar]
- Marchionni, G.; Bassi, M.; Fontana, G.; Maccone, P. Ajroldi, J. Some physical chemical properties of alpha-omega dihydroperfluoropolyethers. J. Fluor. Chem. 1999, 98, 41–54. [Google Scholar] [CrossRef]
- Marchionni, G.; Ajroldi, G.; Righetti, M.C.; Pezzin, G. Molecular interactions in perfluorinated and hydrogenated compounds: Linear paraffins and ethers. Macromolecules 1993, 26, 1751–1757. [Google Scholar] [CrossRef]
- Marchionni, G.; Avataneo, M.; de Patto, U.; Maccone, P.; Pezzin, G. Physical properties of four alpha-omega dimethoxyfluoropolyethers. J. Fluor. Chem. 2005, 126, 465–473. [Google Scholar]
- Bondi, A. Van der waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Le, T.D.; Weers, J.G. Group contribution-additivity and quantum mechanical models for predicting the molar refractions, indexes of refraction, and boiling points of fluorochemicals. J. Phys. Chem. 1995, 99, 13909–13916. [Google Scholar] [CrossRef]
- Blowers, P.; Moline, D.M.; Tetrault, K.F.; Wheeler, R.R.; Tuchawena, S.L. Prediction of radiative forcing values for hydrofluoroethers using density functional theory methods. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef]
- Blowers, P.; Moline, D.M.; Tetrault, K.F.; Wheeler, R.R.; Tuchawena, S.L. Global warming potentials of hydrofluoroethers. Environ. Sci. Technol. 2008, 42, 1301–1307. [Google Scholar]
- Blanco, M.B.; Teruel, M.A. Atmospheric degradation of fluoroesters (FESs): Gas-phase reactivity study towards OH radicals at 298 K. Atmos. Environ. 2007, 41, 7330–7338. [Google Scholar] [CrossRef]
- Blanco, M.B.; Bejan, I.; Barnes, I.; Wiesen, P.; Teruel, M.A. Kinetics of the reactions of chlorine atoms with selected fluoroacetates at atmospheric pressure and 298 K. Chem. Phys. Lett. 2008, 453, 18–23. [Google Scholar] [CrossRef]
- Hudlicky, M. Chemistry of Organic Fluorine Compounds, 2nd ed; Ellis Horwood: Chichester, UK, 1976; pp. 255–257. [Google Scholar]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Atmospheric Chemistry: Fundamentals and Experimental Techniques; John Wiley and Sons: New York, NY, USA, 1986. [Google Scholar]
- Calvert, J.G.; Pitts, J.N., Jr. Photochemistry; John Wiley: New York, NY, USA, 1966. [Google Scholar]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Crowley, J.N.; Hampson, R.F.; Hynes, R.G.; Jenkin, M.E.; Rossi, M.J.; Troe, J.; Wallington, T.J. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume IV—Gas phase reactions of organic halogen species. Atmos. Chem. Phys. 2008, 8, 4141–4496. [Google Scholar] [CrossRef]
- AFEAS Workshop, Kinetics and Mechanisms for the Reaction of Halogenated Organic Compounds in the Troposphere; AFEAS Workshop: Dublin, Ireland, 1993.
- Pinnock, S.; Hurley, M.D.; Shine, K.P.; Wallington, T.J.; Smyth, T.J. Radiative forcing of climate by hydrochloroflurocarbons and hydrofluorocarbons. J. Geophys. Res. Atmos. 1995, 100, 23227–23238. [Google Scholar]
- Bunyard, W.C.; Kadla, J.F.; de Simone, M.S. Viscosity effects on the thermal decomposition of bis(perfluoro-2-N-propoxypropionyl) peroxide in dense carbon dioxide and fluorinated solvents. J. Am. Chem. 2001, 123, 7199–7206. [Google Scholar]
- Sansotera, M.; Bianchi, C.L.; Lecardi, G.; Marchionni, G.; Metrangolo, P. Resnati, G.; Navarrini, W. Highly hydrophobic carbon black obtained by covalent linkage of perfluorocarbon and perfluoropolyether chains on the carbon surface. Chem. Mater. 2009, 21, 4498–4504. [Google Scholar]
- Bravo, I.; Diaz-de-mera, Y.; Aranda, A.; Moreno, E.; Nutt, D.R.; Marston, G. Radiative efficiencies for fluorinated esters: Indirect global warming potentials of hydrofluoroethers. Phys. Chem. Chem. Phys. 2011, 13, 17185–17193. [Google Scholar]
- Javadiando, S.M.; Nielsen, J.; Wallington, J.T.; Hurley, D.A.; Owens, G.J. Atmospheric chemistry of n-butanol: kinetics, mechanisms, and products of Cl atom and OH radical initiated oxidation in the presence and absence of NO(x). Environ. Sci. Technol. 2007, 41, 7389–7395. [Google Scholar]
- IPCC, Climate Change 2001: The Scientific Basis; IPCC: Geneva, Switzerland, 2001.
- Wallington, T.J.; Hurley, M.D.; Nielsen, O.J. The radiative efficiency of HCF2OCF2OCF2CF2OCF2H (Galden® PFPEs) revisited. Int. J. Chem. Kinet. 2008, 40, 819–825. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, M.; Navarrini, W.; Spataro, G.; Venturini, F.; Sansotera, M. An Environmentally Friendly Class of Fluoropolyether: α,ω-Dialkoxyfluoropolyethers. Appl. Sci. 2012, 2, 351-367. https://doi.org/10.3390/app2020351
Wu M, Navarrini W, Spataro G, Venturini F, Sansotera M. An Environmentally Friendly Class of Fluoropolyether: α,ω-Dialkoxyfluoropolyethers. Applied Sciences. 2012; 2(2):351-367. https://doi.org/10.3390/app2020351
Chicago/Turabian StyleWu, Menghua, Walter Navarrini, Gianfranco Spataro, Francesco Venturini, and Maurizio Sansotera. 2012. "An Environmentally Friendly Class of Fluoropolyether: α,ω-Dialkoxyfluoropolyethers" Applied Sciences 2, no. 2: 351-367. https://doi.org/10.3390/app2020351
APA StyleWu, M., Navarrini, W., Spataro, G., Venturini, F., & Sansotera, M. (2012). An Environmentally Friendly Class of Fluoropolyether: α,ω-Dialkoxyfluoropolyethers. Applied Sciences, 2(2), 351-367. https://doi.org/10.3390/app2020351





