Abstract
Layered double hydroxides (LDH) containing various exchangeable anions were studied to show how X-ray Photoelectron Spectroscopy (XPS) can provide information on the local environments of the different elements within the interlayer anionic groups and their possible influence on the LDH interlayer hydroxide surfaces. As such, XPS can potentially provide additional information about these systems that cannot be obtained by other common spectroscopic methods, such as infrared and Raman spectroscopy. A Mg6Al2X(OH)16. 4H2O with X representing interlayer anions CO32−, PO43−, SO42−, MoO42−, CrO43−, Fe(CN)64−, and Fe(CN)63− was studied. The hydroxide layer structure is characterized by the Mg 2p and Al 2p with a binding energy of around 50.1 and 74.5 eV for the normal CO32− containing LDH. The O 1s contained three peaks related to the layer OH-groups at 531.6 eV, interlayer CO32− at 530.5 eV and interlayer water at 532.4 eV. Similar observations were made for the other interlayer anions showing characteristic P 2p, S 2p, and Mo 3d peaks. Intercalation with CrO43− shows that a significant amount of the Cr6+ has been reduced to Cr3+. Finally, the intercalation of hexacyanoferrate in hydrotalcite showed the potential of XPS in detecting changes in the oxidation state of Fe upon intercalation in the LDH with a change in the Fe 2p peaks with a shift in binding energy and the possibility of determining the amount of reduction of Fe(III) to Fe(II). In general, the XPS high-resolution scans of P 2p, S 2p, Mo 3d, and Cr 2p show that slightly lower binding energies are observed compared to the binding energy values for the corresponding anionic groups as part of a rigid crystal structure, such as in minerals. Overall, the influence of the nature of the interlayer anion on the binding energy of the elements (Mg, Al, O) in the layered double hydroxide structure is minimal and considered to be within the experimental error of XPS. A detailed analysis of XPS data in combination with infrared and Raman spectroscopy shows how XPS can provide additional information that is not readily available via vibrational spectroscopy. XPS can simultaneously account for both surface and bulk properties of LDH that are not available through common vibrational spectroscopic methods.
1. Introduction
Hydrotalcites, also referred to as layered double hydroxides (LDHs), are categorized as anionic clays due to their distinctive layered architecture [1,2]. This structure is characterized by positively charged hydroxy layers, in stark contrast to cationic (silicate) clays such as smectites, which possess a negative layer charge. Within the interlayer space of these positively charged layers, anions are present to balance the overall charge. A more comprehensive understanding of hydrotalcite’s structure reveals positively charged hydroxide layers that resemble those found in brucite [Mg(OH)2]. In this configuration, a portion of the Mg2+ ions can be substituted with trivalent metals, such as Al3+, while charge-compensating anions, typically in a hydrated form, occupy the interstitial regions between the hydroxide layers (Figure 1) [2,3].
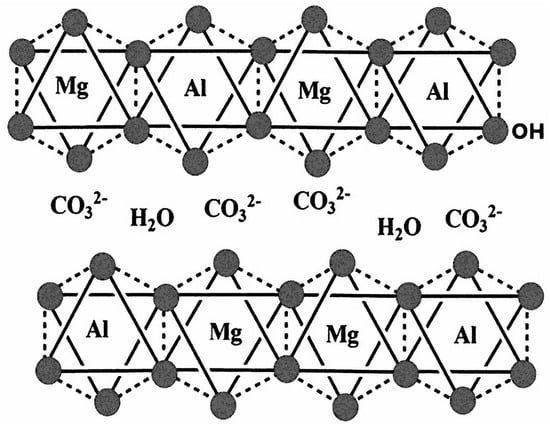
Figure 1.
Simplified structure of LDH showing the Mg/Al hydroxide layers with the OH-groups (filled grey spheres) on its surfaces exposed to the interlayer space filled with water and interlayer anions such as CO32−, in this case.
The composition of LDH is very versatile, commonly represented by the formula [M2+1−xM3+ x (OH)2][An−]x/n·yH2O, where M2+ and M3+ denote the divalent and trivalent cations residing in octahedral coordination within the hydroxide layers. The variable x usually ranges from 0.17 to 0.33, indicating the proportion of M3+ ions. Notably, it is imperative that the ionic radii of M2+ and M3+ do not exhibit significant disparity—specifically, a deviation of no more than 30%—to maintain structural integrity. For instance, the ionic radii for Mg2+ and Al3+ are approximately 0.65 Å and 0.45 Å, respectively. Furthermore, sound chemical principles dictate that the solubility products, S1 for M2+(OH)2 and S2 for M2+CO3, must adhere to the conditions where the difference pS1–pS2 is maintained within the range of 0 and 10, ensuring the stability of the hydrotalcite phase [2,3,4].
The variety of anions that can exist within the LDH framework is relatively unconstrained, provided that these anions do not engage in complexation with the cations within the octahedral sheets during the synthesis process. Such complexation would inhibit the formation of a viable LDH structure [5]. The inherent flexibility in both cationic and anionic compositions of hydrotalcite enables the engineering of tailor-made materials suitable for a broad array of applications. These may include their utilization as basic catalysts or precursors for synthesizing mixed metal oxide catalysts. Additionally, hydrotalcites serve functional roles as adsorbents, fillers, UV stabilization agents, chloride scavengers, and thermal stabilizers, positioning them as versatile components in various industrial and technological contexts [6,7,8,9,10,11].
Infrared spectroscopy, along with the less frequently employed Raman spectroscopy, has proven instrumental in elucidating the structural and compositional characteristics of LDH containing varied cations and anions. These spectroscopic techniques have facilitated comprehensive examinations of anionic intercalation and thermal degradation processes in these materials, primarily serving to identify a range of exchangeable anions. Among the anions detected in such studies are carbonate (CO32−), chloride (Cl−), perchlorate (ClO4−), nitrate (NO3−), sulfate (SO42−), arsenate (AsO43−), vanadate (VO43−), molybdate (MoO42−) and chromate (CrO42−) [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Moreover, the investigation has extended to more complex anionic entities, including anionic silica (SiO(OH)3−) [28] and larger polyoxometalate ions, as well as hexacyanoferrate complexes (Fe(CN)6n−) (see e.g., [29,30,31,32,33,34,35]).
The unique vibrational fingerprints associated with each of these anions are discernible through their respective infrared and Raman spectra. These spectral signatures provide crucial insights into the interactions and substitutions occurring within the hydrotalcite matrix. Through rigorous analysis using these spectroscopic techniques, researchers have been able to correlate the presence of specific anions with structural modifications within the LDH framework. This knowledge not only enhances the understanding of the properties of LDH but also informs potential applications in fields such as catalysis, ion exchange, and environmental remediation, where the selective uptake and release of ions play a critical role. Such advancements underscore the relevance of infrared and Raman spectroscopy in characterizing the multi-faceted nature of hydrotalcite and their layered structures.
X-ray photoelectron spectroscopy (XPS) has established itself as the predominant technique for investigating surface phenomena. However, there remains a notable paucity of research focusing on the bulk atomic structure and chemical states of minerals. This oversight is particularly striking given that the vast majority—often exceeding 90%—of the intensity of XPS lines is derived from the bulk material when employing conventional laboratory X-ray sources, such as the Al Kα source at 1486.6 eV. This discrepancy invites further inquiry into the collective properties of minerals that are not fully captured by surface-centric methodologies.
Historically, the XPS analysis of various mineralogical components has been documented in the literature, encompassing a diverse range of materials, including feldspars, clays, aluminum oxy(hydroxide) phases, arsenates, and phosphates. Notable contributions have highlighted the unique electronic and structural characteristics present in these minerals, but comprehensive studies that simultaneously account for both surface and bulk properties remain limited. It is essential to broaden the scope of investigation to include these often-ignored bulk properties to achieve a better understanding of mineral behavior. So far, limited use has been made of XPS as an analytical tool to study LDH in detail, and it generally stops at just providing binding energy values (see e.g., [36,37,38,39,40,41,42]). As part of an ongoing study on LDH, this study aims to better understand the X-ray photoelectron spectroscopy (XPS) of the interlayer anions in LDH to complement the existing Infrared and Raman spectral data of LDH.
2. Materials and Methods
The synthesis of hydrotalcite, characterized by the theoretical composition Mg6Al2(OH)16CO3.nH2O, was performed utilizing the procedure outlined by Kloprogge and Frost [43]. The procedure involves the gradual simultaneous introduction of a solution containing aluminum nitrate (0.25 M) and magnesium nitrate (0.75 M) alongside a mixed solution of NaOH (2.00 M) and Na2CO3 (0.125 M) during vigorous stirring. The pH is maintained at approximately 10 throughout the process. The product was washed to eliminate excess salt and dried at 60 °C.
To minimize the incorporation of carbonate during the synthesis of hydrotalcite with other anions, several measures were employed: deionized water was boiled prior to use, NaOH pellets were thoroughly rinsed, and the synthesis was conducted in a nitrogen atmosphere. A mixed solution containing aluminum and magnesium nitrates was prepared with concentrations of [Al3+] at 0.25 M and [Mg2+] at 0.75 M. Additionally, a separate mixed solution of sodium hydroxide was formulated with a hydroxide concentration of [OH−] at 2 M, incorporating the desired anion at the specified concentration. Both solutions were transferred into distinct vessels and subjected to nitrogen purging for 20 min, with all solutes dissolved in freshly decarbonated water. The cationic solution was delivered to the anionic phase using a peristaltic pump at a flow rate of 40 mL/min while maintaining a pH above 9. The resulting mixture was subjected to aging at 75 °C for 18 h in a nitrogen atmosphere. Following this, the precipitate was filtered using room-temperature decarbonated water to eliminate nitrate ions and subsequently dried in a vacuum desiccator over several days. This method facilitated the synthesis of hydrotalcite containing various anions in the interlayer. Phase composition was evaluated via X-ray diffraction, and chemical composition was determined through energy-dispersive X-ray spectroscopy (EDXA) analyses [44].
