Modeling the Chemical Hydrolysis of Mesquite (Prosopis laevigata) Seed Husk Using Response Surface Methodology and Artificial Neural Networks
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining the Sample
2.2. Chemical Hydrolysis
2.3. Enzymatic Hydrolysis
2.4. Data Adjustment Techniques
2.4.1. Data Adjustment via Response Surface Methodology
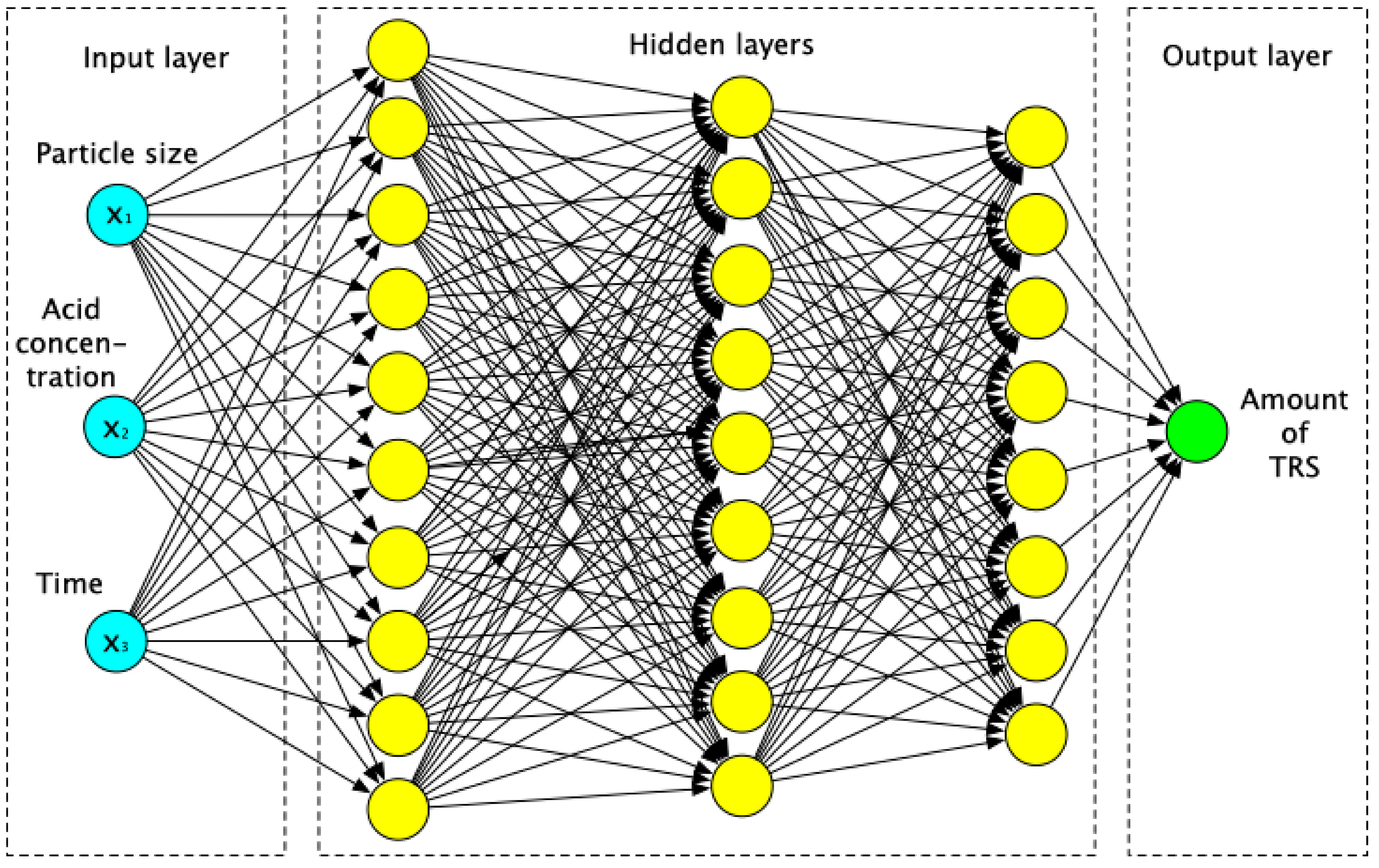
2.4.2. Data Adjustment via Artificial Neural Network
2.5. Comparison of RSM and ANN Models
3. Results
3.1. Experimental Values
3.1.1. Effect of Particle Size, Acid, and Reaction Time on Obtaining TRS
3.1.2. Effect of Chemical and Enzymatic Hydrolysis on Mesquite Husk
3.2. Response Surface Methodology
3.3. Artificial Neural Network
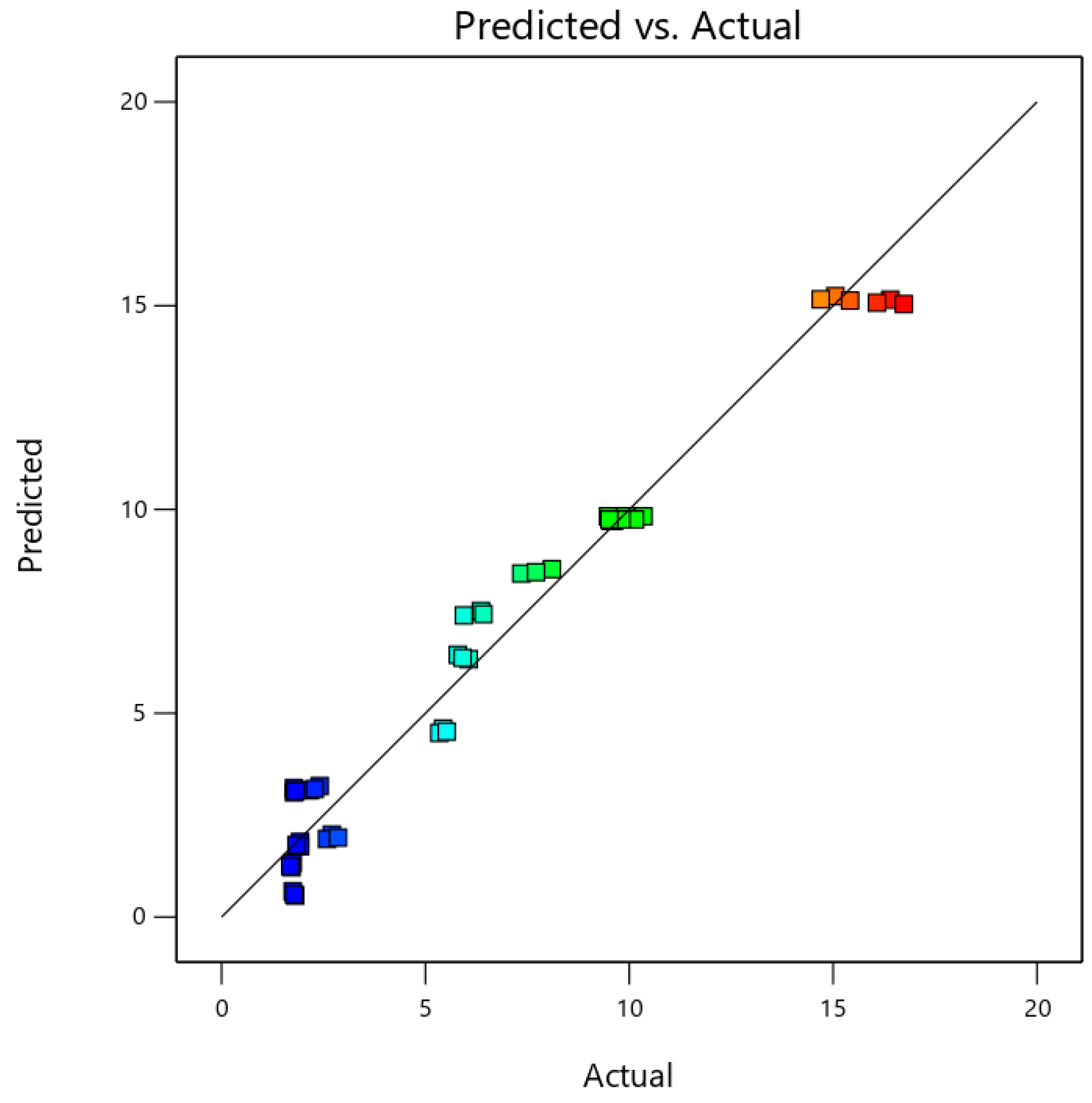
3.4. Model Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallett, J.P.; Eckert, C.A.; Ragauskas, A.J.; Nagy, M.; Kim, D.H.; Liotta, C.L. From wood to fuels: Integrating biofuels and pulp production. Ind. Biotechnol. 2006, 2, 55–65. [Google Scholar] [CrossRef]
- Betiku, E.; Taiwo, A.E. Modeling and optimization of bioethanol production from breadfruit starch hydrolyzate vis-á-vis response surface methodology and artificial neural network. Renew. Energy 2015, 74, 87–94. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Sanggari, V. Bioethanol Production from Sugarcane Bagasse using Fermentation Process. Orient. J. Chem. 2014, 30, 507–513. [Google Scholar] [CrossRef][Green Version]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Petersson, A.; Thomsen, M.; Hauggaard-Nielsen, H.; Thomsen, A. Potential bioethanol and biogas production using lignocellulosic biomass from winter rye, oilseed rape and faba bean. Biomass Bioenergy 2007, 31, 812–819. