1. Introduction
Out-of-hospital cardiac arrest is a significant medical emergency, affecting more than 350,000 individuals annually in the United States. Despite extensive advancements in emergency care, the survival rates for these events remain low: less than 23% of patients who experience out-of-hospital cardiac arrest survive long enough to be admitted to the hospital, and only about 10% ultimately survive to hospital discharge [
1]. Among those who do survive, brain injury remains a leading cause of morbidity and mortality, with over two-thirds of survivors enduring irreversible neurological damage during their hospital stay. These dire outcomes highlight the urgent need for a deeper understanding of the physiological changes in the brain during and after cardiac arrest. Recent reviews of clinical trials registered on ClinicalTrials.gov highlight that most of the out-of-hospital cardiac arrest (OHCA) studies focus on interventional procedures, devices, and drugs, with a growing emphasis on neurological monitoring strategies [
2].
The brain’s vulnerability to oxygen deprivation during cardiac arrest makes it particularly susceptible to ischemic injury [
3]. Any disruption in oxygen delivery can lead to severe consequences, including secondary brain injury in conditions such as ischemia, severe traumatic brain injury, and sub-arachnoid hemorrhage [
4]. Post-cardiac arrest brain injury is the leading cause of mortality in patients revived from cardiac arrest and the primary cause of long-term disability in survivors [
5]. Cardiac arrest (CA) and cardiopulmonary resuscitation (CPR) introduce significant fluctuations in brain oxygen metabolism, posing a severe challenge to preserving cerebral function during these critical events.
Brain function relies heavily on the precise balance between its metabolic needs, the adequate delivery of oxygen and nutrients, and the efficient removal of cellular waste. Achieving this balance necessitates the constant regulation of the cerebral blood flow (
CBF) [
6]. The rapid and precise restoration of the
CBF and oxygenation is crucial for preserving brain function, yet the complex interactions between cerebral perfusion, oxygen metabolism, and neuronal survival during these critical moments are not fully understood. Monitoring cerebral perfusion parameters—such as the cerebral tissue oxygen saturation, the cerebral blood volume, the
CBF, the cerebral metabolic rate of oxygen (
CMRO2), and redox cytochrome c oxidase (
rCCO) activity—is essential for improving patient outcomes. These metrics offer vital insights into the brain’s oxygen metabolism, a process critical for maintaining cerebral health, especially under the stress conditions of cardiac arrest.
Understanding the relationship between the
CMRO2, mitochondrial activity, and oxygen delivery is crucial for assessing cerebral health and function. Mitochondrial activity, particularly involving
rCCO, plays a key role in oxygen consumption within the electron transport chain, with its oxidation state influenced by factors such as the oxygen availability and electron flow [
4,
7,
8,
9]. This understanding provides insight into neurovascular coupling and how these processes are altered in pathological conditions. It is a key step in neurocritical care, as it can guide interventions aimed at preserving brain function and reducing neurological damage.
Advancements in measurement techniques have enhanced our ability to quantify rCCO, with hyperspectral near-infrared spectroscopy (hNIRS) providing detailed in-sights into its activity. This advancement is critical for understanding how rCCO activity correlates with oxygen delivery and consumption. However, not everyone has access to these sorts of technologies. To overcome this issue, mathematical models play a huge role. Mathematical models have been employed to study the interactions among CBF, CMRO2, and rCCO, providing a framework to understand these relationships more comprehensively. These models help in analyzing how changes in one parameter can affect the others, offering deeper insights into neurovascular coupling and alterations in pathological conditions.
Previous studies have explored the coupling between oxygen consumption, mitochondrial function, and oxygen delivery in the brain. For instance, research utilizing a multimodal NIRS-MRI approach has quantified the interplay between
CBF,
CMRO2, and the redox state of
rCCO in response to physiological challenges such as hypercapnia and varying oxygenation levels [
10]. This research has revealed that the relationship between
CBF and
rCCO oxidation is dynamic and can vary depending on the type of physiological challenge, thereby providing valuable insights into the complex dynamics of neurovascular coupling under different conditions [
10].
Some researchers employed advanced imaging techniques to probe these relationships with greater precision. For example, functional MRI (fMRI) studies in macaques measured the laminar ∆
CMRO2 in the primary visual cortex during visual stimulation, showing that ∆
CMRO2 peaked in the middle cortical layers, which corresponded with autoradiographic measures of metabolism [
11]. This study also highlighted a strong relationship between the peak
CMRO2 and the highest cytochrome oxidase intensity, further refining our understanding of oxygen metabolism in the brain.
In addition, research using an hNIRS-DCS (Diffuse Correlation Spectroscopy) setup has investigated the interdependence of
CBF,
CMRO2, and
rCCO during minor perturbations such as hypoxia [
12]. These studies have focused on the brain’s responses to less extreme conditions, offering a nuanced understanding of how these critical variables interact in relatively stable environments.