X-ray photoelectron spectroscopy (XPS) data were acquired on a Kratos AXIS Ultra (Kratos Analytical, Manchester, UK) utilizing a monochromatic 225 W Al X-ray source and conducted under ultrahigh vacuum conditions. Initial survey scans were executed within the energy range of 0 to 1200 eV, employing a dwell time of 100 milliseconds, a pass energy of 160 eV, and a step size of 1 eV over a single sweep. Subsequent high-resolution analyses were performed by increasing the number of sweeps, reducing the pass energy to 20 eV with a step increment of 100 meV, and extending the dwell time to 250 milliseconds. For calibration purposes, the adventitious C 1s peak at 284.8 eV was utilized to correct all spectra.
3. Results
3.1. Layer Structure and Chemistry
Figure 2 shows the survey scan for a normal LDH with carbonate as the interlayer anion. It is characterized by a single Mg 2p peak at 50.1 eV, a single Al 2p peak at 74.5 eV (Figure 3), as well as a single O 1s peak at 531.6 eV. High-resolution scans of the O 1s exhibit three peaks at 530.5 eV, 531.6 eV and 532.4 eV assigned to oxygen atoms in the interlayer carbonate anion-, hydroxyl groups and interlayer water, respectively. These values are in excellent agreement with the results reported by Kloprogge and Wood [45]. They also showed the Mg 2p for Mg(OH)2 at 49.3 eV, which is at a slightly lower binding energy (BE) than for LDH, as shown here, caused by the partial substitution of Mg2+ by Al3+. The LDH Al 2p peak at 74.5 eV is close to the BE observed for gibbsite, Al(OH)3 (74.4 eV), bayerite, Al(OH)3 (75.0 eV), boehmite (73.9 eV), AlOOH, and pseudoboehmite, AlOOH (74.3 eV) [46]. Peng et al. [47] observed comparable BE values for LDH with Mg 2p at 50.29 eV and Al 2p at 74.31 eV.
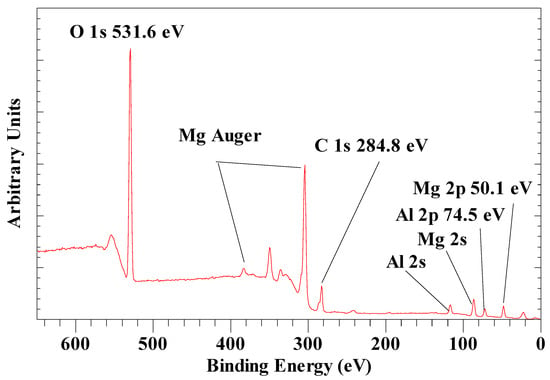
Figure 2.
XPS survey scan of LDH with carbonate as the anion in the BE region between 650 and 0 eV. The region between 1200 and 650 is not shown, as no peaks are observed there.
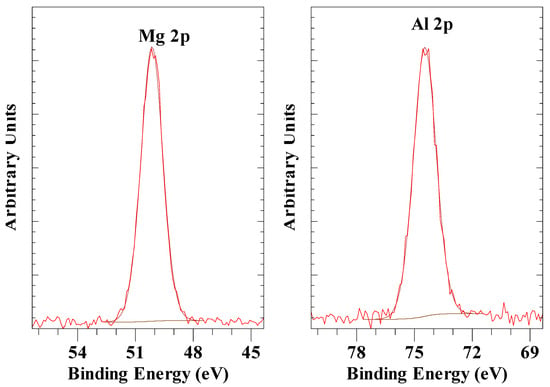
Figure 3.
XPS high-resolution scan of Mg 2p and Al 2p of LDH with carbonate as the anion.
Based on the high-resolution spectra, the chemical composition can be determined as the surface area under the peak is directly proportional to the amount of the element present in the sample. Table 1 provides the chemical composition in atom percent of the Mg and Al in the hydroxide layer as well as the characteristic cation in the interlayer anion as well as the Mg/Al ratio and the Al/anion ratio. In all instances, the Mg/Al ratio is close to 3, as expected based on the starting mixture used for the synthesis. The Al/anion ratios observed are also close to what theoretically would be expected. In the case of the carbonate anion, a slightly lower value is observed due to the small amount of nitrate present in the interlayer, as has been shown in earlier IR and Raman spectroscopy work [19].

Table 1.
Chemical composition in atom % for Al, Mg and interlayer anions.
3.2. Carbonate Anion
The most often studied interlayer anionic group in LDH, due to its strong affinity to the hydroxide layers, is CO32−. Going from the free anion in solution, changes can be anticipated when the CO32− is present in the interlayer space of LDH due to interactions with interlayer H2O and/or OH groups at the interlayer surfaces of the LDH layers.
The C 1s high-resolution spectrum of the carbonate anion shows a peak at a BE value of 288.6 eV (Figure 4 left). This peak is due to the interlayer anion. The other peaks are due to surface-adsorbed organic matter, the so-called “rubbish” carbon. The corresponding O 1 peak was found at 530.5 eV (Figure 4 right), and the ratio of O/C is about 3.10, close to the expected ratio of 3 for CO32−. The BE value is slightly lower compared to pure carbonate minerals such as magnesite, which has a C 1s at 290.0 eV, dolomite at 289.8 eV, and calcite at 289.5 eV [45]. This shift in BE is probably associated with interactions with the interlayer water molecules and the restrictions imposed by the LDH layers.
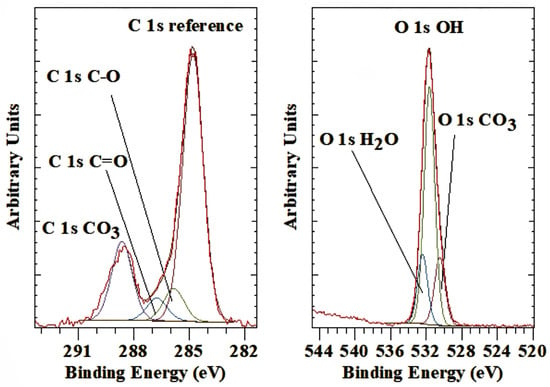
Figure 4.
XPS high-resolution scan of C 1s and O 1s of LDH with carbonate as the interlayer anion.
In earlier work, Kloprogge, Wharton, Hickley and Frost [19] have shown by infrared and Raman spectroscopy that there is an interaction with interlayer water as shown by a water-carbonate bridging mode around 3000–3100 cm−1. Rey et al. [48] observed that heating Mg/Al-LDH to 100 °C resulted in the disappearance of this water-carbonate bridging mode together with the water OH-bending mode around 1616 cm−1 due to dehydration. Relative to free CO32− a shift toward lower wavenumbers was detected. Based on X-ray diffraction, ionic size, and charge density, it has been generally accepted that the CO32− anion is present within the interlayer space with its C3 axis perpendicular to the interlayer surfaces of the LDH hydroxide layers [49]. Due to these restrictions in the interlayer space, the symmetry of the carbonate anion decreased from D3h to C2s, causing the activation of the IR inactive ν1 mode around 1050–1060 cm−1. In addition, the ν3 showed a splitting of 30–60 cm−1. Though IR and Raman clearly show that there are interactions between the interlayer anion and the LDH layers as well as water and that the movement of the interlayer anion is restricted, XPS shows that the binding energy of the C 1s is less than for a pure carbonate mineral indicative that the carbonate anion still has some degree of freedom inside the interlayer space.
3.3. Phosphate Anion
The interlayer phosphate anion is characterized by the P 2p3/1 peak with a BE of 133.0 eV. The corresponding P 2p1/2 is found at a BE value of 132.1 eV (Figure 5 left). The synthesis of the phosphate-containing LDH was performed at three different pH values (9.3, 11.9 and 12.5). No difference in the BE values was observed, but a significantly higher amount of phosphate was present in the interlayer space of the LDH in the lowest pH sample. The BE value is similar to some common phosphate minerals, such as monazite, autunite, vivianite and apatite, which have P 2p3/2 Be values around 133.2 to 134 eV [50]. On the other hand, the BE of H3PO4 is slightly higher than observed here at 135.2 eV. The same is true for NaH2PO4 with a BE of 134.0 to 134.2 eV [51]. Wang, Cai, Han, Fang, Chen and Tan [22] reported that the P 2p was approximately 134 eV for LDH containing hexametaphosphate. At the same time, they observed a shift in the Mg 2p and Al 2p towards higher BE. They ascribed this shift in BE to the fact that the Mg2+/Al3+ in the LDH hydroxide layers can extract electron density from the negatively charged oxygen atoms in hexametaphosphate [52]. Gupta, Saifuddin, Kim and Kim [52] found a BE shift in the Zn 2p of 0.04 eV, which is considered to be within the experimental error of XPS, while the Fe 2p showed a larger shift in BE of about 0.2 eV. In this study, no shift was observed for the Mg 2p, and there was a minor shift toward a higher BE value of 0.1 eV for the Al 2p.
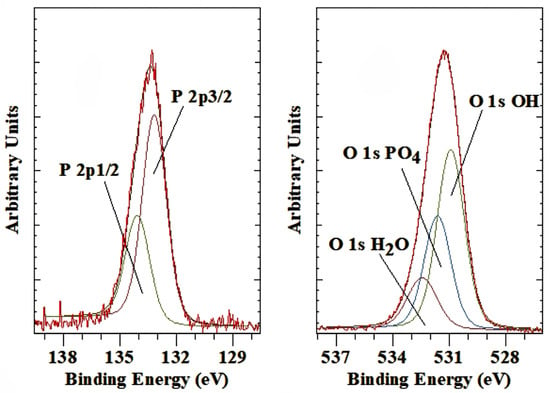
Figure 5.
XPS high-resolution scan of P 2p and O 1s of LDH with phosphate as the interlayer anion.
The O 1s is characterized by three peaks associated with the layer hydroxyl groups at 530.9 eV, interlayer water at 532.4 eV and the interlayer PO42− at 531.6 eV (Figure 5 right). Again, the BE value for the oxygen in the phosphate anion is comparable to that found for various phosphate minerals, e.g., monazite (531.2 eV), vivianite (531.2 eV), amblygonite (531.6 eV) and apatite (531.0 eV) [50]. Similar BE values are also listed in the NIST database, with, e.g., AlPO4 at 532.8 eV [51]. Wang, Cai, Han, Fang, Chen and Tan [22] only reported a general position for the O 1s at about 532 eV but did not attempt to provide details fitting the different oxygen species present. Gupta, Saifuddin, Kim and Kim [52] fitted the O 1s with three bands with the O 1s OH at 530.1 eV, slightly lower than observed here. In contrast, though, the other two peaks are at very different BE values, with an extremely broad peak at 530.9 eV assigned to H2O, -O-Nand O-C, while a third peak at very low BE of 528.4 eV assigned to M-O. No explanation is given for what M is, but presumably, this is the P-O in the phosphate, as there are no M-O modes in the LDH hydroxide layer. These assignments do not seem to be correct as, generally, O 1s for PO4 are always found at much higher BE.
