The Mechanically Destabilizing Effect of Increased Heel Height in Women Is Not Enhanced by Dual-Task Interference
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
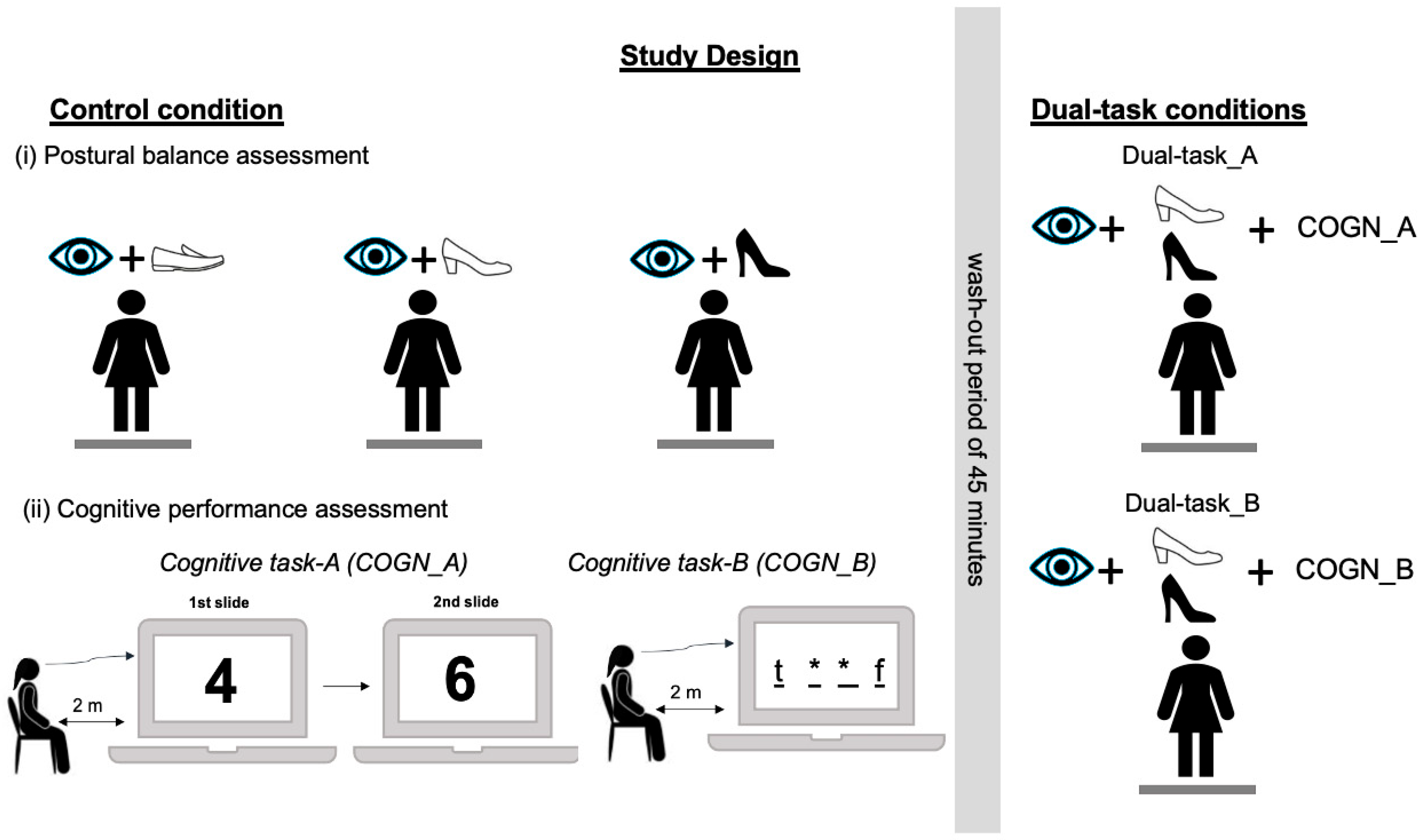
2.2. Experimental Protocol
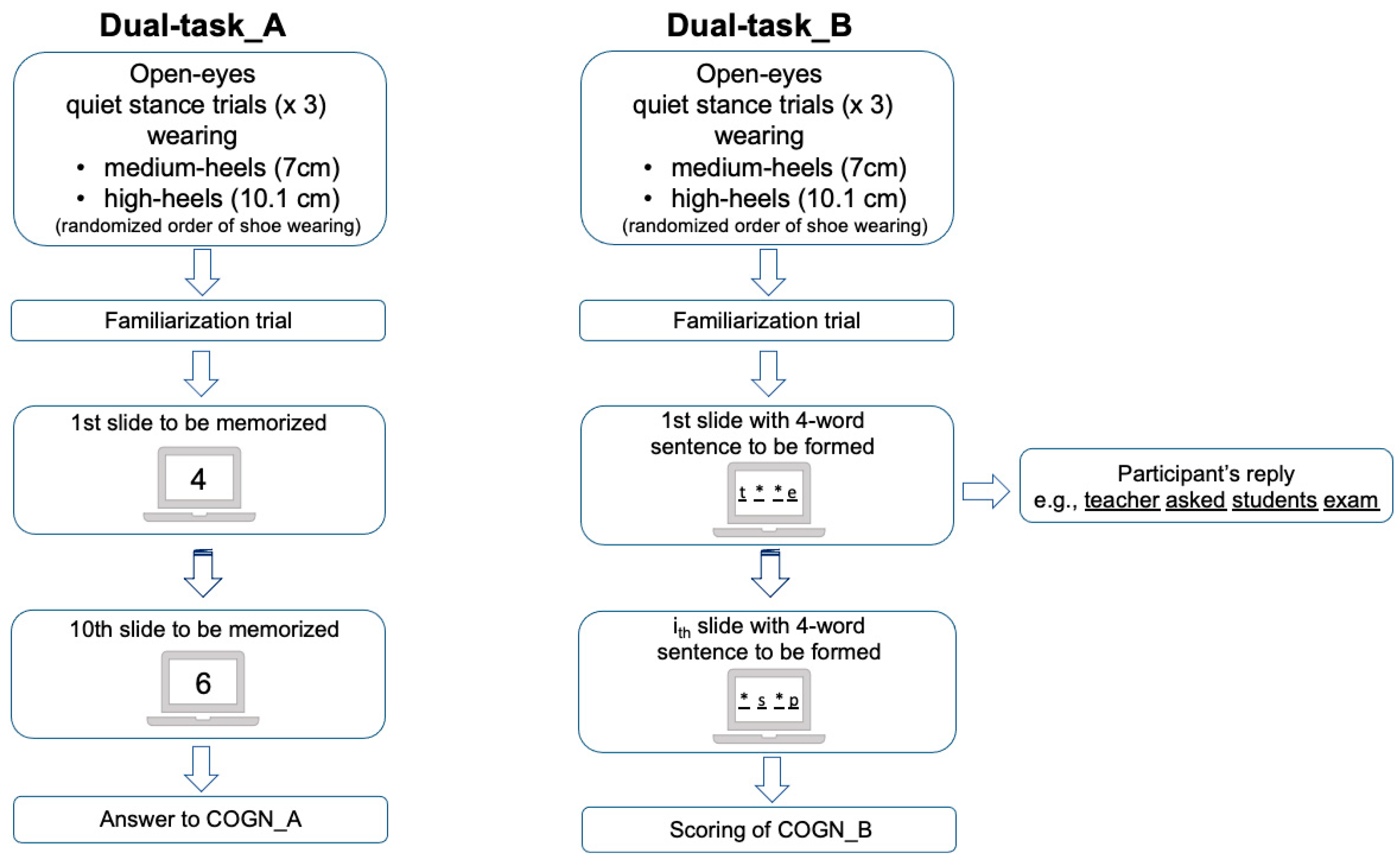
2.2.1. Postural Balance Assessment
2.2.2. Concurrent Cognitive Tasks
2.3. Statistical Analysis
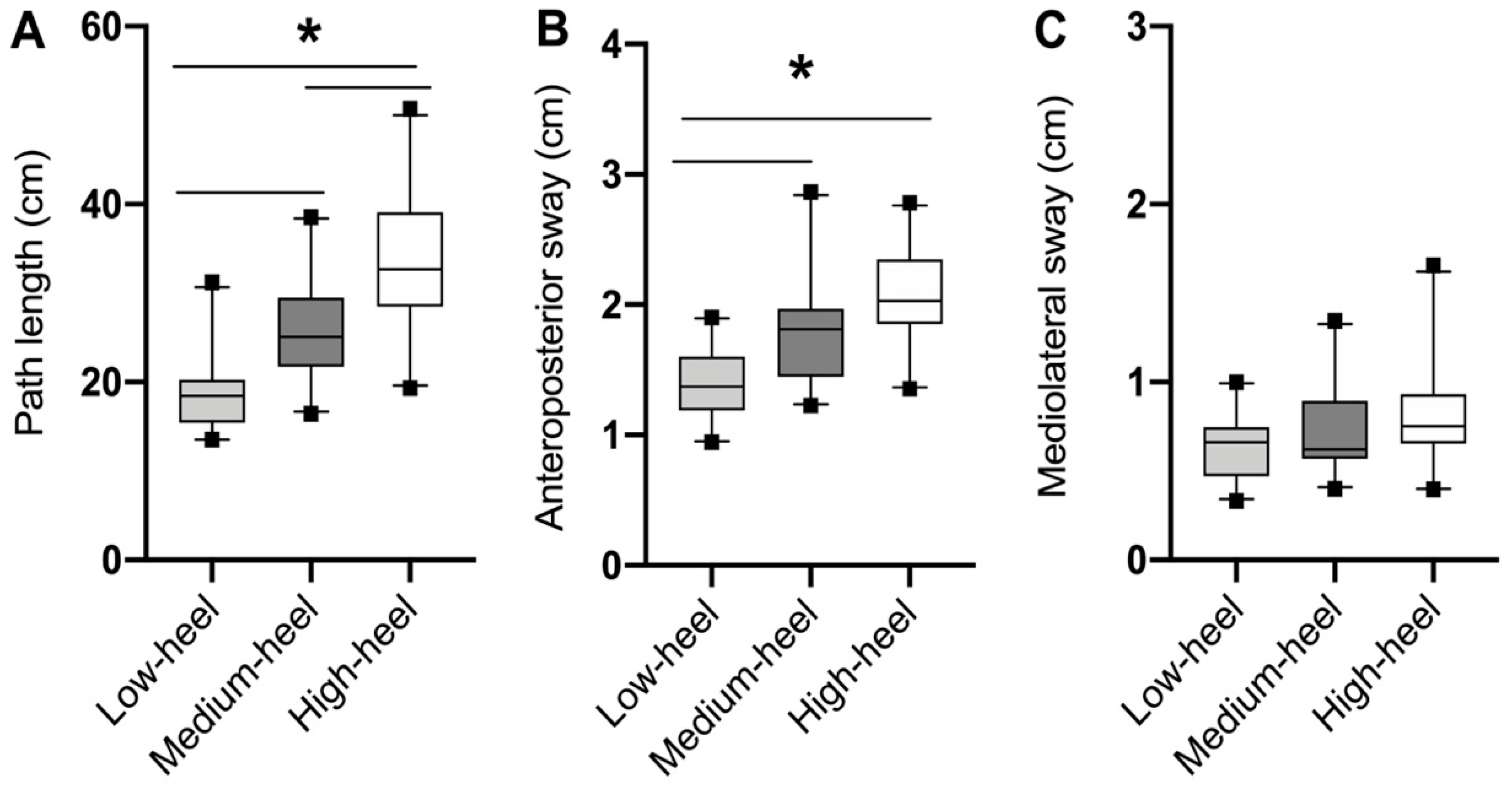
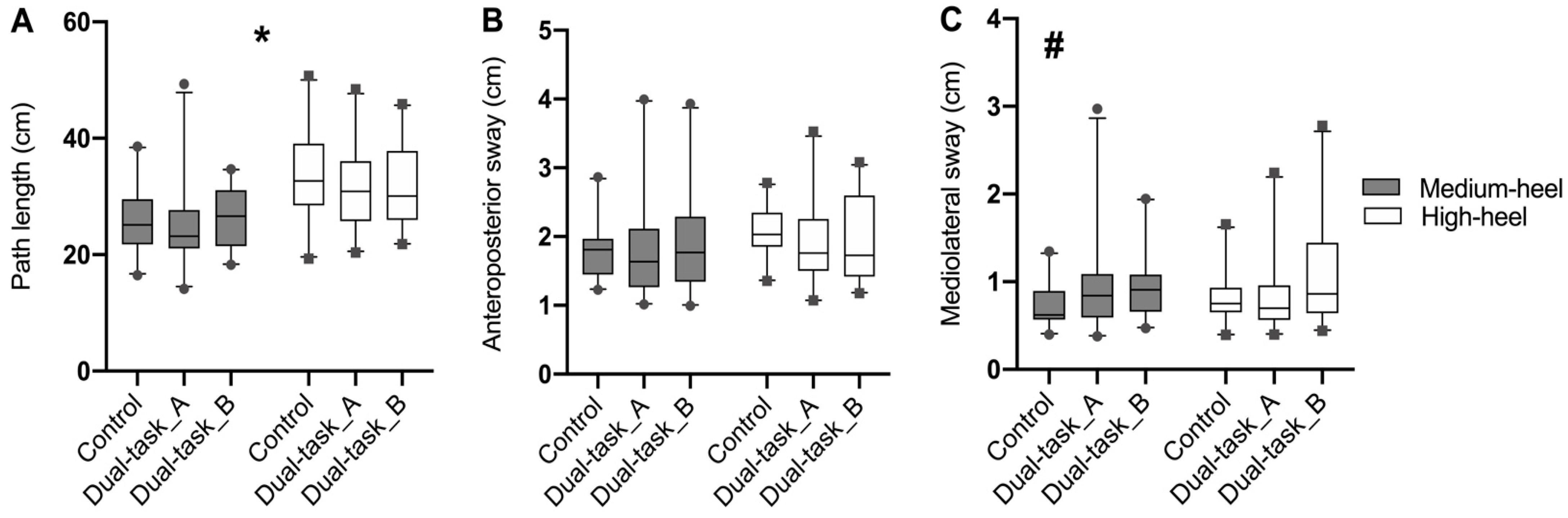
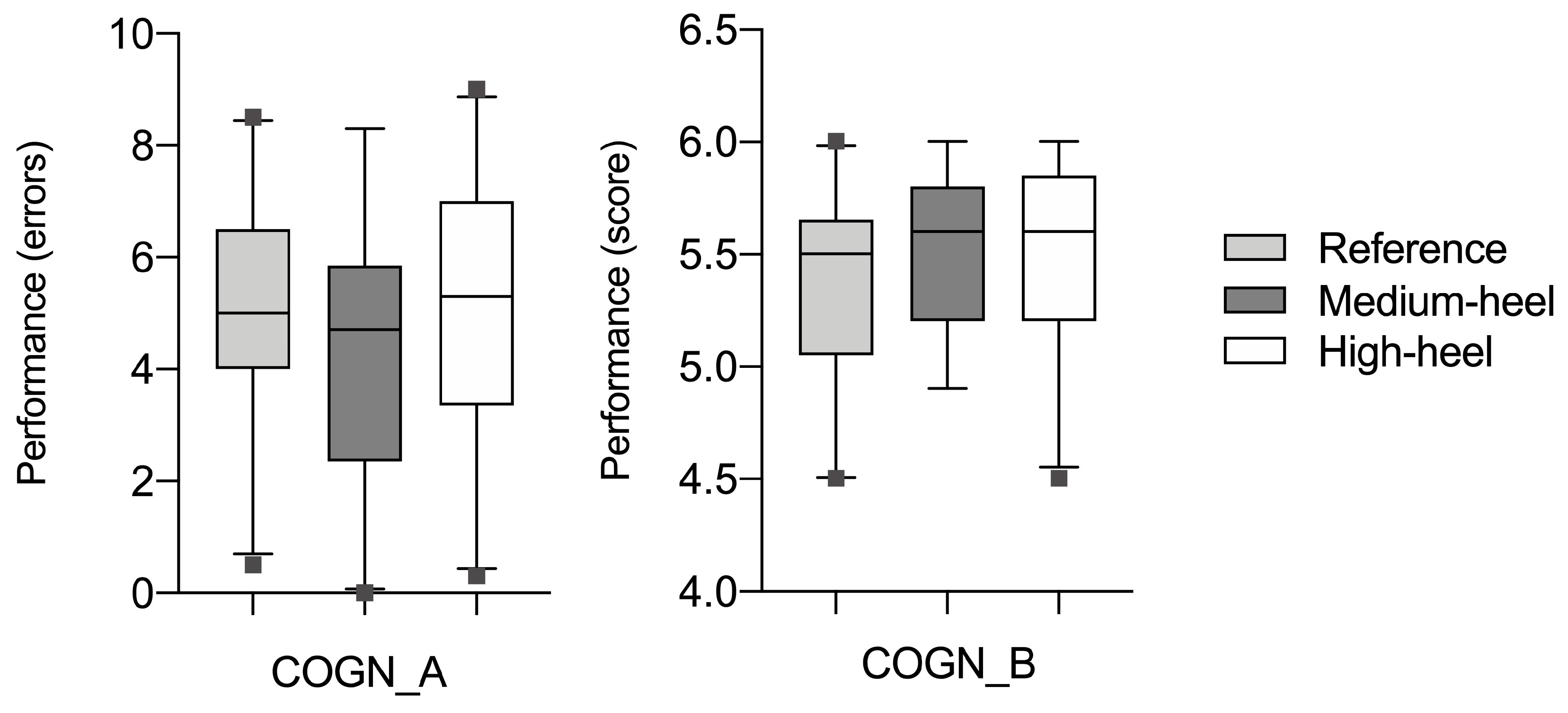
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COGN_A | First cognitive short-term memory task |
| COGN_B | Second cognitive verbal fluency task |
| CoP | Center of pressure |
References
- Opila, K.A.; Wagner, S.S.; Schiowitz, S.; Chen, J. Postural alignment in barefoot and high-heeled stance. Spine 1988, 13, 542–547. [Google Scholar] [CrossRef]
- Snow, R.E.; Williams, K.R. High heeled shoes: Their effect on center of mass position, posture, three-dimensional kinematics, rearfoot motion, and ground reaction forces. Arch. Phys. Med. Rehabil. 1994, 75, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.X.; Wang, L. Influences of heel height on human postural stability and functional mobility between inexperienced and experienced high heel shoe wearers. PeerJ 2020, 8, e10239. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, T.; Buck, F.M.; Jentzsch, T.; Simmen, H.P.; Werner, C.M.; Osterhoff, G. Influence of high-heeled shoes on the sagittal balance of the spine and the whole body. Eur. Spine J. 2016, 25, 3658–3665. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, H.; Quan, W.; Ma, X.; Chon, T.E.; Fernandez, J.; Gusztav, F.; Kovács, A.; Baker, J.S.; Gu, Y. New insights optimize landing strategies to reduce lower limb injury risk. Cyborg. Bionic. Syst. 2024, 5, 0126. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.B.; Costa, R.V.; Grecco, L.A.; Pasini, H.; Marconi, N.F.; Oliveira, C.S. Interference of high-heeled shoes in static balance among young women. Hum. Mov. Sci. 2012, 31, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Oleksy, Ł.; Kielnar, R.; Świerczek, M. The influence of high- and low-heeled shoes on balance in young women. Acta Bioeng. Biomech. 2016, 18, 97–103. [Google Scholar] [PubMed]
- Wan, F.K.W.; Yick, K.L.; Yu, W.W.M. Effects of heel height and high-heel experience on foot stability during quiet standing. Gait Posture 2019, 68, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Yanagawa, A.; Sasagawa, S.; Nakazawa, K.; Ishii, N. Effects of Occasional and Habitual Wearing of High-Heeled Shoes on Static Balance in Young Women. Front. Sports Act. Living 2022, 4, 760991. [Google Scholar] [CrossRef] [PubMed]
- Truszczyńska, A.; Trzaskoma, Z.; Stypinska, Z.; Drzal-Grabiec, J.; Tarnowski, A. Is static balance affected by using shoes of different height? Biomed. Hum. Kinet. 2016, 8, 137–144. [Google Scholar] [CrossRef]
- Albertsen, I.M.; Ghédira, M.; Gracies, J.M.; Hutin, É. Postural stability in young healthy subjects—Impact of reduced base of support, visual deprivation, dual tasking. J. Electromyogr. Kinesiol. 2017, 33, 27–33. [Google Scholar] [CrossRef]
- Dault, M.C.; Geurts, A.C.; Mulder, T.W.; Duysens, J. Postural control and cognitive task performance in healthy participants while balancing on different support-surface configurations. Gait Posture 2001, 14, 248–255. [Google Scholar] [CrossRef]