In the present study, we analyzed the data obtained invasively from a pig model of cardiac arrest to examine the intricate relationships between rCCO, CMRO2, and CBF during CA and CPR. We used laser Doppler flowmetry (LDF) to measure CBF and intracerebral hNIRS to assess changes in oxyhemoglobin ([HbO2]), deoxyhemoglobin ([HHb]), and rCCO. These measurements provided insights into variations in the cerebral blood volume (CBV), rCCO, and the CMRO2 throughout the critical phases of cardiac arrest and resuscitation. By investigating these relationships in a model that closely mimics the human condition, we aimed to deepen the understanding of the brain’s metabolic responses to such critical events. Ultimately, the findings of this research could contribute to the development of more effective strategies for managing brain injury in cardiac arrest patients, potentially improving survival rates and long-term neurological outcomes.
3. Results
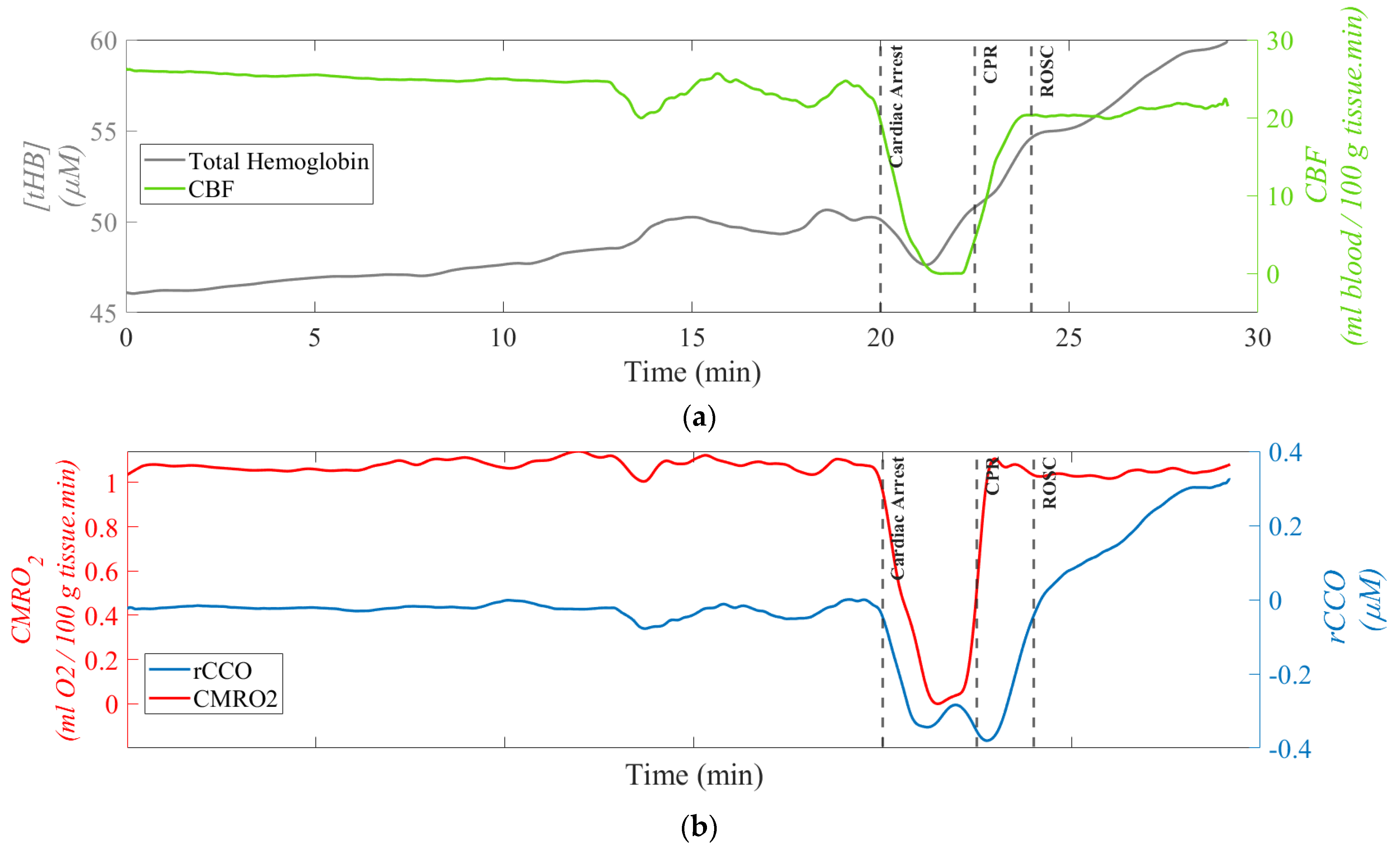
Figure 3 presents representative time traces of
[tHB],
rCCO,
CMRO2, and
CBF for one of the pigs, serving as an example of absolute value calculations as well as the normalized
CBF, rCCO, and
CMRO2 in pairs. The top two plots show
[tHB] versus
CBF and
rCCO versus
CMRO2 in absolute values.
[tHB] and
rCCO values are given in
μM, while
CBF and
CMRO2 are expressed in mL blood/100 g tissue/min and mL
O2/100 g tissue/min, respectively.