LDHs intercalated with PO43− have been shown to form different phases with very different basal spacings: 0.84 nm [20], 0.78 nm, 0.80 nm, 1.19 nm [17] and 1.11 nm [53], depending on the synthesis conditions such as Mg/Al ratio, pH, etc. This spread in basal spacings can be explained by different positions adopted by the PO43− between the LDH hydroxide layers (perpendicular, inclined and planar). Wang, Cai, Han, Fang, Chen and Tan [22] even indicate that the hexametaphosphate complex stays intact within the interlayer space of the LDH after exchange. Mid-infrared and Raman spectra have been reported in a limited number of papers. Benício et al. [54] reported the IR-active bands in the 1050 cm−1, 870 cm−1 and 550 cm−1 regions, which correspond to ν3, ν1, and ν4 vibrations [55,56]. The PO43− anion is tetrahedral with Td symmetry; thus, there are four normal modes of vibration, all of which are Raman active. However, only the triply degenerate ν3 (F2) and ν4 (F2) modes are infrared active. The observation of the ν1 mode must be due to either the presence of some nitrate or carbonate instead [19]. In contrast, Benício, Constantino, Pinto, Vergütz, Tronto and Da Costa [53], in a paper a year earlier, claimed the presence of not only PO43− in the interlayer space but also HPO42−. Though the spectrum provided is of rather low quality and they do not show the band fit, they claim that the broadband in the 1020 cm−1 region consists of the ν3 vibrational mode of PO43− anion and two additional bands assigned to the ν3 and ν2 modes of HPO42− anion [57]. In contrast, Gupta, Saifuddin, Kim and Kim [52] assigned the bands at 1045 cm−1 and 565 cm−1 corresponding to the v3 and v4 band vibrations of HPO42− or H2PO4− without further explanation. Wang, Cai, Han, Fang, Chen and Tan [22] reported only a single band at 1088 cm−1 attributed to the vibration of P-O. Shabanian et al. [58] reported that three bands attributed to HPO42− coalesce into a single broad band around 1056 cm−1. In addition, a shoulder band was detected at 870 cm−1 near the theoretical antisymmetric stretching mode of P–OH. Others also described the characteristic band related to phosphate in the interlayer of Zn/Al LDH at 1040 cm−1 [59] and 1048 cm−1 [60].
In earlier work, we reported on the Raman spectra of phosphate-LDH and addressed the possibility of determining the presence of H2PO4− and HPO42− in addition to PO43− [17]. The Raman spectra will change if hydrogen is linked to the phosphate unit as the symmetry will change from Td to C3v to C2v. This will cause a loss of degeneracy, and other bands may become visible. The sample synthesized at pH 12.5 exhibited a strong, sharp band at 960 cm−1 with a broader band at 1026 cm−1 assigned to the PO43− symmetric and antisymmetric stretching vibrational modes identical to that of the PO43− ion. Similar bands were observed for the pH 11.9 sample at 957 cm−1 and 1032 cm−1. Benício, Eulálio, Guimarães, Pinto, Costa and Tronto [54] reported the formation of bands in the region between 940 cm−1 and 960 cm−1 after exchange with phosphate. Slight changes were also detected in the band positions in the 1050 cm−1 region. A different Raman spectrum was obtained for the pH 9.3 sample with four bands at 964, 989, 1033 and 1138 cm−1. In aqueous systems, the PO43− ion has a strong band at 936 cm−1, while HPO42− anion exhibits a strong band at 990 cm−1. Therefore, the spectrum can be interpreted as a combination of the PO43− anion (964 and 1033 cm−1) and the HPO42− ion (989 and 1138 cm−1). However, it is questionable whether these are actually present in the interlayer space of LDH, as XRD showed the material mainly as amorphous. For the oxyanion PO43− the symmetric bending mode (ν2) and the ν4 mode are observed at 420 cm−1 and 567 cm−1, respectively. For the phosphate LDH at pH 12.5, a band of 474 cm−1 was observed. A similar band was found at 472 cm−1 for the pH 11.9 sample, while a broad band around 441 cm−1 was present in the Raman spectrum of the pH 9.3 sample.
Infrared and Raman spectroscopy of phosphate-LDH has shown that the nature of the anion present in the interlayer space is affected by the synthesis conditions. In this study, three different pH values were studied despite the fact that in the literature, the presence of H2PO4− and HPO42− have been claimed [52,53]. For the two samples synthesized at pH 12.5 and 11.9, Raman spectroscopy showed that this was not the case [17]. This is further confirmed by the XPS results that show only a single P 2p 3/2 peak, as well as by the O/P ratio, which in both cases is close to 4. XPS did not allow us to distinguish between PO43− and HPO42− in the three samples studied.
3.4. Sulphate Anion
In naturally occurring LDH, other than the commonly present CO32− anion, SO42− forms an additional important interlayer anion found in minerals such as honessite (Ni6Fe2SO4(OH)16·4H2O), hydrohonessite (Ni6Fe2SO4(OH)16·7H2O), and carrboydite ((Ni,Cu)14Al9(SO4,CO3)6(OH)43·7H2O) [61,62,63]. Figure 6 shows the S 2p and O 1s of sulphate-LDH. The S 2p is characterized by a single S 2p3/1 peak with a BE of 168.3 eV. This BE is slightly lower than observed for common sulfate minerals such as baryte (169.2 eV), anhydrite (168.9 eV), or gypsum (169.2 eV) [45], indicating that the sulfate in the LDH interlayer space is less strongly interacting with the LDH hydroxide layers compared to a sulfate group in a rigid crystal structure. In the O 1s, a single peak at 532.4 eV is associated not only with the oxygen in the sulfate group but also with oxygen in interlayer water that has a similar BE. This value is comparable with H2SO4, which has an O 1s BE of 532.5 eV and Al2(SO4)3 of 532.4 eV [51]. Similar results were also reported for group 1A sulfates by Wahlqvist and Shchukarev [64].
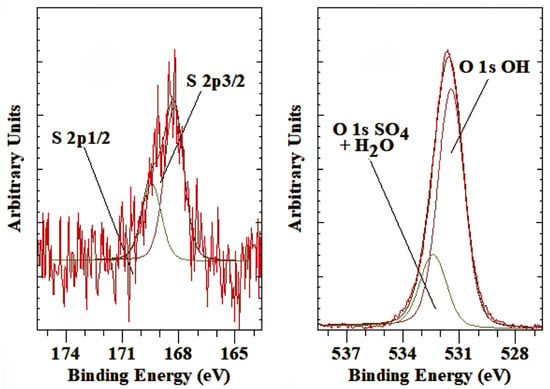
Figure 6.
XPS high-resolution scan of S 2p and O 1s of LDH with phosphate as the interlayer anion.
Bish and Livingstone [61] described for honessite the SO42− ν1, ν2, ν3, and ν4 infrared active modes at 980, 500, 1140, and 650 cm−1, respectively. The ν3 mode is obviously split, though no separate band positions were provided. Since all four modes are infrared active, the symmetry of the SO42− anion was lowered from Td (free anion) to either C3 or C3v, causing the activation of the two infrared inactive modes together with the splitting of the ν3 mode ([65], p. 140). Earlier work in our group has shown that the infrared spectrum of Mg/Al-LDH containing SO42− in the interlayer space exhibits a strong but broad ν3 mode at 1126 cm−1, a ν4 mode at 614 cm−1, and a very weak ν1 mode at 981 cm−1 [19]. The ν2 mode was not observed as a distinct band, in contrast to honessite and hydrohonnesite, due to its overlap with the O-M-O bending vibration of the LDH hydroxide layer around 450 cm−1 [66]. Fahami and Beall [67] reported only a band at a significantly lower wavenumber position around 1066 cm−1, which was attributed to the bending mode of interlayer sulfate ions. Liu and Yang [68] described a single band for Zn/Al-LDH at 1113 cm−1, which is much closer to the value observed by Kloprogge, Wharton, Hickley and Frost [19]. Frost et al. [69] described for glaucocerinite (Zn,Cu)10Al6(SO4)3(OH)32∙18H2O, a naturally occurring LDH mineral, three strong bands at 1053, 1078 and 1109 cm−1, which was attributed to the SO42− ν3 antisymmetric stretching mode. A weak band at 986 cm−1 was assigned to the corresponding ν1 symmetric stretching mode. They also reported that carrboydite exhibited three similar bands at 1088, 1021 and 978 cm−1, with the first two bands due to ν3 antisymmetric stretching modes and the last band to the ν1 symmetric stretching mode.
Kloprogge, Wharton, Hickley and Frost [19] showed that the Raman spectrum of sulphate-LDH consisted of a very intense ν1 mode at 982 cm−1 together with medium intensity ν2 and ν4 modes around 453 and 611 cm−1, respectively. The ν3 mode was not observed as a separate band, even though broadband is visible around 1134 cm−1. They indicated that when SO42− is located in the interlayer space of LDH, the infrared ν2 mode may become inactive, while at the same time, the ν1 mode becomes active. In contrast to the other modes, the ν3 is considerably broader, indicating the possible existence of two overlapping bands due to splitting. Dutta and Puri [70] proposed a D2 site symmetry for the sulfate anion. This is not compatible with the infrared spectrum, where all four modes are present. For analogous reasons, the C3 site symmetry, as proposed by Bish [71], is not compatible with the Raman spectrum. Hence, using the combined observations in both the infrared and Raman spectra Kloprogge, Wharton, Hickley and Frost [19] concluded that the site symmetry is most probably C2v with ν1 infrared and Raman active, ν2 infrared and Raman active, and ν3 and ν4 infrared and Raman active. A similar conclusion was reached by Lin et al. [72] for NixZn6−xAl2(OH)16(SO4)·4H2O, while Frost, Theiss, López and Scholz [69] concluded for glaucocerinite, a reduction in symmetry of the sulfate anion from Td to C2v or even lower symmetry.