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, D.; Zhao, X. Conversion of lignocellulose to biofuels and chemicals via sugar platform: An updated review on chemistry and mechanisms of acid hydrolysis of lignocellulose. Renew. Sustain. Energy Rev. 2021, 146, 111169. [Google Scholar] [CrossRef]
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Agrawal, R.; Verma, A.; Singhania, R.R.; Varjani, S.; Di Dong, C.; Kumar Patel, A. Current understanding of the inhibition factors and their mechanism of action for the lignocellulosic biomass hydrolysis. Bioresour. Technol. 2021, 332, 125042. [Google Scholar] [CrossRef]
- Soto, X.; Fernández, K. Aprovechamiento del Fruto del Mezquite (Prosopis glandulosa y Prosopis spp) en la Zona de San Luis Rio Colorado, Sonora, Para la Elaboración y Comercialización de Harina de alto Valor Nutricional (Use of Mesquite Fruit (Prosopis glandulosa and Prosopis spp) in the area of San Luis Rio Colorado, Sonora, for the Production and Marketing of Flour of High Nutritional Value). In Ciencias Agropecuarias; Aguilera, M., Ramos, V., Eds.; Handbook T-II; ECORFAN®: Valle de Santiago, Mexico, 2014; pp. 1–5. [Google Scholar]