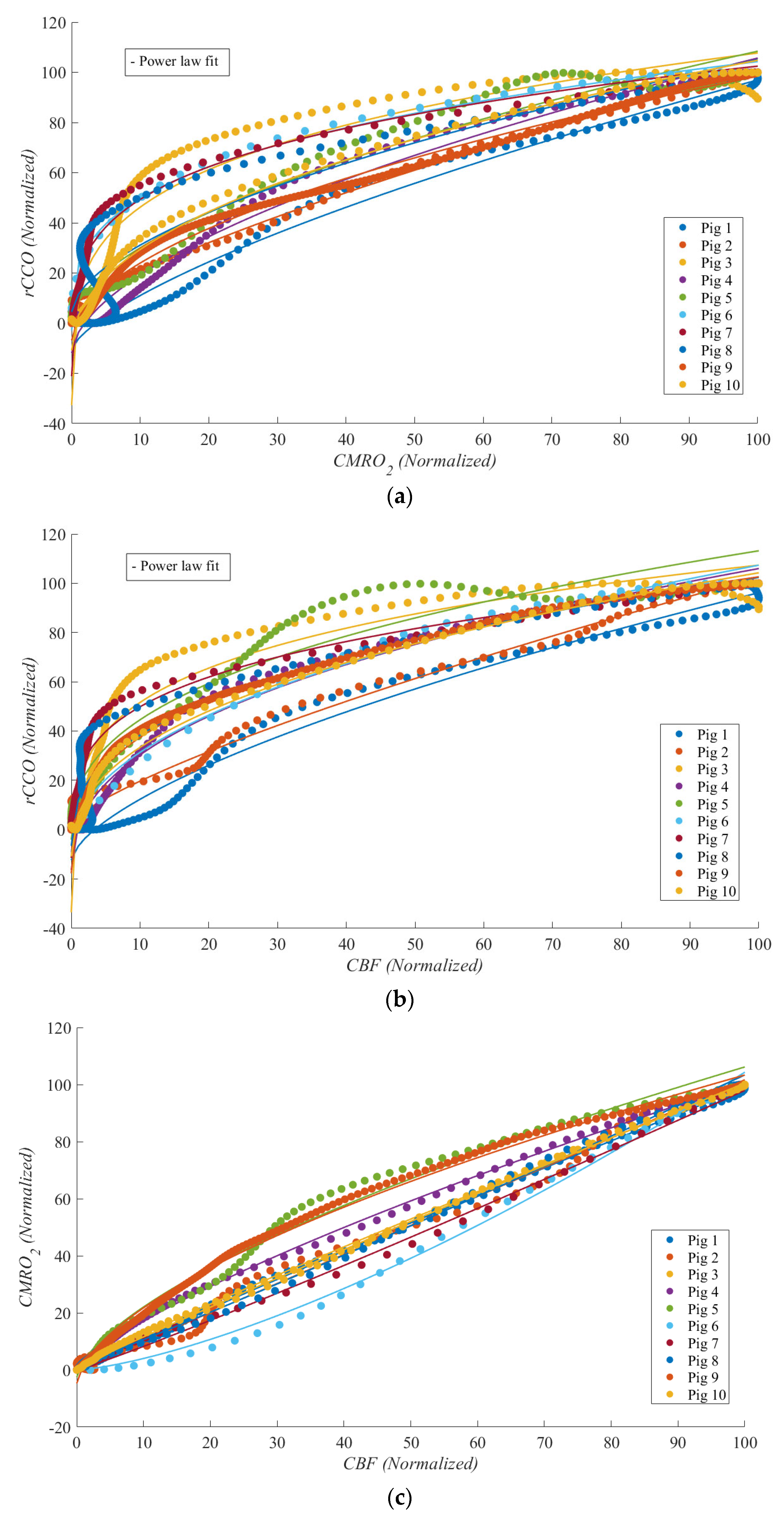
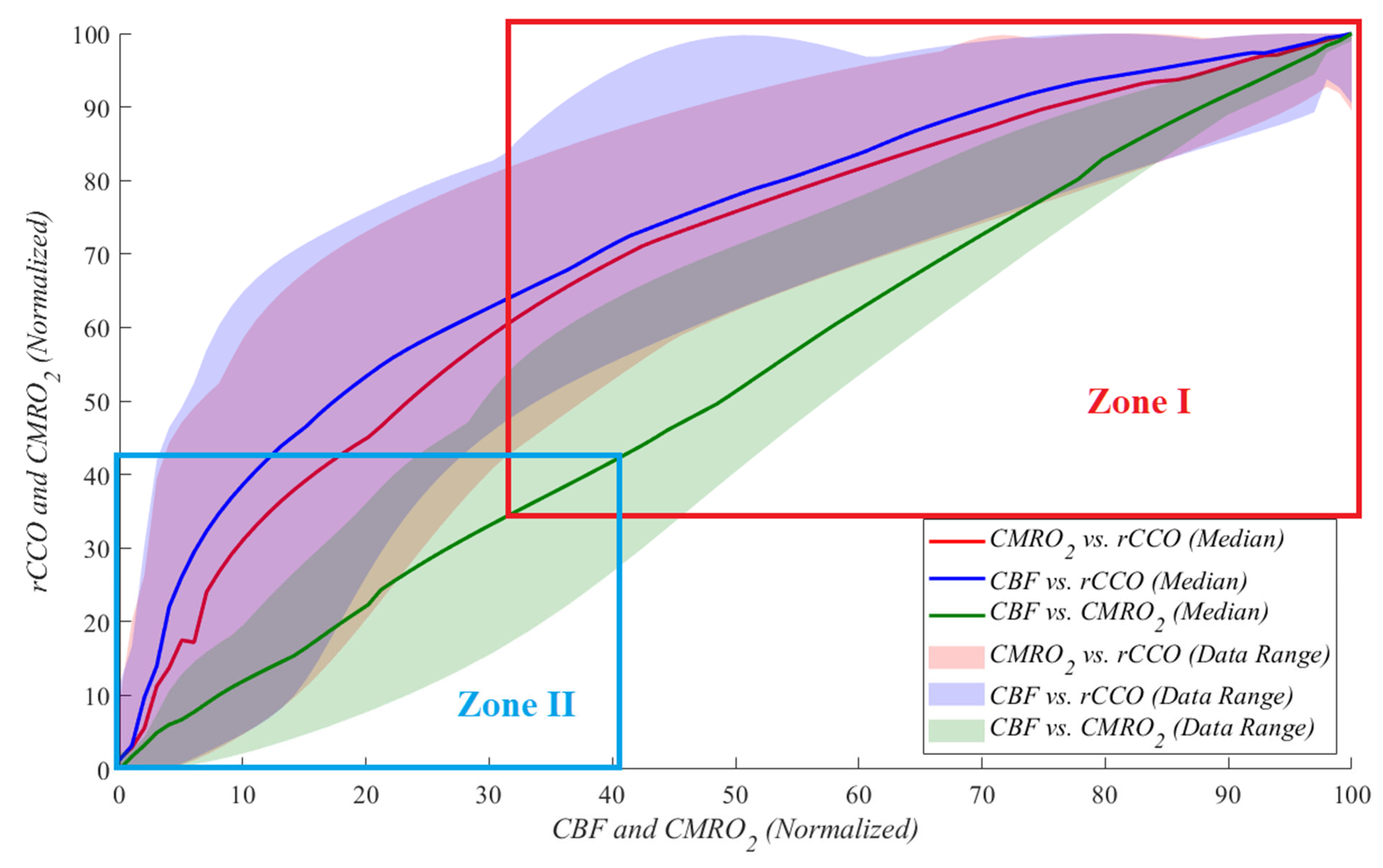
Figure 4 illustrates these relationships during cardiac arrest, with each dataset normalized to its maximum value for comparability. Each relationship is represented by a unique color, with median lines shown as solid lines and data ranges depicted by shaded areas. These plots highlight the linear and non-linear dynamics between
rCCO,
CMRO2, and
CBF under the extreme physiological conditions of cardiac arrest.
Table 1 presents the values of the power law fit
R2, power law
γ means ± standard deviations,
t-test
p-values, and statistical power corresponding to
Figure 4. From
Table 1, one can see that for the
CMRO2 vs.
rCCO and CBF vs.
rCCO (
Figure 4a and
Figure 4b, respectively) the “square-root” type of relationship (
γ ≈ 0.5) was established with high statistical significance and power, despite a relatively low sample size of 10.
To further analyze the data shown in
Figure 5 and to compare them to similar studies reporting approximately 60-70% reductions from the baseline, the data obtained were investigated by dividing them into two zones. During cardiac arrest, zone I represents the period from the baseline to a 70% drop relative to the baseline, while zone II corresponds to a 60% to 100% drop (complete cessation of blood flow). The results indicate that as the blood flow approached zero, the parameters exhibited increasingly random behavior. This is reflected in the standard deviation of the power law
γ values, with
CMRO2 vs.
rCCO showing a standard deviation of 0.388,
CBF vs.
rCCO of 0.350, and
CBF vs.
CMRO2 of 0.314. These findings highlight the variability and instability of physiological responses during severe reductions in perfusion. This suggests that during severe ischemia, the relationships between these parameters become less predictable, reflecting the breakdown of normal regulatory mechanisms. While variability at this level is expected under extreme conditions, it highlights the need for careful interpretation and potentially more complex modeling to accurately capture metabolic dynamics during CA.
Table 2 provides the median and standard deviation values for
γ across both zones and for all three relationships.
Due to the volatile conditions during CPR, characterized by intense movements and mechanical compressions, analyzing physiological markers was challenging compared to doing so for stable periods for most experiments. As a result, no clear relationships between
rCCO and other variables were observed, and the data appeared random. This is reflected in the low
R2 values for the power law fits.