It is clear from the infrared and Raman data that the sulfate anion, when present in the interlayer space, is restricted in its movement and has a lower site symmetry due to the interactions with the LDH hydroxide layers. This is also reflected in the XPS data, where the S 2p clearly shows a slightly lower BE compared to structural sulfate in mineral structures. This confirms that the sulfate anion is no longer a completely free anion, but it is, to a certain extent, interacting with the hydroxide layers.
3.5. Molybdate Anion
The MoO4-LDH is characterized by a single peak for the Mo 3d5/2 at a BE of 229.6 eV (Figure 7 left). This BE value is lower than, e.g., molybdenite, MoS2, with a BE of 230.1 eV or wulfenite, PbMoO4, with a BE of 231.9 eV [3]. Several molybdates, such as Al2(MoO4)3 (232.5 eV), CaMoO4 (232.8 eV), NiMoO4 (233.0), etc. all have higher BE than observed for intercalated molybdate ions in LDH. This is similar to what has been observed before, an indication that the interactions between the molybdate anion and the LDH hydroxide layers are not as strong as when the molybdate is part of a rigid crystal structure. The corresponding O 1s peak is observed at 530.3 eV (Figure 7 right), which compares well with the value observed for wulfenite at 530.0 eV. The NIST database reports for H2MoO4 a BE of 530.7 eV, CaMoO4 at 530,6 eV, and NiMoO4 at 530.9 eV, while for Al2(MoO4), the BE ranges from 530.7 to 531.0 eV [51]. Thao et al. [73] assigned the Mo 3d5/2 peak at 232.6 eV to Mo(VI) in MoO42−. The hardly visible shoulder at 231.7 eV was attributed to Mo(V) species, possibly caused by a charge transfer between (Mo6+ + O2−) and (Mo5+ + O−) [74,75]. This shoulder was not observed in this study. Thao, Trung and Van Long [73] also reported the BE value of O 1s at 532.4 eV for the fresh molybdate-LDH assigned to the O2− [35,38,45]. They concluded that it is probably composed of two overlapping photoelectron peaks at 532.4 and 531.5 eV. The peak at 532.4 eV was assigned to the metal hydroxides in the LDH layers, while the other peak at 531.6 eV was attributed to oxygen O− in the oxomolybdenum. Both the Mo 3d5/2 and O 1s peaks for the MO42− anion are at slightly higher BE values than observed in this study and in comparison with other molybdate structures such as those listed in Kloprogge and Wood [45] and the NIST database [51].
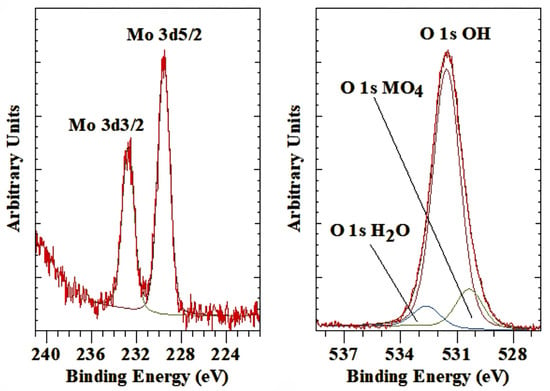
Figure 7.
XPS high-resolution scan of Mo 3d and O 1s of LDH with molybdate as the interlayer anion.
Previously, infrared and Raman spectroscopy on the same sample studied here have shown that molybdate anions are not polymerized in the interlayer space of the LDH because of the synthesis under alkaline conditions. The splitting of the vibrational modes of the molybdate anion in the vibrational spectra suggests a symmetry lowering due to interactions of the molybdate anion with the LDH hydroxide surfaces and interlayer water [16]. Palmer, Soisonard and Frost [21] reported a sharp Raman band at around 900 cm−1, which was assigned to the MoO42− symmetric stretching mode. A single broad IR band at approximately 830 cm−1 was reported as characteristic of the MoO42− anion, by Klemkaitė-Ramanauskė et al. [76]. Mitchell and Wass [77] confirmed the finding of Adebajo, Musumeci, Kloprogge, Frost and Martens [16] that at pH above 7–8, the prevalent molybdate species was the MoO42− ion characterized by an IR band at ca. 830 cm−1, while LDH prepared at pH 4.5 and with high molybdate loadings had an IR band at ca. 920–930 cm−1 and bands or shoulders near 890 cm−1 suggesting the presence of polymolybdate. Nejati et al. [78] reported a single band in the IR spectrum at 806 cm−1 assigned to the antisymmetric stretching mode of Mo–O. Yu et al. [79] observed a similar IR band attributed to the antisymmetric mode of Mo–O–Mo in MoO42− at 834 cm−1. Their Raman spectrum showed the Mo–O symmetrical stretching mode in MoO42− at 910 cm−1, while two bands at 320 and 220 cm−1 were attributed to the Mo = O bending and Mo–O–Mo deformation mode, respectively. A tetrahedral ion like MoO42− has four vibrational modes if it has full Td symmetry. These consist of ν1 (A1), ν2 (E), ν3, and ν4 (F2). The A1 symmetric stretching mode and the E bending mode are Raman active only, while the F2 stretching and bending modes are both IR and Raman active. For MoO42− the fundamental modes are found at 894 cm−1 (ν1), 381 cm−1 (ν2), 833 cm−1 (ν3), and 318 cm−1 (ν4) [80]. Based on these fundamental modes, it seems incorrect to attribute the IR bands at 823 cm−1 and 635 cm−1 to antisymmetric and symmetric stretching vibrations of Mo-O bonds, as suggested Colombo et al. [81]. The most complete description has probably been provided by Thao, Trung and Van Long [73]. In the IR spectrum, they observed a broad band at 920 cm−1, which was attributed to the vibrations of Mo = O in polymolybdate Mo7O246−. A band at 670 cm−1 with a shoulder at 856 cm−1 was assigned to the Mo–O–Mo stretching mode of Mo O42− in the interlayer space of LDH. It is more likely that the 670 cm−1 band is associated with NO3−—in the system, which is supported by the band at 1370 cm−1. In the Raman spectra, the MoO42− symmetric stretching modes in the LDH were observed at 908 and 892 cm−1. They were interpreted as being associated with two different MoO42− anionic species; the first one is hydrated, and the other one is bonded to the brucite-like hydroxide surface of the LDH within the interlayer space [18]. A broad shoulder at 823 cm−1 was ascribed to the MoO42− antisymmetric stretching mode, while a band at 325 cm−1 was attributed to the Mo-O bending mode [79].
Infrared and Raman spectroscopy have shown that the nature of the molybdate anion can change depending on the synthesis conditions, in particular, the pH. Under alkaline conditions, the MoO42− anion is dominant, either in a hydrated state or forming a stronger interaction with the hydroxide layer of the LDH within the interlayer space, as shown by the slight shift in band positions. Such small changes can not be observed in the XPS spectrum, which only shows that the Mo 3d5/2 peak is found at a slightly lower BE than for molybdate in a rigid crystal structure, indicating that, although there is some interaction with the LDH layer structure, the interaction is not as strong as in molybdate minerals such as wulfenite or other crystalline molybdate compounds [45,51]. In addition, no proof was found for the existence of charge transfer between (Mo6+ + O2−) and (Mo5+ + O−), as suggested in some papers [74,75].
3.6. Chromate Anion
The Cr 2p high-resolution spectrum of the chromate intercalated LDH differs from the other anionic groups shown earlier in this paper in the sense that not one but two Cr 2p3/2 peaks are present (Figure 8 left). The first is a rather broad peak with a BE of 579.0 eV, while the second is much sharper and has a BE of 580.2 eV. The second peak is characteristic of Cr6+ in the CrO42− anion, while the first peak at lower BE is associated with the presence of Cr3+, indicative of substantial reduction of the CrO4 with time. Approximately 60% of all Cr has been reduced based on the peak surface area ratio, which is directly proportional to the amounts present. The mineral crocoite PbCrVIO4 showed a Cr 2p3/2 at 579.3 eV [45], while a compound such as BaCrVIO4 has a BE of 579.1 eV. In contrast, CrIII(OH)3 has a lower BE of 577.1–577.4 eV [51]. Alidokht et al. [82] observed that upon adsorption of chromate using LDH, complete reduction of the CrVI to CrIII took place with Cr 2p3/2 peak at a BE of 277.9 eV. A similar shift was also observed for the reduction of sodium chromate during ion etching in the XPS instrument by Treverton and Davies [83]. The Cr3+ is probably initially present in the form of Cr(OH)63− but this anion shows a strong affinity for carbonate [84], so the exchange of OH− for CO32− in the anion over time is well possible within the LDH interlayer space given that LDH itself also has a strong affinity for carbonate adsorption. The O 1s peak for the chromate is found around 530.2 eV, which is the same as that of crocoite, PbCrO4 [45], and comparable to compounds such as Na2CrO4 at 530.0–530.3 eV, CaCrO4 at 529.5 eV and Li2CrO4 at 530.3 eV [51]. The O 1s peak for CrIII compounds occurs at slightly high BE and can not be distinguished from the O 1s OH peak of the LDH layer structure (Figure 8 right). The NIST database reports BE values of 530.8 to 531.6 for Cr(OH)3 [51].
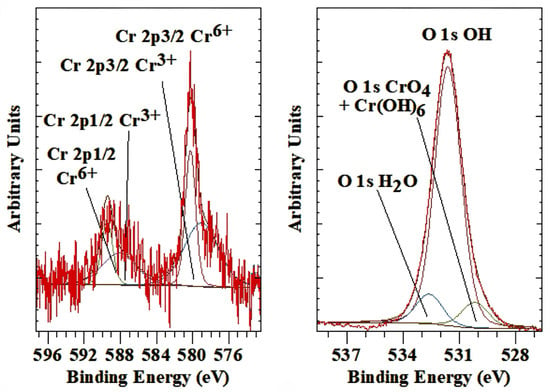
Figure 8.
XPS high-resolution scan of Cr 2p and O 1s of LDH with chromate as the interlayer anion.