- Sauceda, E.N.R.; Martínez, G.E.R.; Valverde, B.R.; Ruiz, R.M.; Hermida, M.d.l.C.C.; Torres, S.M.M.; Ruiz, H.H.P. Análisis técnico del árbol del mezquite (Prosopis laevigata Humb. & Bonpl. ex Willd.) en México (Technical analysis of the mesquite tree (Prosopis laevigata Humb. & Bonpl. ex Willd.) in Mexico). Ra Ximhai 2014, 10, 173–194. [Google Scholar] [CrossRef]
- Luna Castañón, R. Variabilidad Morfologica y Genética del Mezquite Prosopis laevigata (Humb. & Bonpl. ex Willd.) en el Estado de Aguascalientes (Morphological and Genetic Variability of Mesquite Prosopis laevigata (Humb. & Bonpl. ex Willd.) in the State of Aguascalientes). Master’s Thesis, Universidad Autonoma de Aguascalientes, Aguascalientes, Mexico, 2014. Available online: http://bdigital.dgse.uaa.mx:8080/xmlui/bitstream/handle/11317/305/398549.pdf?sequence=1&isAllowed=y (accessed on 10 October 2024).
- Peña-Avelino, L.; Pinos-Rodríguez, J.; Yáñez-Estrada, L.; Juárez-Flores, B.; Mejia, R.; Andrade-Zaldivar, H. Chemical composition and in vitro degradation of red and white mesquite (Prosopis laevigata) pods. S. Afr. J. Anim. Sci. 2014, 44, 298. [Google Scholar] [CrossRef]
- Mom, M.P. Caracterización Estructural y Propiedades Funcionales de las Harinas de Los Frutos de Prosopis alba Griseb., P. chilensis (Molina) Stuntz Emend. Burkart y P. flexuosa DC. Desarrollo de un Proceso de Secado, Molienda y Mezcla Para Optimizar la Calidad del Producto. Ph.D. Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 17 December 2012. Available online: https://bibliotecadigital.exactas.uba.ar/greenstone3/exa/collection/tesis/document/tesis_n5217_Mom (accessed on 24 September 2024).
