Non-Thermal Plasma-Catalytic Conversion of Biogas to Value-Added Liquid Chemicals via Ni-Fe/Al2O3 Catalyst
Abstract
1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
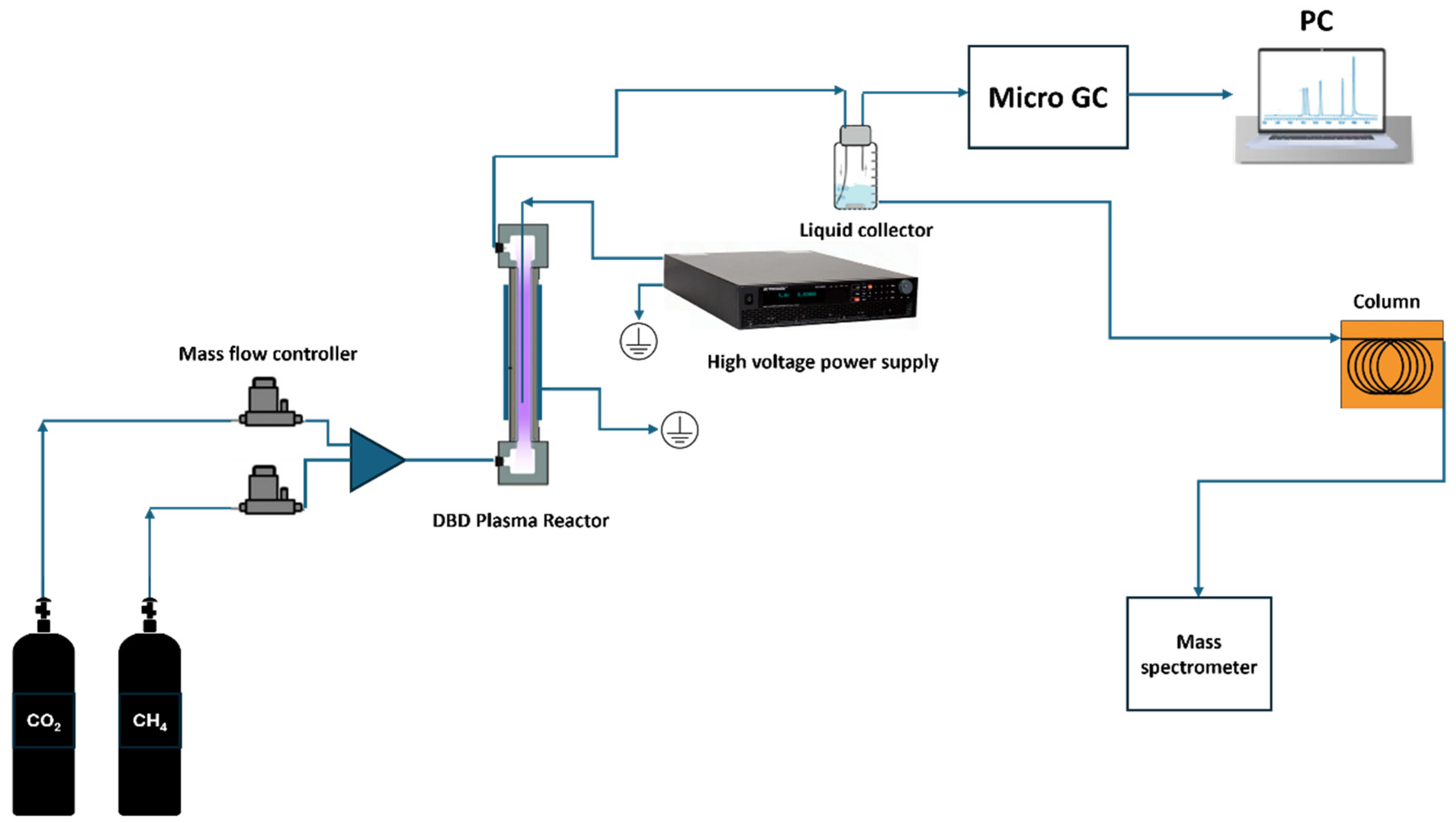
2.2. NTP-Catalytic Conversion of Biogas
2.3. Catalyst Characterization Techniques
3. Results and Discussion
3.1. Catalyst Characterization
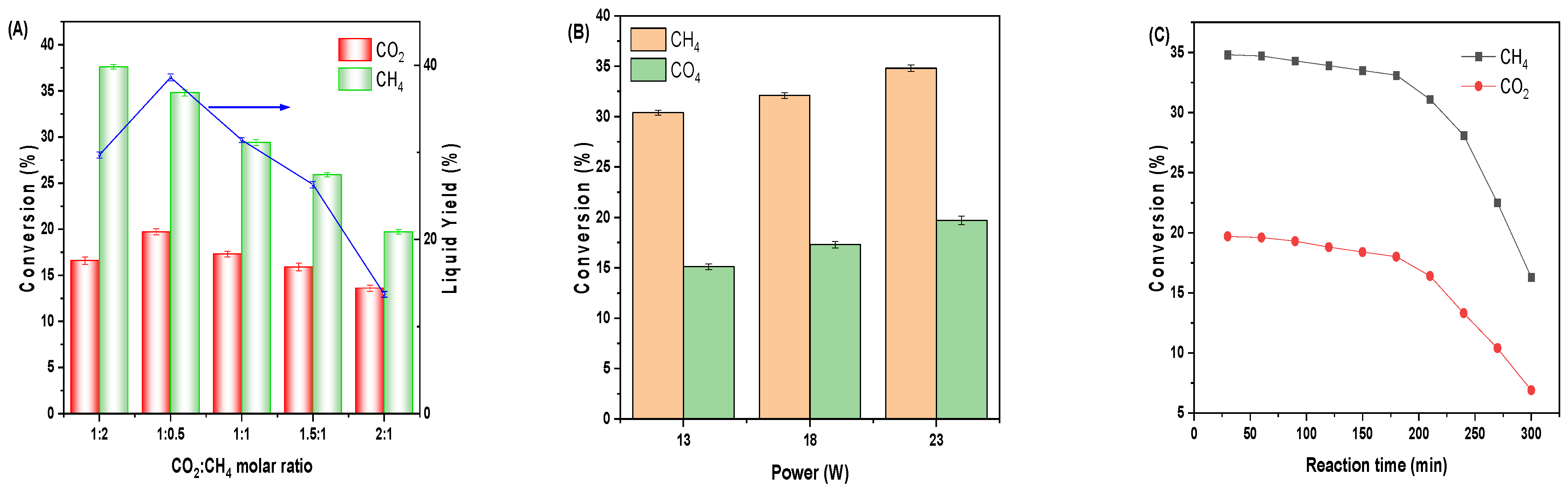
3.2. Plasma-Catalytic Conversion of Biogas
3.3. Comparison with Previous Plasma Catalytic Studies and Other Conversion Technologies
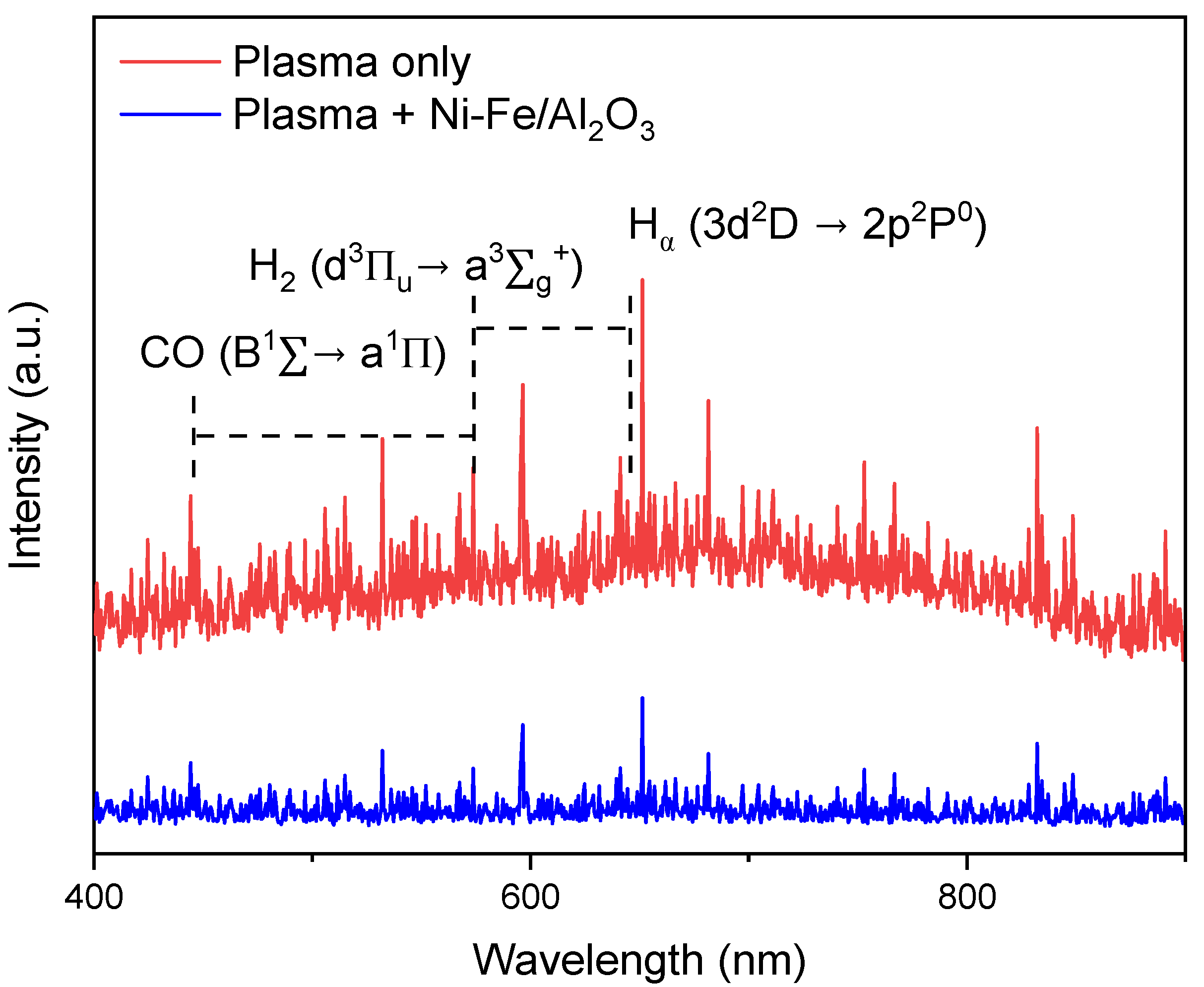
3.4. In Situ OES in Plasma-Catalytic Biogas Conversion
3.5. Sustainability and Scalability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turan, V.; Schröder, P.; Bilen, S.; Insam, H.; Juárez, M.F.-D. Co-inoculation effect of Rhizobium and Achillea millefolium L. oil extracts on growth of common bean (Phaseolus vulgaris L.) and soil microbial-chemical properties. Sci. Rep. 2019, 9, 15178. [Google Scholar] [CrossRef] [PubMed]
- Marun, C.; Conde, L.D.; Suib, S.L. Catalytic oligomerization of methane via microwave heating. J. Phys. Chem. A 1999, 103, 4332–4340. [Google Scholar] [CrossRef]
- Domínguez, A.; Fidalgo, B.; Fernández, Y.; Pis, J.J.; Menéndez, J.A. Microwave-assisted catalytic decomposition of methane over activated carbon for CO2-free hydrogen production. Int. J. Hydrogen Energy 2007, 32, 4792–4799. [Google Scholar] [CrossRef]
- Puliyalil, H.; Jurković, D.L.; Dasireddy, V.D.B.C.; Likozar, B. A review of plasma-assisted catalytic conversion of gaseous carbon dioxide and methane into value-added platform chemicals and fuels. RSC Adv. 2018, 8, 27481–27508. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, A.; Tu, X.; Whitehead, J.C.; Centi, G.; Lefferts, L.; Guaitella, O.; Azzolina-Jury, F.; Kim, H.-H.; Murphy, A.B.; Schneider, W.F. The 2020 plasma catalysis roadmap. J. Phys. D Appl. Phys. 2020, 53, 443001. [Google Scholar] [CrossRef]