Vibrational spectroscopy (Raman and FTIR) results on the fresh sample showed that the initial chromate anions were not polymerized to Cr2O72− in the LDH interlayer space [16]. The Raman spectrum of the chromate anion in solution reveals distinct vibrational modes: the symmetric stretching mode (ν1) is observed at 848 cm−1, the bending mode (ν2) at 348 cm−1, the asymmetric stretching mode (ν3) at 884 cm−1, and the out-of-plane bending mode (ν4) at 363 cm−1 [80]. Frost, Musumeci, Martens, Adebajo and Bouzaid [18] reported the Raman spectrum of the same freshly prepared sample as later used in this study for the XPS analyses. They observed a strong band at 848 cm−1 attributed to the ν1 symmetric stretching vibrational mode, while two bands at 884 and 928 cm−1 were assigned to the ν3 antisymmetric stretching vibrational mode. The band at 474 cm−1 was assigned to the ν4 bending mode. Finally, two bands at 363 and 237 cm−1 were interpreted as being the ν2 bending modes. The splitting of the ν3 and ν2 modes is suggestive of an initial lowering of the symmetry of the chromate anions in the LDH interlayer space prior to partial reduction. The symmetry lowering must be taken into account through the interaction of the CrO42− anions with both interlayer water and the LDH hydroxide layer surfaces on both sides of the interlayer space. They did not give an explanation for a broad band at 821 cm−1. Although this is lower than reported for fresh Cr(OH)3 around 850 cm−1 [85], it is possible that this band is associated with the start of the reduction of the Cr6+ to Cr3+ and represents the Cr-OH symmetric and antisymmetric stretching mode and the shift is due to the restricted space within the LDH interlayer space. The IR interlayer CrO42− υ3 (Cr-O) mode was reported at 870 cm−1 by Del Arco et al. [86], while Prasanna and Vishnu Kamath [87] found the same band at 866 cm−1. After heating between 100 and 200 °C, a split of this band was observed, and two new bands appeared around 874 and 930 cm−1, supporting a change in the anion symmetry from Td to C3v as a result of the interaction between the CrO42− and the LDH hydroxide layers similar to what was observed in the Raman spectra by Frost, Musumeci, Martens, Adebajo and Bouzaid [18]. Prasanna et al. [88] also observed in the mid-IR spectrum of intercalated CrO42− two bands at 866 and 917 cm−1. The tetrahedral chromate ion is characterized by two distinct IR-active vibrational modes: the antisymmetric stretch (ν3) and the symmetric deformation mode (ν4). The ν4 is expected to be observed around 330 cm−1, i.e., outside the mid-IR range and therefore not observed. The two bands represent the triply degenerate (F2) mode of the chromate. With C3v symmetry, the antisymmetric stretching vibration splits into two modes (A1 + E) ([65], p. 414).
The XPS Cr 2p high-resolution scan of the chromate-LDH provided evidence that upon aging, the nature of the interlayer anion changed, resulting in the reduction of Cr6+ to Cr3+. Since the Raman spectrum of the same sample was taken shortly after synthesis, the spectrum did not show any significant evidence of this reduction [18]. Instead, the Raman spectrum is dominated by the CrO42− anion with a reduced symmetry, as has also been observed in the infrared spectra of fresh chromate-LDH samples. The XPS data were obtained about 6 months after the Raman spectra, and by then, approximately 60% of the Cr6+ was reduced to Cr3+.
3.7. Hexacyanoferrate Anions
In earlier work by Kloprogge, Ponce and Ortillo [50] for the hexacyanoferrate(II) anion intercalated in LDH, the N 1s peak was found at 397.1 eV for Fe(CN)64− while for Fe(CN)63− it was observed at 397.4 eV (Figure 9 left). The NIST database shows that Fe(CN)64 compounds have N 1s BE values of around 397.4 to 398.0 eV, while K3Fe(CN)6 had a BE of 398.1 eV [51], indicating that there is a minor difference in BE for N 1s in these two anions.
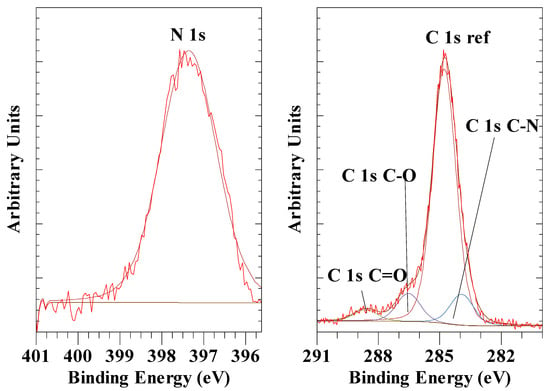
Figure 9.
XPS high-resolution scan of N 1s and C1s of LDH with Fe(CN)64− as the interlayer anion.
There were significant differences in the Fe 2p3/2 high-resolution spectra, with the Fe(CN)63− exhibiting the main peak at a BE of 707.7 eV and a second peak at a BE of 709.5 eV. In contrast, the Fe(CN)64 spectrum exhibited only one peak at 707.8 eV (see Figure 14 in [50]). For K4Fe(CN)6, the NIST database shows a BE value of 707.1 and 708. 5 eV, while for K3Fe(CN)6, it was reported at 709.6 eV [51]. Holgado et al. [89] used Fe-XANES analysis to show that partial reduction of the Fe(III) in the Fe(CN)64 intercalated LDH had taken place. Moreover, Idemura, Suzuki and Ono [32] showed the reduction of Fe(III) in Fe(CN)64 complexes by Mössbauer spectroscopy. Yamashita and Hayes [90] found that the Fe 2p3/2 for Fe(II) has a BE of about 1 eV lower than Fe(III) in their oxides. The same is true for the potassium hexacyanoferrates and in this study of intercalated LDH with hexacyanoferrates. Kloprogge, Ponce and Ortillo [50] concluded that the Fe 2p3/2 peak at 707.7 eV is due to Fe(II) caused by partial reduction in the initial Fe(III) observed at 709.46 eV. In their study, they did not report on the C 1s results for these two samples; for the Fe(CN)64− LDH, the C 1s of the C-N bond was observed at a BE value of 283.5 eV while for Fe(CN)63− LDH at was observed at 283.9 eV (Figure 9 right). These BE values, and the small difference between the two compare well with the values and differences reported for K4Fe(CN)6 at 283.5 eV and K3Fe(CN)6 at 283.9 eV [51].
The infrared and Raman spectra of hexacyanoferrate intercalated LDH have been reported for the last 30 years. Kikkawa and Koizumi [91] reported a band around 2000 cm−1 was assigned to the CN stretching mode. In contrast, Idemura, Suzuki and Ono [32] observed for the anion-exchanged LDH in an aqueous solution of K3Fe(CN)6 resulted in two bands at 2120 and 2040 cm−1 in the CN stretching region, indicating that part of the cyanoferrate(III) complex was reduced to cyanoferrate(II) complex during the intercalation. They based this on the fact that pure K3Fe(III)(CN)6 and K4Fe(II)(CN)6.3H2O produce IR active bands at 2120 and 2040 cm−1, respectively. After the intercalation of Fe(CN)64− in the LDH, no change in the oxidation state of Fe was detected. A band at 2040 ± 4 cm−1 was the stretching vibration of ν(CN) of Fe(CN)64− intercalated in LDH by Mao et al. [92]. Amini, Rahimpour and Jouyban [29] reported two bands at 2038 and 2113 cm−1 for Fe(CN)63− intercalated Ni/Al–LDH assigned to CN bound to Fe(II) and to CN bound to Fe(III), respectively. Panda et al. [93] described an intense sharp band at 2094 cm−1 attributed to the CN stretching mode of hexacyanoferrate(III) anions, but in addition, two weak bands were observed around 2002 and 2163 cm−1. The band at 2002 cm−1 was interpreted as being due to the partial reduction of Fe3+ to Fe2+. The weak band at 2163 cm−1 was thought to be the result of the formation of nickel ferricyanide, in which the hexacyanoferrate(III) anion is free from the LDH layers. Likewise, Meng et al. [94] observed a band at 2111 cm−1 was assigned to the CN stretching mode of hexacyanoferrate(III), and a second weak band at 2034 cm−1 was attributed to the CN stretching mode of hexacyanoferrate(II), suggesting that a small amount of Fe3+ was reduced to Fe2+ in the LDH interlayer space. Holgado, Rives, Sanromán and Malet [89] described two sharp bands at 2035 ±1 cm−1 assigned to the CN stretching mode of hexacyanoferrate(II) and at 2120–2086 cm−1 attributed to the CN stretching mode of hexacyanoferrate(III) intercalated in LDH.
Hansen and Koch [31] detected the CN-stretching mode of hexacyanoferrate(II) and (III) at 2036 and 2112 cm−1, respectively. In addition, they observed a band at 2080 cm−l, which they attributed to the presence of free cyanide anions in the interlayer space of the LDH, indicating that the hexacyanoferrate(II,III) cyanide ligands can be replaced with either water or hydroxyls. In addition, oxidation and ligand substitution of the hexacyanoferrate(II) were observed by Mössbauer spectroscopy. In contrast, Yao et al. [95] attributed a band at 2030 cm−1 when Fe(CN)63− was incorporated in LDH to a CN− group in which the bond strength of CN− was weaker due to the interaction with the LDH hydroxide layer. The intensity of the band at 2030 cm−1 was shown to increase upon aging. Crespo, Barriga, Rives and Ulibarri [35] remarked that regardless of the nature of the starting hexacyanoferrate, two separate or overlapping bands were observed in all cases. Furthermore, the two bands are found closer to each other (maximum Δν = 46 cm−1) than for the pure potassium salts (Δν = 73 cm−1), indicating that the samples underwent redox processes resulting in a mixture of Fe2+ and Fe3+ species. They indicated that it is common knowledge from coordination chemistry that the exact band position of the ν(CN) stretching mode for hexacyanoferrate(II) varies with the nature of the counter-cation. In the case of intercalated LDH, hydrogen bonding between interlayer water molecules or the LDH hydroxide layer hydroxyl groups and the cyano groups may likewise have an effect on the exact band position. Fernández et al. [96] observed the most intense band at 2118 cm−1. In addition, a weak band was observed at 2042 cm−1, while even weaker shoulders were detected at 2089 and 2060 cm−1. The νCN band, at 2042 cm−1, indicates a partial Fe3+ to Fe2+ reduction within the LDH interlayer space, similar to other studies. It is known that hexacyanoferrate anions are outer-sphere electron-transfer reductants or oxidants [97,98], based upon which the origin of the two weaker bands at 2089 and 2060 cm−1 can be assigned. Based on Jones [99] the A1g, Eg and T1u νCN modes required by the Oh point group for Fe(CN)64− are found at 2094, 2062 and 2044 cm−1 in aqueous solution. The first two modes are infrared-forbidden, but in the restricted interlayer space of the LDH, they can become partially activated due to a decrease in symmetry, resulting in the two weak bands at 2089 and 2060 cm−1, respectively. If grafting had happened (if the calculated interlayer space height was smaller than the size of the Fe(CN)64− anion along the C3 axis), the decrease in symmetry would have been much more dramatic, and the infrared spectrum much more complicated. Braterman et al. [100] and Boclair et al. [101] studied oriented samples of hexacyanoferrate intercalated LDH and compared those to randomly oriented samples. The random-oriented intercalated LDH exhibited two overlapping bands of similar intensity, with the higher wavenumber band being the broader of the two. This higher wavenumber band at 2041 cm−l was still visible in the oriented sample, with unaltered band shape and width, but the sharper, lower wavenumber band at 2035 cm−1 was no longer visible. Therefore, both these intense bands were interpreted to correlate with T1u in Oh, and their separation is proof of a local reduction of symmetry. They attributed this effect to a change to D3d symmetry by the ferrocyanide anion, resulting in a splitting of this mode into Eu and A2u components. The symmetry reduction is a result of the orientation of the ferrocyanide anions in the interlayer space of the LDH, which lies with two opposed triangular faces of the coordination octahedron parallel to the internal surfaces of the LDH hydroxide layers. Under these circumstances, the Eu component is likely to be broader than the A2u component. Furthermore, the Eu component is x,y-polarized in the molecular axis system, while the A2u component is z-polarized. This explains the detected selectivity. The molecular three-fold z-axis, the crystallographic c-axis, and the direction of light propagation are all parallel in the oriented LDH sample.