- Hapsari, V.D.; Xiong, S. Effects of high heeled shoes wearing experience and heel height on human standing balance and functional mobility. Ergonomics 2016, 59, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Gefen, A.; Megido-Ravid, M.; Itzchak, Y.; Arcan, M. Analysis of muscular fatigue and foot stability during high-heeled gait. Gait Posture 2002, 15, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Fraizer, E.V.; Mitra, S. Methodological and interpretive issues in posture-cognition dual-tasking in upright stance. Gait Posture 2008, 27, 271–279. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. Cortical control of postural responses. J. Neural Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Riccio, G.E.; Stoffregen, T.A. Affordances as constraints on the control of stance. Hum. Mov. Sci. 1988, 7, 265–300. [Google Scholar] [CrossRef]
- Wickens, C.D. Processing resources and attention. In Multiple-Task Performance, 1st ed.; Damos, D., Ed.; Taylor-Francis: London, UK, 1991; pp. 3–34. [Google Scholar]
- Schaefer, S. The ecological approach to cognitive-motor dual-tasking: Findings on the effects of expertise and age. Front. Psychol. 2014, 5, 1167. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, Y.; Okada, H.; Yoshikawa, E.; Nobezawa, S.; Futatsubashi, M. Brain activation during maintenance of standing postures in humans. Brain 1999, 122, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, Y.; Okada, H.; Yoshikawa, E.; Futatsubashi, M.; Nobezawa, S. Absolute changes in regional cerebral blood flow in association with upright posture in humans: An orthostatic PET study. J. Nucl. Med. 2001, 42, 707–712. [Google Scholar]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2001, 35, ii7–ii11. [Google Scholar] [CrossRef] [PubMed]