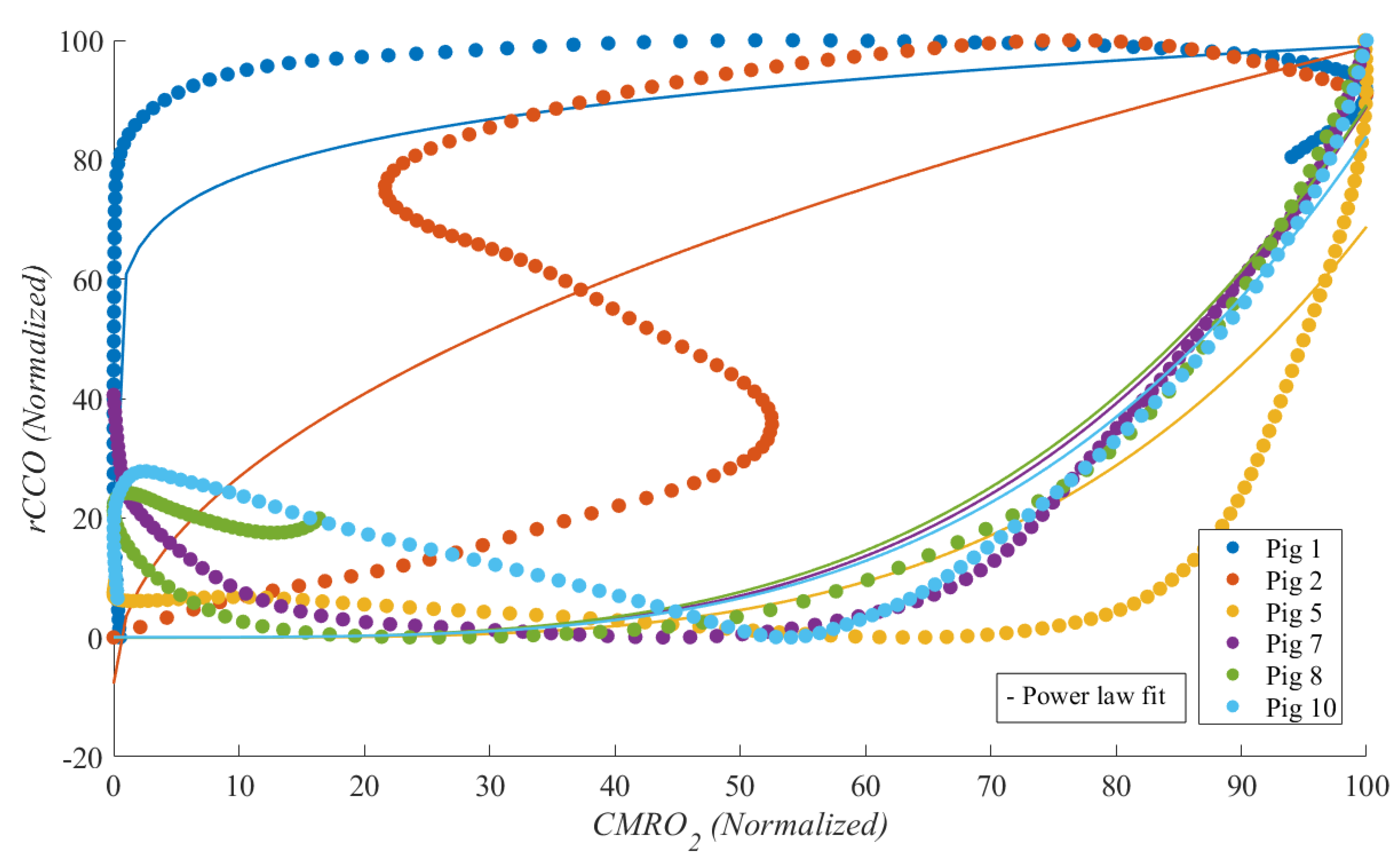
Figure 6 shows a scatter plot of
CMRO2 vs.
rCCO, illustrating this lack of a correlation. However, as expected,
CBF and
CMRO2 maintained a strong linear relationship. In most experiments, MATLAB was unable to fit the power law model for
rCCO-related data, resulting in fewer than 10 pigs being represented in
Figure 6. Nonetheless,
R2 values and the median power law
γ, along with their standard deviations, are presented in
Table 3.
Table 3 summarizes the
R2 values, power law
γ means with standard deviations,
t-test
p-values, and statistical powers for each relationship during CPR. From
Table 3, one can see that during CPR the interactions involving
rCCO exhibited significant non-linear dynamics with
γ>1, underscoring the complexity of cerebral metabolism during cardiac arrest. However,
p-values greater than 0.05 indicated relatively low statistical significance. The statistical power for the values of
γ in
Table 3 was below 50%.