- Heintze, M.; Pietruszka, B. Plasma catalytic conversion of methane into syngas: The combined effect of discharge activation and catalysis. Catal. Today 2004, 89, 21–25. [Google Scholar] [CrossRef]
- Mao, S.; Tan, Z.; Zhang, L.; Huang, Q. Plasma-assisted biogas reforming to syngas at room temperature condition. J. Energy Inst. 2018, 91, 172–183. [Google Scholar] [CrossRef]
- Mei, D.; Ashford, B.; He, Y.; Tu, X. Plasma-catalytic reforming of biogas over supported Ni catalysts in a dielectric barrier discharge reactor: Effect of catalyst supports. Plasma Process. Polym. 2017, 14, 1600076. [Google Scholar] [CrossRef]
- Yap, D.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. Catalyst assisted by non-thermal plasma in dry reforming of methane at low temperature. Catal. Today 2018, 299, 263–271. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Wu, C.; Guo, H.; Tu, X. One-step reforming of CO2 and CH4 into high-value liquid chemicals and fuels at room temperature by plasma-driven catalysis. Angew. Chem. 2017, 129, 13867–13871. [Google Scholar] [CrossRef]
- Sentek, J.; Krawczyk, K.; Młotek, M.; Kalczewska, M.; Kroker, T.; Kolb, T.; Schenk, A.; Gericke, K.-H.; Schmidt-Szałowski, K. Plasma-catalytic methane conversion with carbon dioxide in dielectric barrier discharges. Appl. Catal. B 2010, 94, 19–26. [Google Scholar] [CrossRef]