- Peterka, R.J.; Loughlin, P.J. Dynamic regulation of sensorimotor integration in human postural control. J. Neurophysiol. 2004, 91, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.D.M. Understanding Balance: The Mechanics of Posture and Locomotion; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Lacour, M.; Bernard-Demanze, L.; Dumitrescu, M. Posture control, aging, and attention resources: Models and posture-analysis methods. Neurophysiol. Clin. 2008, 38, 411–421. [Google Scholar] [CrossRef]
- Vuillerme, N.; Nafati, G. How attentional focus on body sway affects postural control during quiet standing. Psychol. Res. 2007, 71, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Redfern, M.S.; Jennings, J.R.; Martin, C.; Furman, J.M. Attention influences sensory integration for postural control in older adults. Gait Posture 2001, 14, 211–216. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Woollacott, M. Attentional demands and postural control: The effect of sensory context. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M10–M16. [Google Scholar] [CrossRef] [PubMed]
- Salihu, A.T.; Hill, K.D.; Jaberzadeh, S. Effect of cognitive task complexity on dual task postural stability: A systematic review and meta-analysis. Exp. Brain Res. 2022, 240, 703–731. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effects of dual tasks and dual-task training on postural stability: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 557–577. [Google Scholar] [CrossRef]
- Barra, J.; Bray, A.; Sahni, V.; Golding, J.F.; Gresty, M.A. Increasing cognitive load with increasing balance challenge: Recipe for catastrophe. Exp. Brain Res. 2006, 174, 734–745. [Google Scholar] [CrossRef]