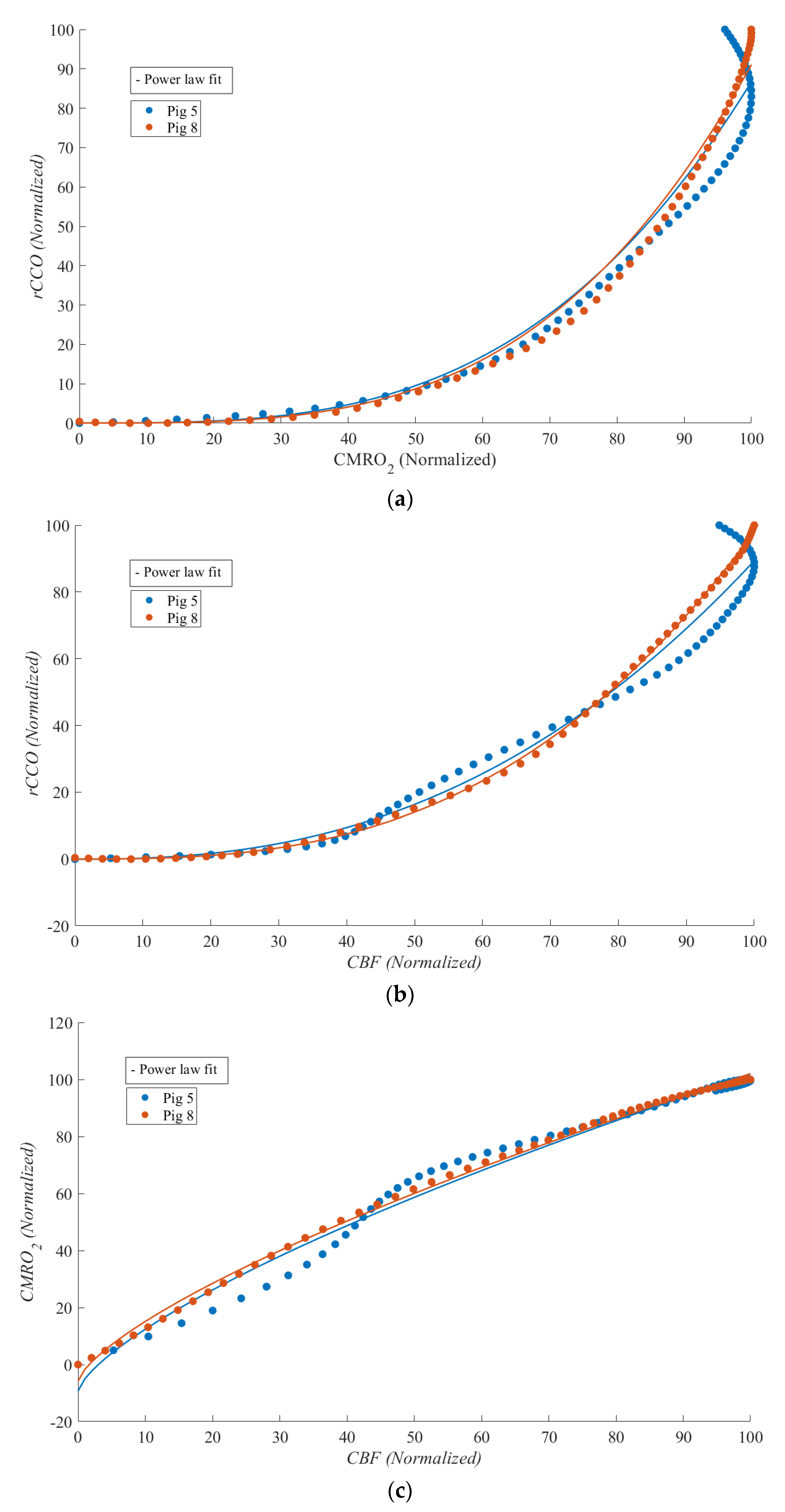
Figure 7 shows
CBF vs.
CMRO2,
CBF vs.
rCCO, and
CMRO2 vs.
rCCO for two of the experiments during which ROSC was achieved, enabling the study of physiological relationships during this phase. Although statistical analysis was not feasible due to the limited sample size, strong non-linear relationships were observed between
rCCO and
CMRO2, as well as between
rCCO and
CBF. Notable differences compared to the cardiac arrest phase included the emergence of a weak non-linear relationship between
CBF and
CMRO2 and a marked change in curvature for the other two relationships (with
γ shifting from approximately 0.5 during cardiac arrest to around 3 during ROSC).
In addition to the power law exponent
γ, the intercept
a and the scaling factor
k in Equation (7) were calculated for each relationship during cardiac arrest and CPR. A one-sample
t-test confirmed that
k and γ were positive and significantly different from zero (
p < 0.05) during cardiac arrest, reflecting strong coupling between
CMRO2,
CBF, and
rCCO under ischemic conditions. The
p-values for γ ≠ 1 (non-linearity) and
γ ≠ 0 (presence of coupling) are presented in
Table 2 and
Table 3, indicating significant deviations from linearity during cardiac arrest (
p < 0.05), except for in the
CBF vs.
CMRO2 relationship, where
γ≈1, suggesting a predominantly linear flow–metabolism coupling. Conversely, during CPR,
a and
k were not significantly different from zero (
p > 0.05) in the
CBF vs.
rCCO and
CMRO2 vs.
rCCO relationships, underscoring the absence of meaningful coupling. However,
k in the
CBF vs.
CMRO2 relationship remained significant (
p < 0.05), reinforcing that partial linearity persisted between the flow and metabolism, even under the dynamic and mechanically unstable conditions introduced by chest compressions. These findings highlight the non-linear and disrupted nature of cerebral metabolism during CPR, with preservation of linear flow–metabolism interactions between
CBF and
CMRO2.