- Pasiecznik, N.M.; Felker, P.; Harris, P.J.C.; Harsh, L.N.; Cruz, G.; Tewari, J.C.; Cadoret, K.; Maldonado, L.J. The Prosopis Juliflora—Prosopis Pallida Complex: A Monograph; HDRA: Coventry, UK, 2001. [Google Scholar]
- Azpeitia, L.G.; Labrada-Delgado, G.J.; Montalvo-González, E.; Loza-Cornejo, S. Morphometric and anatomical characters of fruits and seeds of a population of Prosopis laevigata (Fabaceae) in Lagos de Moreno, Jalisco, Mexico. Acta Bot. Mex. 2022, 129, e2057. [Google Scholar] [CrossRef]
- Asanza, W.R.; Olivo, B.M. Redes Neuronales Artificiales Aplicadas al Reconocimiento de Patrones (Artificial Neural Networks Applied to Pattern Recognition); Editorial UTMACH: Machala, Ecuador, 2018. [Google Scholar]
- Chouaibi, M.; Daoued, K.B.; Riguane, K.; Rouissi, T.; Ferrari, G. Production of bioethanol from pumpkin peel wastes: Comparison between response surface methodology (RSM) and artificial neural networks (ANN). Ind. Crops Prod. 2020, 155, 112822. [Google Scholar] [CrossRef]
- Igwilo, C.N.; Ude, N.C.; Onoh, I.M.; Enekwe, C.B.; Alieze, B.A. RSM, ANN and ANFIS applications in modeling fermentable sugar production from enzymatic hydrolysis of Colocynthis Vulgaris Shrad seeds shell. Bioresour. Technol. Rep. 2022, 18, 101056. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, S.; Jaiswal, A.K. Artificial neural networks in valorization process modeling of lignocellulosic biomass. Biofuels Bioprod. Biorefining 2022, 16, 1849–1868. [Google Scholar] [CrossRef]
- Vibha, R.; Sandesh, K.; Ujwal, P.; Shet, V.B. RSM-and ANN-based modeling for a novel hydrolysis process of lignocellulose residues to produce cost-effective fermentable sugars. Biomass Convers. Biorefinery 2024, 14, 24181–24196. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Pérez-Cadena, R.; Espinosa-Solares, T.; Medina-Moreno, S.A.; Martínez, A.; Lizardi-Jiménez, M.A.; Téllez-Jurado, A. Effect of the age of Opuntia ficus-indica cladodes on the release of simple sugars. Biocatal. Agric. Biotechnol. 2021, 33, 102010. [Google Scholar] [CrossRef]
- Liu, W.; Wu, R.; Wang, B.; Hu, Y.; Hou, Q.; Zhang, P.; Wu, R. Comparative study on different pretreatment on enzymatic hydrolysis of corncob residues. Bioresour. Technol. 2020, 295, 122244. [Google Scholar] [CrossRef]
- Aggarwal, C.C. Neural Networks and Deep Learning; Springer: Cham, Switzerland, 2018; Volume 10, p. 3. [Google Scholar]
- Zhang, Z.; Feng, Q.; Huang, J.; Guo, Y.; Xu, J.; Wang, J. A local search algorithm for k-means with outliers. Neurocomputing 2021, 450, 230–241. [Google Scholar] [CrossRef]
- Hagan, M.T.; Demuth, H.B.; Beale, M.H.; De Jesus, O. Neural Network Design, 2nd ed.; Oklahoma State University: Stillwater, OK, USA, 2014; Available online: http://hagan.okstate.edu/NNDesign.pdf (accessed on 3 September 2024).