- Dault, M.C.; Yardley, L.; Frank, J.S. Does articulation contribute to modifications of postural control during dual-task paradigms? Brain Res. Cogn. Brain Res. 2003, 16, 434–440. [Google Scholar] [CrossRef]
- Kerr, B.; Condon, S.M.; McDonald, L.A. Cognitive spatial processing and the regulation of posture. J. Exp. Psychol. Hum. Percept. Perform. 1985, 11, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Mujdeci, B.; Turkyilmaz, D.; Yagcioglu, S.; Aksoy, S. The effects of concurrent cognitive tasks on postural sway in healthy subjects. Braz. J. Otorhinolaryngol. 2016, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Woollacott, M.; Kerns, K.A.; Baldwin, M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52A, M232–M240. [Google Scholar] [CrossRef] [PubMed]
- Mersmann, F.; Bohm, S.; Bierbaum, S.; Dietrich, R.; Arampatzis, A. Young and old adults prioritize dynamic stability control following gait perturbations when performing a concurrent cognitive task. Gait Posture 2013, 37, 373–377. [Google Scholar] [CrossRef]
- Small, G.H.; Neptune, R.R. Task-prioritization and balance recovery strategies used by young healthy adults during dual-task walking. Gait Posture 2022, 95, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ruffieux, J.; Keller, M.; Lauber, B.; Taube, W. Changes in Standing and Walking Performance Under Dual-Task Conditions Across the Lifespan. Sports Med. 2015, 45, 1739–1758. [Google Scholar] [CrossRef] [PubMed]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Mov. Disord. 2012, 27, 765–770. [Google Scholar] [CrossRef]