Two Raman spectroscopic studies on hexacyanoferrate intercalated LDH were published by Kloprogge et al. [102] and Frost, Musumeci, Bouzaid, Adebajo, Martens and Theo Kloprogge [30]. Details about the symmetry of the hexacyanoferrate anion and its reduction and oxidation behavior after intercalation in LDH can be obtained by comparing the Raman spectra with the infrared spectra. Free Fe(CN)64− will exhibit three vibrational modes with A1g at 2098 cm−1 and Eg at 2062 cm−1 in the Raman spectrum, and T1u at 2044 cm−1 in the infrared spectrum. For the intercalated LDH, they detected bands in the Raman spectrum at 2136, 2094 and 2065 cm−1 for hexacyanoferrate(II) intercalated LDH and at 2164, 2136, 2094 and 2059 cm−1 for hexacyanferrate(III) intercalated LDH, clearly supporting the fact that a change in the site symmetry had taken place as well as partial reduction. Lowering the site symmetry to C3 after intercalation in LDH would predict four bands in the Raman spectra for both the hexacyanoferrate (II) and hexacyanoferrate(III) anions, resulting in theoretically eight modes. In reality, it is expected that a number of these modes will overlap, causing the detection of fewer bands.
It is clear that the case of hexacyanoferrate(III) intercalation in LDH generally results in a partial reduction of Fe3+ to Fe2+. In contrast to the other anionic groups studied here, the reduction of hexacyanoferrate(III) results in clear changes in the infrared and Raman spectra. These changes are also clearly visible in the XPS Fe 2p spectra. Integration of the peak areas for both the Fe 2p3/2 of Fe(II) and Fe(III) allows for a direct measure of the amount of reduction. In this sample, 45% of the Fe(III) was reduced to Fe(II).
3.8. Influence of Interlayer Anions on the LDH Layer Structure
From the previous sections, it is clear that the nature of the interlayer anion can be studied not only by vibrational spectroscopy (infrared and Raman) but also by XPS, and additional information can be obtained. In most instances, the BE of the non-oxygen atom is slightly lower than that of minerals, in which the anion forms part of the rigid crystal structure (Table 2). In several instances, evidence was found for partial reduction of the metal upon intercalation, but this was not detected by vibrational spectroscopy. Since the interlayer anions interaction, most likely through hydrogen bonds with the hydroxide layers of the LDH within the interlayer space, it is of interest to see if the atoms that make up the LDH layer structure are in any way affected by the nature of the interlayer anion. Table 1 provides an overview of the BE values obtained for Al 2p, Mg 2p and O 1s for the hydroxyl group. In all instances, the BE values are very close, though it seems that the MoO42− and CrO42− ions, with their much heavier metals, result in slightly lower BE values for Al 2p, Mg 2p and O 1s. The differences, however, are so small that they may be considered to be within the experimental error of XPS.

Table 2.
BE values of Al 2p, Mg 2p and O 1s for the LDH layer structure as a function of the interlayer anion.
3.9. Limitations of XPS
X-ray Photoelectron Spectroscopy (XPS) is a powerful surface-sensitive analytical technique that provides valuable insight into the elemental composition and chemical state of materials. However, it is not without its limitations. One significant drawback of XPS is its relatively low spatial resolution, typically on the order of 1–10 μm. This constraint can be particularly problematic when investigating heterogeneous materials—such as layered structures or materials with fine features—where localized variations in composition must be accurately assessed. Additionally, because XPS only probes the top 1–10 nanometers of a sample, it may not provide a comprehensive understanding of bulk properties or subsurface characteristics.
In the context of analyzing layered double hydroxides (LDHs), XPS exhibits specific challenges that may affect the reliability of the data obtained. The presence of moisture or atmospheric contamination can introduce artifacts that may skew the results, highlighting the need for stringent sample preparation and vacuum conditions. Additionally, the understanding of the oxidation states and coordination environments of the metal species within LDH structures can be intricate, often requiring complementary techniques to corroborate XPS findings.
Another noteworthy limitation of XPS is its relative insensitivity to light elements such as hydrogen and lithium, which may be critical for some LDH systems. The inherent constraints of XPS in detecting low atomic number elements can lead to an incomplete picture of the chemical environment, particularly when metal cations or interlayer anions play a significant role in the material’s properties and reactivity. Consequently, while XPS remains a valuable tool for elucidating the surface chemistry of LDHs, researchers must acknowledge these drawbacks and adopt a multi-faceted approach—utilizing complementary analytical techniques such as atomic force microscopy (AFM), scanning electron microscopy (SEM), or Fourier-transform infrared spectroscopy (FTIR)—to obtain a more robust understanding of the material’s characteristics.
4. Conclusions
This comprehensive analysis of Layered Double Hydroxides (LDHs) intercalated with various anionic species elucidates the intricate interplay between interlayer anions and LDH structural dynamics. X-ray photoelectron spectroscopy (XPS) characterized the distinct binding energies for key elements, confirming the presence of carbonate, phosphate, sulfate, molybdate, chromate, and hexacyanoferrate anions within the interlayer space. The carbonate anion exhibited a notable reduction in binding energy compared to common carbonate minerals, attributed to interactions with interlayer water and hydroxyl groups, suggesting semi-constrained behavior within the LDH framework. In contrast, phosphate intercalation demonstrated stability without shifts in binding energies, while sulfate ions indicated limited movement, suggesting moderate interaction with hydroxide layers. Molybdate displayed weaker interactions than crystalline counterparts, reflecting the influence of LDH synthesis conditions. A significant observation was the reduction of chromate ions from Cr6+ to Cr3+, indicating dynamic redox behavior within the interlayer space. Furthermore, hexacyanoferrate anions revealed partial reduction, with distinctive spectral differences indicating site symmetry alterations. Collectively, these findings articulate the necessity for considering anion identity and synthesis parameters in the application and functional design of LDHs, highlighting their potential roles in catalysis, ion exchange, and environmental remediation. Further investigations into the relationships between interlayer anions and LDH stability are warranted to broaden the understanding of their reactivity and utility in advanced materials science.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The author acknowledges the facilities and the scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland. The authors also acknowledge the provision of library resources and access to journals by the University of the Philippines System.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural Aspects of Layered Double Hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–87. [Google Scholar]
- Wypych, F.; de Freitas, R.A. Chapter 10—Layered double hydroxides and hydroxide salts: Structure and properties. In Developments in Clay Science; Wypych, F., de Freitas, R.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 10, pp. 317–350. [Google Scholar]
- Miyata, S.; Kumura, T. Synthesis of new hydrotalcite-like compounds and their physico-chemical properties. Chem. Lett. 1973, 2, 843–848. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and Catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Farhan, A.; Khalid, A.; Maqsood, N.; Iftekhar, S.; Sharif, H.M.A.; Qi, F.; Sillanpää, M.; Asif, M.B. Progress in layered double hydroxides (LDHs): Synthesis and application in adsorption, catalysis and photoreduction. Sci. Total Environ. 2024, 912, 169160. [Google Scholar] [CrossRef]
- Kameliya, J.; Verma, A.; Dutta, P.; Arora, C.; Vyas, S.; Varma, R.S. Layered Double Hydroxide Materials: A Review on Their Preparation, Characterization, and Applications. Inorganics 2023, 11, 121. [Google Scholar] [CrossRef]
- Karmakar, A.K.; Hasan, M.S.; Sreemani, A.; Das Jayanta, A.; Hasan, M.M.; Tithe, N.A.; Biswas, P. A review on the current progress of layered double hydroxide application in biomedical sectors. Eur. Phys. J. Plus 2022, 137, 801. [Google Scholar] [CrossRef]
- Tang, S.; Yao, Y.; Chen, T.; Kong, D.; Shen, W.; Lee, H.K. Recent advances in the application of layered double hydroxides in analytical chemistry: A review. Anal. Chim. Acta 2020, 1103, 32–48. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhou, B.; Meng, F.; Wang, Y.; Wen, G. Recent advance of layered double hydroxides materials: Structure, properties, synthesis, modification and applications of wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 111191. [Google Scholar] [CrossRef]