- Li, D.; Rohani, V.; Fabry, F.; Ramaswamy, A.P.; Sennour, M.; Fulcheri, L. Direct conversion of CO2 and CH4 into liquid chemicals by plasma-catalysis. Appl. Catal. B 2020, 261, 118228. [Google Scholar] [CrossRef]
- Li, J.; Dou, L.; Gao, Y.; Hei, X.; Yu, F.; Shao, T. Revealing the active sites of the structured Ni-based catalysts for one-step CO2/CH4 conversion into oxygenates by plasma-catalysis. J. CO2 Util. 2021, 52, 101675. [Google Scholar] [CrossRef]
- Wang, A.; Harrhy, J.H.; Meng, S.; He, P.; Liu, L.; Song, H. Nonthermal plasma-catalytic conversion of biogas to liquid chemicals with low coke formation. Energy Convers. Manag. 2019, 191, 93–101. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Dai, J.; Zhang, Z.; Zhang, Z.; Yu, H.; Liu, L. CH4 and CO2 conversion over boron nitride-supported Ni catalysts with BO defects in DBD plasma. Fuel Process. Technol. 2023, 242, 107655. [Google Scholar] [CrossRef]
- Martín-Espejo, J.L.; Gandara-Loe, J.; Odriozola, J.A.; Reina, T.R.; Pastor-Pérez, L. Sustainable routes for acetic acid production: Traditional processes vs a low-carbon, biogas-based strategy. Sci. Total Environ. 2022, 840, 156663. [Google Scholar] [CrossRef]
- Wu, H.; Guo, L.; Ma, F.; Wang, Y.; Mo, W.; Fan, X.; Li, H.; Yu, Y.; Mian, I.; Tsubaki, N. Structure and surface characteristics of Fe-promoted Ni/Al2O3 catalysts for hydrogenation of 1,4-butynediol to 1,4-butenediol in a slurry-bed reactor. Catal. Sci. Technol. 2019, 9, 6598–6605. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, F.; Li, X.; Wen, J.Z.; Li, Z. Factors controlling nanosized Ni–Al2O3 catalysts synthesized by solution combustion for slurry-phase CO methanation: The ratio of reducing valences to oxidizing valences in redox systems. Catal. Sci. Technol. 2016, 6, 7800–7811. [Google Scholar] [CrossRef]