- Linder, S.M.; Koop, M.M.; Ozinga, S.; Goldfarb, Z.; Alberts, J.L. A Mobile Device Dual-Task Paradigm for the Assessment of mTBI. Mil. Med. 2019, 184, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.C.; Woollacott, M.H. Attentional demands of postural control: The ability to selectively allocate information-processing resources. Gait Posture 2007, 25, 121–126. [Google Scholar] [CrossRef]
- Bourlon, C.; Lehenaff, L.; Batifoulier, C.; Bordier, A.; Chatenet, A.; Desailly, E.; Fouchard, C.; Marsal, M.; Martinez, M.; Rastelli, F.; et al. Dual-tasking postural control in patients with right brain damage. Gait Posture 2014, 39, 188–193. [Google Scholar] [CrossRef]
- Al-Yahya, E.; Dawes, H.; Smith, L.; Dennis, A.; Howells, K.; Cockburn, J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 715–728. [Google Scholar] [CrossRef]
- Riley, M.A.; Baker, A.A.; Schmit, J.M.; Weaver, E. Effects of visual and auditory short-term memory tasks on the spatiotemporal dynamics and variability of postural sway. J. Mot. Behav. 2005, 37, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, G.; Georgoudis, G.; Papandreou, M.; Spyropoulos, P.; Georgakopoulos, D.; Kalfakakou, V.; Evangelou, A. Reliability measures of the short International Physical Activity Questionnaire (IPAQ) in Greek young adults. Hell. J. Cardiol. 2009, 50, 283–294. [Google Scholar]
- Prieto, T.E.; Myklebust, J.B.; Hoffmann, R.G.; Lovett, E.G.; Myklebust, B.M. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Wickens, C.D. Multiple resources and performance prediction. Theor. Issues Ergonom. Sci. 2002, 3, 159–177. [Google Scholar] [CrossRef]
- Blanca, M.J.; Arnau, J.; García-Castro, F.J.; Alarcón, R.; Bono, R. Repeated measures ANOVA and adjusted F-tests when sphericity is violated: Which procedure is best? Front. Psychol. 2023, 14, 1192453. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: Oxfordshire, UK, 1988. [Google Scholar]