4. Discussion
Near-infrared spectroscopy (NIRS) has demonstrated the potential to detect cardiac arrest (CA) and monitor tissue oxygenation even in the absence of a pulse waveform. This suggests its application in identifying CA outside hospital settings and continuously monitoring patients until circulation is restored [
32]. In this study, CA and cardiopulmonary resuscitation (CPR) in pigs using hyperspectral NIRS combined with laser Doppler flowmetry (LDF) were investigated. Broadband NIRS captures the full NIR spectrum at each time point, providing (1) the ability to detect chromophores beyond [
HbO2] and
[HHb], such as cytochrome c oxidase (
CCO) and water; (2) improved accuracy in determining the chromophore concentration by resolving full spectral features; and (3) the capability to determine the path length and absolute concentrations using second differential spectral methods [
33].
Our study aimed to analyze the relationship between redox cytochrome c oxidase (rCCO), the cerebral metabolic rate of oxygen (CMRO2), and the cerebral blood flow (CBF) during CA, CPR, and the return of spontaneous circulation (ROSC). Our results, obtained invasively directly from the brain, reveal distinct patterns of interaction between these variables, shedding light on the non-linear dynamics governing cerebral metabolism under extreme physiological conditions. The findings of this study emphasize the potential for advanced neuro-monitoring techniques to guide resuscitation strategies, particularly for critical care physicians managing post-cardiac arrest patients. Future clinical practice could integrate the continuous real-time monitoring of cerebral oxygen metabolism to optimize CPR’s effectiveness and improve long-term neurological recovery.
The strong linear relationship observed between CBF and CMRO2, with R2 values approaching 1.0, aligns with expectations based on Fick’s principle, indicating a robust coupling between oxygen delivery and consumption. This consistency underscores the fundamental role of CBF in sustaining cerebral oxygen metabolism. In contrast, the relationships involving rCCO deviated from linearity, particularly during CA. The power law fits for CMRO2 vs. rCCO and CBF vs. rCCO exhibited lower R2 values and γ exponents of around 0.5, suggesting that mitochondrial function and oxygen utilization become less predictable as the blood flow diminishes. During cardiac arrest, the scaling factor k and power law exponent γ were consistently positive and significant (p < 0.05), reflecting strong coupling between CMRO2, CBF, and rCCO, while the intercept a was not significant for CBF vs. CMRO2, suggesting proportional flow–metabolism coupling without a baseline offset. In contrast, during CPR, a and k were not significant for rCCO-related relationships, indicating disrupted mitochondrial coupling, likely due to mechanical chest compressions. However, k remained significant for CBF vs. CMRO2, highlighting the partial preservation of a linear flow–metabolism interaction even under unstable conditions. This suggests that while cerebral blood flow and oxygen metabolism coupling persists during CPR, mitochondrial dynamics are more sensitive to resuscitative efforts, emphasizing the need to protect mitochondrial function for better neurological outcomes.
Interestingly, during CPR, the relationships between rCCO and CMRO2 or CBF weakened, likely due to the physical disturbances and hemodynamic fluctuations induced by mechanical chest compressions. The low R2 values during CPR reflect the challenges of accurately capturing cerebral metabolism under such turbulent conditions. This finding highlights the need for refined measurement techniques to mitigate motion artifacts and enhance data reliability.
The p-values for γ ≠ 1 across the entire cardiac event reveal distinct patterns in the relationships between CMRO2, CBF, and rCCO during CA, CPR, and ROSC. The relationship between CBF and CMRO2 consistently showed a p > 0.05 (p = 0.428 during CA and p = 0.831 during CPR), indicating that the coupling between oxygen delivery and consumption persisted even during mechanical compressions. Conversely, the relationships involving rCCO demonstrated a p < 0.05 for γ ≠ 1 during CA and ROSC (p = 0.000 and p = 0.015, respectively), reflecting significant deviations from linearity. This suggests that mitochondrial function follows non-linear behavior under ischemic and reperfusion conditions. During CPR, the relationships between rCCO and CMRO2 or CBF weakened, as indicated by a p > 0.05, which may reflect the hemodynamic instability introduced by chest compressions. Despite this, p-values for γ ≠ 0 remained highly significant (p < 0.001), highlighting the ongoing metabolic contribution of rCCO even during turbulent periods.
The scaling factor k, which represents the proportional relationship between the variables of interest, was consistently positive and statistically significant across all phases of the study (CA, CPR, and ROSC). This finding aligns with physiological expectations, suggesting that even during severe reductions in the cerebral blood flow, a positive relationship between CMRO2, CBF, and rCCO persists, reflecting the brain’s ongoing metabolic activity and mitochondrial function. The significance of k during CPR highlights the resilience of cerebral metabolism, even under the dynamic and mechanically unstable conditions introduced by chest compressions. This reinforces the importance of preserving mitochondrial function during resuscitation efforts, as it may play a critical role in improving neurological outcomes post-ROSC.