- Salmasi, M.Z.; Kazemeini, M.; Sadjadi, S.; Nematollahi, R. Spinel MgAl2O4 nanospheres coupled with modified graphitic carbon nitride nanosheets as an efficient Z-scheme photocatalyst for photodegradation of organic contaminants. Appl. Surf. Sci. 2022, 585, 152615. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Wang, L.; Wu, C.F.; Wang, J.Q.; Shen, B.X.; Tu, X. Low temperature reforming of biogas over K-, Mg-and Ce-promoted Ni/Al2O3 catalysts for the production of hydrogen rich syngas: Understanding the plasma-catalytic synergy. Appl. Catal. B 2018, 224, 469–478. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Dry reforming of methane using different dielectric materials and DBD plasma reactor configurations. Energy Convers. Manag. 2017, 144, 262–274. [Google Scholar] [CrossRef]
- Tu, X.; Whitehead, J.C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: Understanding the synergistic effect at low temperature. Appl. Catal. B 2012, 125, 439–448. [Google Scholar] [CrossRef]
- Zou, J.-J.; Zhang, Y.; Liu, C.-J.; Li, Y.; Eliasson, B. Starch-enhanced synthesis of oxygenates from methane and carbon dioxide using dielectric-barrier discharges. Plasma Chem. Plasma Process. 2003, 23, 69–82. [Google Scholar] [CrossRef]
- Tu, X.; Gallon, H.J.; Twigg, M.V.; Gorry, P.A.; Whitehead, J.C. Dry reforming of methane over a Ni/Al2O3 catalyst in a coaxial dielectric barrier discharge reactor. J. Phys. D Appl. Phys. 2011, 44, 274007. [Google Scholar] [CrossRef]
- Zhang, K.; Mukhriza, T.; Liu, X.; Greco, P.P.; Chiremba, E. A study on CO2 and CH4 conversion to synthesis gas and higher hydrocarbons by the combination of catalysts and dielectric-barrier discharges. Appl. Catal. A Gen. 2015, 502, 138–149. [Google Scholar] [CrossRef]