- Boisgontier, M.P.; Beets, I.A.; Duysens, J.; Nieuwboer, A.; Krampe, R.T.; Swinnen, S.P. Age-related differences in attentional cost associated with postural dual tasks: Increased recruitment of generic cognitive resources in older adults. Neurosci. Biobehav. Rev. 2013, 37, 1824–1837. [Google Scholar] [CrossRef] [PubMed]
- Bustillo-Casero, P.; Villarrasa-Sapiña, I.; García-Massó, X. Effects of dual task difficulty in motor and cognitive performance: Differences between adults and adolescents. Hum. Mov. Sci. 2017, 55, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Tokur, D.; Grimmer, M.; Seyfarth, A. Review of balance recovery in response to external perturbations during daily activities. Hum. Mov. Sci. 2020, 69, 102546. [Google Scholar] [CrossRef]
- Jakubowski, K.L.; Martino, G.; Beck, O.N.; Sawicki, G.S.; Ting, L.H. Center of mass states render multijoint torques throughout standing balance recovery. J. Neurophysiol. 2025, 133, 206–221. [Google Scholar] [CrossRef]
- Bohm, S.; Mademli, L.; Mersmann, F.; Arampatzis, A. Predictive and reactive locomotor adaptability in healthy elderly: A systematic review and meta-analysis. Sports Med. 2015, 45, 1759–1777. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, A.; Wollesen, B.; Asamoah, J.; Delbaere, K.; Li, K. The impact of cognitive-motor interference on balance and gait in hearing-impaired older adults: A systematic review. Eur. Rev. Aging. Phys. Act. 2024, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, Z.G.; Yilmaz, U.; Celik, H.; Arpinar-Avsar, P.; Kirazci, S. Effect of individualized cognitive and postural task difficulty levels on postural control during dual task condition. Gait Posture 2022, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Potvin-Desrochers, A.; Richer, N.; Lajoie, Y. Cognitive tasks promote automatization of postural control in young and older adults. Gait Posture 2017, 57, 40–45. [Google Scholar] [CrossRef]