- Nalawade, P.; Aware, B.N.; Kadam, V.J.; Hirlekar, R.S. Layered double hydroxides: A review. J. Sci. Ind. Res. 2009, 68, 267–272. [Google Scholar]
- Evana, E.; Marchidan, R.; Mănăila, R. NO3−–CO32− anion exchange during washing of Ni-Al hydroxy-compounds. Bull. Soc. Chim. Belge 1992, 2, 101–107. [Google Scholar] [CrossRef]
- Miyata, S. The synthesis of hydrotalcite-like compounds and their structures and physico-chemical properties—I: The systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Miyata, S.; Okada, A. Synthesis of hydrotalcite-like compounds and their physico-chemical properties—The system Mg2+–Al3+–SO42– and Mg2+–Al3+–CrO42–. Clays Clay Miner. 1977, 25, 14–18. [Google Scholar] [CrossRef]
- Reichle, W.T.; Kang, S.Y.; Everhardt, D.S. The nature of the thermal decomposition of a catalytically active anionic clay mineral. J. Catal. 1986, 101, 352–359. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Musumeci, A.W.; Kloprogge, J.T.; Frost, R.L.; Martens, W.N. Synthesis and characterization of hydrotalcites containing interlayer sulphate, molybdate and chromate anions. In Proceedings of the 13th International Clay Conference, Tokyo, Japan, 21–27 August 2005; p. 127. [Google Scholar]
- Frost, R.L.; Musumeci, A.W.; Kloprogge, J.T.; Adebajo, M.O.; Martens, W.N. Raman spectroscopy of hydrotalcites with phosphate in the interlayer: Implications for the removal of phosphate from water. J. Raman Spectrosc. 2006, 37, 733–741. [Google Scholar] [CrossRef]
- Frost, R.L.; Musumeci, A.W.; Martens, W.N.; Adebajo, M.O.; Bouzaid, J. Raman spectroscopy of hydrotalcites with sulphate, molybdate and chromate in the interlayer. J. Raman Spectrosc. 2005, 36, 925–931. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wharton, D.; Hickley, L.; Frost, R.L. Infrared and Raman study of interlayer anions CO32–, NO3−, SO42− and ClO4− in Mg/Al-hydrotalcite. Amer. Miner. 2002, 87, 623–629. [Google Scholar] [CrossRef]
- Ookubo, A.; Ooi, K.; Tani, F.; Hayashi, H. Phase Transition of Cl−-Intercalated Hydrotalcite-like Compound during Ion Exchange with Phosphates. Langmuir 1994, 10, 407–411. [Google Scholar] [CrossRef]
- Palmer, S.J.; Soisonard, A.; Frost, R.L. Determination of the mechanism(s) for the inclusion of arsenate, vanadate, or molybdate anions into hydrotalcites with variable cationic ratio. J. Colloid Interface Sci. 2009, 329, 404–409. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Y.; Han, T.; Fang, M.; Chen, K.; Tan, X. Phosphate functionalized layered double hydroxides (phos-LDH) for ultrafast and efficient U(VI) uptake from polluted solutions. J. Hazard. Mater. 2020, 399, 123081. [Google Scholar] [CrossRef]
- Pálinkó, I.; Sipos, P.; Berkesi, O.; Varga, G. Distinguishing Anionic Species That Are Intercalated in Layered Double Hydroxides from Those Bound to Their Surface: A Comparative IR Study. J. Phys. Chem. C 2022, 126, 15254–15262. [Google Scholar] [CrossRef]
- Radha, S.; Vishnu Kamath, P. Electronic spectra of anions intercalated in layered double hydroxides. Bull. Mater. Sci. 2013, 36, 923–929. [Google Scholar] [CrossRef][Green Version]
- Mora, M.; Jiménez-Sanchidrián, C.; Rafael Ruiz, J. Raman spectroscopy study of layered-double hydroxides containing magnesium and trivalent metals. Mater. Lett. 2014, 120, 193–195. [Google Scholar] [CrossRef]
- Lv, S.; Zhao, Y.; Zhang, L.; Zhang, T.; Dong, G.; Li, D.; Cheng, S.; Ma, S.; Song, S.; Quintana, M. Anion regulation strategy of lithium-aluminum layered double hydroxides for strengthening resistance to deactivation in lithium recovery from brines. Chem. Eng. J. 2023, 472, 145026. [Google Scholar] [CrossRef]
- Ciocan, C.E.; Dumitriu, E.; Cacciaguerra, T.; Fajula, F.; Hulea, V. New approach for synthesis of Mo-containing LDH based catalysts. Catal. Today 2012, 198, 239–245. [Google Scholar] [CrossRef]
- Schutz, A.; Biloen, P. Interlamellar chemistry of hydrotalcites. I. Polymerzation of silcate anions. J. Solid State Chem. 1987, 68, 360–368. [Google Scholar] [CrossRef]
- Amini, R.; Rahimpour, E.; Jouyban, A. An optical sensing platform based on hexacyanoferrate intercalated layered double hydroxide nanozyme for determination of chromium in water. Anal. Chim. Acta 2020, 1117, 9–17. [Google Scholar] [CrossRef]
- Frost, R.L.; Musumeci, A.W.; Bouzaid, J.; Adebajo, M.O.; Martens, W.N.; Theo Kloprogge, J. Intercalation of hydrotalcites with hexacyanoferrate(II) and (III)—A thermoRaman spectroscopic study. J. Solid State Chem. 2005, 178, 1940–1948. [Google Scholar] [CrossRef]
- Hansen, H.C.B.; Koch, C.B. Synthesis and Properties of Hexacyanoferrate Interlayered in Hydrotalcite. I. Hexacyanoferrate(II). Clays Clay Miner. 1994, 42, 170–179. [Google Scholar] [CrossRef]
- Idemura, S.; Suzuki, E.; Ono, Y. Electronic State of Iron Complexes in the Interlayer of Hydrotalcite-Like Materials. Clays Clay Miner. 1989, 37, 553–557. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Y.; Wang, R.C.; Colon, J.L.; Cearfield, A. Systematic preparation of polyoxometalate pillared layered double hydroxides via direct aqueous reaction. Mater. Res. Soc. Symp. Proc. 1991, 233, 63–80. [Google Scholar] [CrossRef]
- Carpani, I.; Berrettoni, M.; Ballarin, B.; Giorgetti, M.; Scavetta, E.; Tonelli, D. Study on the intercalation of hexacyanoferrate(II) in a Ni, Al based hydrotalcite. Solid State Ion. 2004, 168, 167–175. [Google Scholar] [CrossRef]
- Crespo, I.; Barriga, C.; Rives, V.; Ulibarri, M.A. Intercalation of iron hexacyano complexes in zn,al-hydrotalcite. Solid State Ion. 1997, 101–103, 729–735. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, B.; Chen, Q.; Peng, X.; Yang, D.; Qiu, F. Layered double hydroxide functionalized biomass carbon fiber for highly efficient and recyclable fluoride adsorption. Appl. Biol. Chem. 2019, 62, 12. [Google Scholar] [CrossRef]
- Bao, W.; Tang, Y.; Yu, J.; Yan, W.; Wang, C.; Li, Y.; Wang, Z.; Yang, J.; Zhang, L.; Yu, F. Si-doped ZnAl-LDH nanosheets by layer-engineering for efficient photoelectrocatalytic water splitting. Appl. Catal. B Environ. Energy 2024, 346, 123706. [Google Scholar] [CrossRef]
- Lv, H.; Rao, H.; Liu, Z.; Zhou, Z.; Zhao, Y.; Wei, H.; Chen, Z. NiAl layered double hydroxides with enhanced interlayer spacing via ion-exchange as ultra-high performance supercapacitors electrode materials. J. Energy Storage 2022, 52, 104940. [Google Scholar] [CrossRef]
- Li, R.; Xu, J.; Pan, Q.; Ba, J.; Tang, T.; Luo, W. One-Step Synthesis of NiFe Layered Double Hydroxide Nanosheet Array/N-Doped Graphite Foam Electrodes for Oxygen Evolution Reactions. ChemistryOpen 2019, 8, 1027–1032. [Google Scholar] [CrossRef]
- Mahmoud, R.K.; Taha, M.; Zaher, A.; Amin, R.M. Understanding the physicochemical properties of Zn–Fe LDH nanostructure as sorbent material for removing of anionic and cationic dyes mixture. Sci. Rep. 2021, 11, 21365. [Google Scholar] [CrossRef]
- Li, X.; Fortunato, M.; Cardinale, A.M.; Sarapulova, A.; Njel, C.; Dsoke, S. Electrochemical study on nickel aluminum layered double hydroxides as high-performance electrode material for lithium-ion batteries based on sodium alginate binder. J. Solid State Electrochem. 2022, 26, 49–61. [Google Scholar] [CrossRef]
- Shen, W.; Hu, T.; Liu, X.; Zha, J.; Meng, F.; Wu, Z.; Cui, Z.; Yang, Y.; Li, H.; Zhang, Q.; et al. Defect engineering of layered double hydroxide nanosheets as inorganic photosensitizers for NIR-III photodynamic cancer therapy. Nat. Commun. 2022, 13, 3384. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Fourier Transform Infrared and Raman spectroscopic study of the local structure of Mg, Ni and Co — hydrotalcites. J. Solid State Chem. 1999, 146, 506–515. [Google Scholar] [CrossRef]
- Frost, R.L.; Musumeci, A.W.; Kloprogge, J.T.; Weier, M.; Adebajo, M.; Martens, W.N. Thermal decomposition of hydrotalcite with hexacyanoferrate(II) and hexacyanoferrate(III) anions in the interlayer. J. Therm. Anal. Cal. 2006, 86, 205–209. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wood, B.J. Handbook of Mineral Spectroscopy Volume 1 X-Ray Photoelectron Spectra; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, p. 505. [Google Scholar]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS study of the major minerals in bauxite: Gibbsite, bayerite and (pseudo-)boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Yu, J.; Zhao, Z.; Dai, J.; Fu, J.; Zhao, M.; Wang, W. Synthesis and Properties of a Clean and Sustainable Deicing Additive for Asphalt Mixture. PLoS ONE 2015, 10, e0115721. [Google Scholar] [CrossRef]
- Rey, F.; Fornes, V.; Rojo, J.M. Thermal decomposition of hydrotalcites. An infrared and nuclear magnetic resonance spectroscopic study. J. Chem. Soc. Faraday Trans. 1992, 88, 2233–2238. [Google Scholar] [CrossRef]
- Alzamora, L.E.; Ross, J.R.; Kruissink, E.C.; Reijen, L.L.v. Coprecipitated Nickel-Alumina catalysts for methanation at high temperature. J. Chem. Soc. Faraday Trans. I 1981, 77, 665–681. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Ponce, C.P.; Ortillo, D.O. X-ray Photoelectron Spectroscopic Study of Some Organic and Inorganic Modified Clay Minerals. Materials 2021, 14, 7115. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-Ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20. Available online: https://srdata.nist.gov/xps (accessed on 29 November 2024).