- Zambrano, G.; Riascos, H.; Prieto, P.; Restrepo, E.; Devia, A.; Rincón, C. Optical emission spectroscopy study of rf magnetron sputtering discharge used for multilayers thin film deposition. Surf. Coat. Technol. 2003, 172, 144–149. [Google Scholar] [CrossRef]
- Kholodkov, A.V.; Golant, K.M.; Nikolin, I.V. Nano-scale compositional lamination of doped silica glass deposited in surface discharge plasma of SPCVD technology. Microelectron. Eng. 2003, 69, 365–372. [Google Scholar] [CrossRef]
- Czerwiec, T.; Gavillet, J.; Belmonte, T.; Michel, H.; Ricard, A. Determination of O atom density in Ar-O2 and Ar-O2-H2 flowing microwave discharges. Surf. Coat. Technol. 1998, 98, 1411–1415. [Google Scholar] [CrossRef]
- Granier, A.; Vervloet, M.; Aumaille, K.; Vallée, C. Optical emission spectra of TEOS and HMDSO derived plasmas used for thin film deposition. Plasma Sources Sci. Technol. 2003, 12, 89. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, X.; Jafarzadeh, A.; Wang, L.; Liu, P.; He, B.; Yan, J.; Zhang, R.; Zhang, H.; Liu, X. Plasma-catalytic ammonia reforming of methane over Cu-based catalysts for the production of HCN and H2 at reduced temperature. ACS Catal. 2021, 11, 1765–1773. [Google Scholar] [CrossRef]

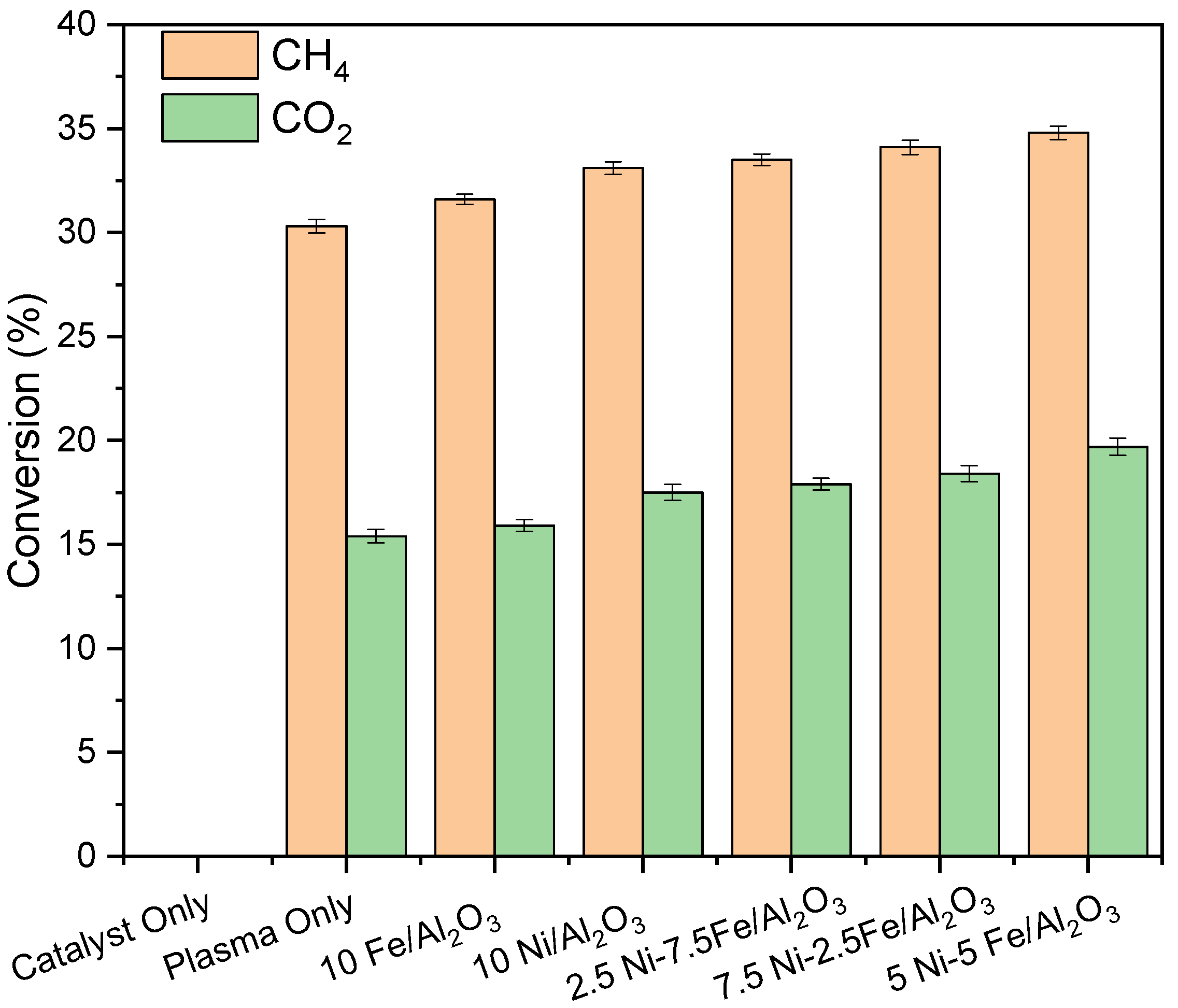
| Catalyst | Nominal Loading (wt/wt) % | Experimental Loading (wt/wt) % | ||
|---|---|---|---|---|
| Ni | Fe | Ni | Fe | |
| Ni/Al2O3 | 10 | 0 | 7.1 | 0 |
| Fe/Al2O3 | 0 | 10 | 0 | 6.9 |
| Ni-Fe/Al2O3 | 5 | 5 | 3.1 | 3.0 |
| Catalyst | Weak (<250 °C) mmol/g | Medium and Strong (>250 °C) mmol/g | Total Acid Sites (mmol/g) |