- Polskaia, N.; Richer, N.; Dionne, E.; Lajoie, Y. Continuous cognitive task promotes greater postural stability than an internal or external focus of attention. Gait Posture 2015, 41, 454–458. [Google Scholar] [CrossRef]

| Variable | |
|---|---|
| Age (years) | 37.8 ± 10.7 |
| Body Mass (kg) | 66.5 ± 10.2 |
| Height (cm) | 169.0 ± 6.3 |
| Body Mass Index (kg/m2) | 23.2 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaidou, M.-E.; Dervenioti, A.; Schroll, A.; Arampatzis, A. The Mechanically Destabilizing Effect of Increased Heel Height in Women Is Not Enhanced by Dual-Task Interference. Appl. Sci. 2025, 15, 4485. https://doi.org/10.3390/app15084485
Nikolaidou M-E, Dervenioti A, Schroll A, Arampatzis A. The Mechanically Destabilizing Effect of Increased Heel Height in Women Is Not Enhanced by Dual-Task Interference. Applied Sciences. 2025; 15(8):4485. https://doi.org/10.3390/app15084485
Chicago/Turabian StyleNikolaidou, Maria-Elissavet, Aikaterini Dervenioti, Arno Schroll, and Adamantios Arampatzis. 2025. "The Mechanically Destabilizing Effect of Increased Heel Height in Women Is Not Enhanced by Dual-Task Interference" Applied Sciences 15, no. 8: 4485. https://doi.org/10.3390/app15084485
APA StyleNikolaidou, M.-E., Dervenioti, A., Schroll, A., & Arampatzis, A. (2025). The Mechanically Destabilizing Effect of Increased Heel Height in Women Is Not Enhanced by Dual-Task Interference. Applied Sciences, 15(8), 4485. https://doi.org/10.3390/app15084485



_Nikolaidou.jpg)