To further analyze these relationships, we separated the data obtained during CA into two zones: zone I (baseline to a ≈70% drop) and zone II (60% to 100% drop in the blood flow). The results revealed increasing randomness in physiological responses as the blood flow approached zero, with greater variability in the power law γ values observed in zone II. The standard deviations of γ for CMRO2 vs. rCCO (0.388), CBF vs. rCCO (0.350), and the CBF vs. CMRO2 (0.314) suggest heightened instability in mitochondrial and metabolic responses near the complete cessation of perfusion.
The segmentation of the data into zone I and zone II enabled a more detailed analysis of metabolic responses. This division allowed for a direct comparison with prior non-invasive studies in small piglets by Milej et al. [
12], which focused on smaller perturbations in
CBF from the baseline values. In zone I, for the
CMRO2 vs.
rCCO relationship,
p = 0.07, suggesting near-linearity. This result corresponds closely to those of [
12], where the relationship between
rCCO and
CMRO2 was found to be not significantly different from one. The relationship between
CMRO2 and
CBF remained linear with
γ = 0.975 ± 0.339 and
p = 0.813 for
γ ≠ 1, indicating that oxygen delivery and consumption are tightly coupled under moderate reductions in blood flow. Interestingly, this result contrasts with the findings of [
12], where the same relationship exhibited mild non-linearity (
γ = 1.39 ± 0.31) during carotid occlusion. One possible explanation for this difference is the nature of the ischemic event. In Milej et al. [
12], ischemia was induced gradually under anesthesia, which may have allowed for compensatory mechanisms that introduced non-linearity. In contrast, our study involved CA, characterized by a rapid and complete cessation of blood flow, likely overwhelming autoregulatory mechanisms and preserving a direct linear relationship between
CBF and
CMRO2 during the initial phase of ischemia.
However, in zone II, as the blood flow approached zero, non-linear responses emerged. The relationships between
CBF and
rCCO (
p = 0.023) showed significant deviations from linearity, reflecting increased variability in mitochondrial responses under severe ischemia. These findings contrast with the linearity observed during moderate occlusion in [
12] and emphasize the need for non-linear modeling to fully capture metabolic dynamics during extreme reductions in blood flow. The comparison between zone I and zone II highlights the shift from linear to non-linear behavior as ischemia deepens. While prior studies concentrated on milder ischemic conditions, our data illustrate the breakdown of linear relationships during extreme drops in blood flow, emphasizing the need for non-linear modeling to fully capture the metabolic dynamics of CA and CPR.
Motion artifacts from mechanical chest compressions during CPR pose challenges to accurate data recording. In our study, we minimized these effects by invasively placing LDF probes and hNIRS optodes on the dura mater through cranial drilling and securing them with sutures, providing an ideal setup for data acquisition. However, in real-life clinical scenarios, non-invasive sensor placements would be more affected by motion. Future research should explore modeling techniques, such as advanced signal processing or machine learning, to mitigate motion artifacts and improve the reliability of cerebral oxygen monitoring in practical applications.
Unfortunately, our study observed only two ROSC cases out of a total of ten experiments. During ROSC, the non-linear relationship between CMRO2 and rCCO persisted but exhibited a significant shift, with γ values increasing to approximately 3. This sharp contrast to the values of γ observed during CA suggests dynamic changes in mitochondrial activity and oxygen utilization as circulation is restored. The observed non-linearity may reflect the brain’s attempt to rapidly restore homeostasis following prolonged ischemia. However, the differences in metabolic and mitochondrial responses between CA, CPR, and ROSC phases warrant further investigation in larger cohorts.
Additionally, in this study we focused on the relative changes in rCCO rather than on the absolute values, as we did not have baseline measurements of oxidized and reduced CCO concentrations. This limitation should be considered when interpreting the results, as absolute values would provide a more comprehensive understanding of the physiological changes occurring during CA and CPR. To advance our understanding, leveraging mathematical models presents several advantages. By identifying and quantifying the mathematical relationships between physiological variables, we can predict one variable based on measurements of another. This approach becomes particularly valuable in situations where advanced measurement technologies are unavailable or impractical. Mathematical models can provide meaningful insights and predictions in such contexts.
In our future research, we will focus on evaluating the validity and applicability of existing hemometabolic models to describe the mathematical relationships between
rCCO,
CMRO2, and
CBF. A key part of this effort will involve applying the
BrainSignal model [
34], which provides a comprehensive framework for analyzing these interactions. Unlike CHS, which lacks the capacity to calculate
rCCO,
BrainSignal incorporates mitochondrial metabolism, allowing for the quantification of
rCCO. In this study, the availability of hNIRS enabled us to directly measure
rCCO, offering insights into mitochondrial function during CA and CPR.
Moving forward, we aim to apply the BrainSignal model to mathematically estimate rCCO in contexts where direct measurements are not feasible. By integrating this model with our experimental data, we seek to uncover more precise correlations between rCCO, CMRO2, and CBF under various physiological conditions. This dual approach—combining experimental data from hNIRS with predictive modeling—will not only deepen our understanding of cerebral metabolism but also enhance the development of tools capable of predicting cerebral hemodynamic responses in both clinical and research settings. Ultimately, these efforts could bridge the gap between experimental measurements and real-time predictive monitoring, furthering the potential for the non-invasive assessment of brain metabolism in critical care environments.