|---|---|---|---|
| Al2O3 | 0.31 | 0.59 | 0.90 |
| Ni/Al2O3 | 0.35 | 0.13 | 0.48 |
| Fe/Al2O3 | 0.32 | 0.11 | 0.43 |
| Ni-Fe/Al2O3 | 0.33 | 0.09 | 0.42 |
| Run | H2 Yield (%) | CO Yield (%) | CxHy Yield (%) | Liquid Yield (%) | Coke (%) |
|---|---|---|---|---|---|
| Catalyst only | 0 | 0 | 0 | 0 | 0 |
| Plasma only | 48.9 | 29.2 | 26.7 | 0 | 44.1 |
| 10Fe/Al2O3 | 44.2 | 28.4 | 25.9 | 30.9 | 14.8 |
| 10Ni/Al2O3 | 43.3 | 27.8 | 25.1 | 34.0 | 13.1 |
| 2.5Ni-7.5Fe/Al2O3 | 42.4 | 27.5 | 24.6 | 35.6 | 12.3 |
| 7.5Ni-2.5Fe/Al2O3 | 41.7 | 27.3 | 24.3 | 36.7 | 11.7 |
| 5Ni-5Fe/Al2O3 | 40.1 | 27.1 | 23.9 | 38.6 | 10.4 |
| Chemical | Formaldehyde | Isopropyl Alcohol | Ethanol | Methanol | Propanoic Acid | Acetic Acid |
|---|---|---|---|---|---|---|
| Selectivity (%) | 10.5 | 15.7 | 14.5 | 19.1 | 16.3 | 23.9 |
| Catalyst | Parameters | CH4 Conv. (%) | CO2 Conv. (%) | Remarks | Ref. |
|---|---|---|---|---|---|
| SiO2 | 6 g, 9.25 kV P-P | 17.0 | 10.9 | The plasma-assisted catalytic biogas conversion was studied for value-added liquid chemicals; 0% liquid yield was achieved. | [14] |
| Ag/CZSM5 | 6 g, 9.25 kV P-P | 18.7 | 13.6 | The plasma-assisted catalytic biogas conversion was studied for value-added liquid chemicals; 33.1% liquid yield was achieved. | [14] |
| Ir/CZSM5 | 6 g, 9.25 kV P-P | 21.2 | 12.8 | The plasma-assisted catalytic biogas conversion was studied for value-added liquid chemicals; 25.0% liquid yield was achieved. | [14] |
| Ni/Al2O3 | 0.25 g, 50 W | 18 | 13 | The plasma-assisted catalytic DRM was investigated for the discharge behavior characteristics. The Ni-dispersed catalyst contributes to the expansion of discharges and enhancement of DRM activity with enhanced product distribution. | [24] |