- Geyikçi, F.; Kılıç, E.; Çoruh, S.; Elevli, S. Modelling of lead adsorption from industrial sludge leachate on red mud by using RSM and ANN. Chem. Eng. J. 2012, 183, 53–59. [Google Scholar] [CrossRef]
- Alokika, A.; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and hemicellulosic fractions of sugarcane bagasse: Potential, challenges and future perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Chundawat, S.P.S.; Venkatesh, B.; Dale, B.E. Effect of particle size based separation of milled corn stover on AFEX pretreatment and enzymatic digestibility. Biotechnol. Bioeng. 2007, 96, 219–231. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Kumar, S.; Singh, J.; Kumar, P. Bioethanol production from sesame (Sesamum indicum L.) plant residue by combined physical, microbial and chemical pretreatments. Bioresour. Technol. 2020, 297, 122484. [Google Scholar] [CrossRef]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef]
- Kapoor, M.; Semwal, S.; Satlewal, A.; Christopher, J.; Gupta, R.P. The impact of particle size of cellulosic residue and solid loadings on enzymatic hydrolysis with a mass balance. Fuel 2019, 245, 514–520. [Google Scholar] [CrossRef]
- Baksi, S.; Ball, A.K.; Sarkar, U.; Banerjee, D.; Wentzel, A.; Preisig, H.A.; Kuniyal, J.C.; Birgen, C.; Saha, S.; Wittgens, B.; et al. Efficacy of a novel sequential enzymatic hydrolysis of lignocellulosic biomass and inhibition characteristics of monosugars. Int. J. Biol. Macromol. 2019, 129, 634–644. [Google Scholar] [CrossRef]
- Infanzón-Rodríguez, M.I.; Ragazzo-Sánchez, J.A.; del Moral, S.; Calderón-Santoyo, M.; Aguilar-Uscanga, M.G. Enzymatic hydrolysis of lignocellulosic biomass using native cellulase produced by Aspergillus niger ITV02 under liquid state fermentation. Biotechnol. Appl. Biochem. 2021, 69, 198–208. [Google Scholar] [CrossRef]

| No. | Particle Size (A) | Acid (N) (B) | Time (min) (C) | TRS (g/L) Experimental |
|---|---|---|---|---|
| 1 | 30 (0) | 0.25 (0) | 20 (0) | 9.75 |
| 2 | 16 (−1) | 0 (−1) | 20 (0) | 1.79 |
| 3 | 50 (+1) | 0 (−1) | 20 (0) | 2.72 |
| 4 | 16 (−1) | 0.5 (+1) | 20 (0) | 7.72 |
| 5 | 50 (+1) | 0.5 (+1) | 20 (0) | 16.41 |
| 6 | 16 (−1) | 0.25 (0) | 10 (−1) | 1.78 |
| 7 | 50 (+1) | 0.25 (0) | 10 (−1) | 2.29 |
| 8 | 30 (0) | 0.25 (0) | 20 (0) | 9.91 |
| 9 | 16 (−1) | 0.25 (0) | 30 (+1) | 5.43 |
| 10 | 50 (+1) | 0.25 (0) | 30 (+1) | 6.24 |
| 11 | 30 (0) | 0 (−1) | 10 (−1) | 1.90 |
| 12 | 30 (0) | 0.5 (+1) | 10 (−1) | 5.92 |
| 13 | 30 (0) | 0 (−1) | 30 (+1) | 1.72 |
| 14 | 30 (0) | 0.5 (+1) | 30 (+1) | 15.06 |
| 15 | 30 (0) | 0.25 (0) | 20 (0) | 9.65 |
| Coefficient of Determination r2 | Root Mean Square Error (RMSE) | Mean Absolute Percentage Error (MAPE) | |||
|---|---|---|---|---|---|
| RSM | ANN | RSM | ANN | RSM | ANN |
| 0.93 | 0.99 | 1.48 | 0.44 | 26.22% | 3.65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Cadena, R.; Vázquez-Maldonado, S.; Téllez-Jurado, A.; Serna-Diaz, M.G.; Medina-Marin, J. Modeling the Chemical Hydrolysis of Mesquite (Prosopis laevigata) Seed Husk Using Response Surface Methodology and Artificial Neural Networks. Appl. Sci. 2025, 15, 1419. https://doi.org/10.3390/app15031419
Pérez-Cadena R, Vázquez-Maldonado S, Téllez-Jurado A, Serna-Diaz MG, Medina-Marin J. Modeling the Chemical Hydrolysis of Mesquite (Prosopis laevigata) Seed Husk Using Response Surface Methodology and Artificial Neural Networks. Applied Sciences. 2025; 15(3):1419. https://doi.org/10.3390/app15031419
Chicago/Turabian StylePérez-Cadena, Rogelio, Silvana Vázquez-Maldonado, Alejandro Téllez-Jurado, Maria Guadalupe Serna-Diaz, and Joselito Medina-Marin. 2025. "Modeling the Chemical Hydrolysis of Mesquite (Prosopis laevigata) Seed Husk Using Response Surface Methodology and Artificial Neural Networks" Applied Sciences 15, no. 3: 1419. https://doi.org/10.3390/app15031419
APA StylePérez-Cadena, R., Vázquez-Maldonado, S., Téllez-Jurado, A., Serna-Diaz, M. G., & Medina-Marin, J. (2025). Modeling the Chemical Hydrolysis of Mesquite (Prosopis laevigata) Seed Husk Using Response Surface Methodology and Artificial Neural Networks. Applied Sciences, 15(3), 1419. https://doi.org/10.3390/app15031419