| BaTiO3 | 0.2 g, 86 W | 28 | 17 | While BaTiO3 led to a higher hydrogen (H2) yield, Ni/SiO2 and Ni-Fe/SiO2 catalysts demonstrated greater selectivity for carbon monoxide (CO), indicating the enhancement of the reverse water-gas shift (RWGS) reaction. The catalyst was packed within the 100 cm3 discharge volume of the reactor used in this investigation. | [25] |
| Ni/SiO2 | 0.2 g, 86 W | 26 | 16 | ||
| Ni-Fe/SiO2 | 0.2 g, 86 W | 22 | 14 | ||
| 5Ni-5Fe/Al2O3 | 0.5 g, 23 W | 34.8 | 19.7 | The plasma-assisted catalytic biogas conversion was studied for value-added liquid chemicals; 38.6% liquid yield was achieved. | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmasi, M.Z.; Es’haghian, R.; Omidkar, A.; Song, H. Non-Thermal Plasma-Catalytic Conversion of Biogas to Value-Added Liquid Chemicals via Ni-Fe/Al2O3 Catalyst. Appl. Sci. 2025, 15, 4248. https://doi.org/10.3390/app15084248
Salmasi MZ, Es’haghian R, Omidkar A, Song H. Non-Thermal Plasma-Catalytic Conversion of Biogas to Value-Added Liquid Chemicals via Ni-Fe/Al2O3 Catalyst. Applied Sciences. 2025; 15(8):4248. https://doi.org/10.3390/app15084248
Chicago/Turabian StyleSalmasi, Milad Zehtab, Razieh Es’haghian, Ali Omidkar, and Hua Song. 2025. "Non-Thermal Plasma-Catalytic Conversion of Biogas to Value-Added Liquid Chemicals via Ni-Fe/Al2O3 Catalyst" Applied Sciences 15, no. 8: 4248. https://doi.org/10.3390/app15084248
APA StyleSalmasi, M. Z., Es’haghian, R., Omidkar, A., & Song, H. (2025). Non-Thermal Plasma-Catalytic Conversion of Biogas to Value-Added Liquid Chemicals via Ni-Fe/Al2O3 Catalyst. Applied Sciences, 15(8), 4248. https://doi.org/10.3390/app15084248