- Gupta, N.K.; Saifuddin, M.; Kim, S.; Kim, K.S. Microscopic, spectroscopic, and experimental approach towards understanding the phosphate adsorption onto Zn–Fe layered double hydroxide. J. Mol. Liquids 2020, 297, 111935. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Constantino, V.R.L.; Pinto, F.G.; Vergütz, L.; Tronto, J.; Da Costa, L.M. Layered Double Hydroxides: New Technology in Phosphate Fertilizers Based on Nanostructured Materials. ACS Sustain. Chem. Eng. 2017, 5, 399–409. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Eulálio, D.; Guimarães, L.D.M.; Pinto, F.G.; Costa, L.M.D.; Tronto, J. Layered Double Hydroxides as Hosting Matrices for Storage and Slow Release of Phosphate Analyzed by Stirred-Flow Method. Mater. Res. 2018, 21, e20171004. [Google Scholar] [CrossRef]
- Yang, K.; Yan, L.-g.; Yang, Y.-m.; Yu, S.-j.; Shan, R.-r.; Yu, H.-q.; Zhu, B.-c.; Du, B. Adsorptive removal of phosphate by Mg–Al and Zn–Al layered double hydroxides: Kinetics, isotherms and mechanisms. Sep. Purif. Technol. 2014, 124, 36–42. [Google Scholar] [CrossRef]
- Ross, S.D. Phosphates and other Oxy-anions of Group V. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society of Great Britain and Ireland: London, UK, 1974; Volume 4, pp. 383–422. [Google Scholar]
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Shabanian, M.; Hajibeygi, M.; Raeisi, A. 2—FTIR characterization of layered double hydroxides and modified layered double hydroxides. In Layered Double Hydroxide Polymer Nanocomposites; Thomas, S., Daniel, S., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 77–101. [Google Scholar]
- Cheng, X.; Huang, X.; Wang, X.; Sun, D. Influence of calcination on the adsorptive removal of phosphate by Zn–Al layered double hydroxides from excess sludge liquor. J. Hazard. Mater. 2010, 177, 516–523. [Google Scholar] [CrossRef]
- He, H.; Kang, H.; Ma, S.; Bai, Y.; Yang, X. High adsorption selectivity of ZnAl layered double hydroxides and the calcined materials toward phosphate. J. Colloid Interface Sci. 2010, 343, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Bish, D.L.; Livingstone, A. The crystal chemistry and paragenesis of honessite and hydrohonessite: The sulfate analogues of reevesite. Miner. Mag. 1981, 44, 339–343. [Google Scholar] [CrossRef]
- Nickel, E.H.; Clarke, R.M. Carrboydite, a hydrated sulfate of nickel and aluminum: A new mineral from Western Australia. Amer. Miner. 1976, 61, 366–372. [Google Scholar]
- Nickel, E.H.; Wildman, J.E. Hydrohonessite—A new hydrated Ni-Fe hydroxy-sulphate mineral; its relationship to honessite, carrboydite, and minerals of the pyroaurite group. Miner. Mag. 1981, 44, 333–337. [Google Scholar] [CrossRef]
- Wahlqvist, M.; Shchukarev, A. XPS spectra and electronic structure of Group IA sulfates. J. Electr. Spectros. Rel. Phenom. 2007, 156–158, 310–314. [Google Scholar] [CrossRef]
- Ross, S.D. Inorganic Infrared and Raman Spectra; McGraw-Hill Book Company: London, UK, 1972; pp. 140, 414. [Google Scholar]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. Synthesis and spectroscopic characterization of deuterated hydrotalcite. J. Mater. Sci. Lett. 2001, 21, 603–605. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W. Mechanosynthesis and characterization of Hydrotalcite like Mg–Al–SO4-LDH. Mater. Lett. 2016, 165, 192–195. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z. Intercalation of sulfate anions into a Zn–Al layered double hydroxide: Their synthesis and application in Zn–Ni secondary batteries. RSC Adv. 2016, 6, 68584–68591. [Google Scholar] [CrossRef]
- Frost, R.L.; Theiss, F.L.; López, A.; Scholz, R. Vibrational spectroscopic study of the sulphate mineral glaucocerinite (Zn,Cu)10Al6(SO4)3(OH)32⋅18H2O—A natural layered double hydroxide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 127, 349–354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dutta, P.K.; Puri, M. Anion exchange in lithium aluminate hydroxides. J. Phys. Chem. 1989, 93, 376–381. [Google Scholar] [CrossRef]
- Bish, D.L. Anion-exchange in takovite: Applications to other hydroxide minerals. Bull. Miner. 1980, 103, 170–175. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Adebajo, M.O.; Kloprogge, J.T.; Martens, W.N.; Frost, R.L. X-ray diffraction and Raman spectroscopic studies of Zn-substituted carrboydite-like compounds. Mater. Chem. Phys. 2006, 100, 174–186. [Google Scholar] [CrossRef][Green Version]
- Thao, N.T.; Trung, N.D.; Van Long, D. Activity of Molybdate-Intercalated Layered Double Hydroxides in the Oxidation of Styrene with Air. Catal. Lett. 2016, 146, 918–928. [Google Scholar] [CrossRef]
- Behera, G.C.; Parida, K.M. A comparative study of molybdenum promoted vanadium phosphate catalysts towards epoxidation of cyclohexene. Appl. Catal. A Gen. 2013, 464–465, 364–373. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Mendoza-Sanchez, B.; Fernandez, V.; Veenstra, R.; Dukstiene, N.; Roberts, A.; Fairley, N. Generalized molybdenum oxide surface chemical state XPS determination via informed amorphous sample model. Appl. Surf. Sci. 2015, 326, 151–161. [Google Scholar] [CrossRef]
- Klemkaitė-Ramanauskė, K.; Žilinskas, A.; Taraškevičius, R.; Khinsky, A.; Kareiva, A. Preparation of Mg/Al layered double hydroxide (LDH) with structurally embedded molybdate ions and application as a catalyst for the synthesis of 2-adamantylidene(phenyl)amine Schiff base. Polyhedron 2014, 68, 340–345. [Google Scholar] [CrossRef]
- Mitchell, P.C.H.; Wass, S.A. Propane dehydrogenation over molybdenum hydrotalcite catalysts. Appl. Catal. A Gen. 2002, 225, 153–165. [Google Scholar] [CrossRef]
- Nejati, K.; Akbari, A.R.; Davari, S.; Asadpour-Zeynali, K.; Rezvani, Z. Zn–Fe-layered double hydroxide intercalated with vanadate and molybdate anions for electrocatalytic water oxidation. New J. Chem. 2018, 42, 2889–2895. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Zhang, M.; Yang, P.; Yang, L.; Cao, D.; Li, J. One-step synthesis of lamellar molybdate pillared hydrotalcite and its application for AZ31 Mg alloy protection. Solid State Sci. 2009, 11, 376–381. [Google Scholar] [CrossRef]
- Ross, S.D. Sulphates and other Oxy-anions of Group VI. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society of Great Britain and Ireland: London, UK, 1974; Volume 4, pp. 423–444. [Google Scholar]
- Colombo, K.; Maruyama, S.; Yamamoto, C.; Wypych, F. Intercalation of Molybdate Ions into Ni/Zn Layered Double Hydroxide Salts: Synthesis, Characterization, and Preliminary Catalytic Activity in Methyl Transesterification of Soybean Oil. J. Brazil. Chem. Soc. 2016, 28, 1315–1322. [Google Scholar] [CrossRef]
- Alidokht, L.; Oustan, S.; Khataee, A.; Neyshabouri, M.; Reyhanitabar, A. Removal of chromate from aqueous solution by reduction with nanoscale Fe–Al layered double hydroxide. Res. Chem. Intermed. 2018, 44, 2319–2331. [Google Scholar] [CrossRef]
- Treverton, J.A.; Davies, N.C. An XPS study of chromate pretreatment of aluminium. Met. Technol. 1977, 4, 480–489. [Google Scholar] [CrossRef]
- Amonette, J.E.; Rai, D. Identification of Noncrystalline (Fe,Cr)(OH)3 by Infrared Spectroscopy. Clays Clay Miner. 1990, 38, 129–136. [Google Scholar] [CrossRef]
- Gomes, A.S.O.; Yaghini, N.; Martinelli, A.; Ahlberg, E. A micro-Raman spectroscopic study of Cr(OH)3 and Cr2O3 nanoparticles obtained by the hydrothermal method. J. Raman Spectrosc. 2017, 48, 1256–1263. [Google Scholar] [CrossRef]
- Del Arco, M.; Carriazo, D.; Martín, C.; Pérez Grueso, A.M.; Rives, V. Characterization of Chromate-Intercalated Layered Double Hydroxides. Mater. Sci. Forum 2006, 514–516, 1541–1545. [Google Scholar] [CrossRef]
- Prasanna, S.V.; Vishnu Kamath, P. Chromate uptake characteristics of the pristine layered double hydroxides of Mg with Al. Solid State Sci. 2008, 10, 260–266. [Google Scholar] [CrossRef]
- Prasanna, S.V.; Rao, R.A.P.; Kamath, P.V. Layered double hydroxides as potential chromate scavengers. J. Colloid Interface Sci. 2006, 304, 292–299. [Google Scholar] [CrossRef]
- Holgado, M.J.; Rives, V.; Sanromán, M.S.; Malet, P. Hexacyanoferrate-interlayered hydrotalcite. Solid State Ion. 1996, 92, 273–283. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Kikkawa, S.; Koizumi, M. Ferrocyanide anion bearing Mg, Al hydroxide. Mater. Res. Bull. 1982, 17, 191–198. [Google Scholar] [CrossRef]
- Mao, G.; Tsuji, M.; Tamaura, Y. Synthesis and CO2 Adsorption Features of a Hydrotalcite-Like Compound of the Mg2+-Al3+-Fe(CN)64− System with High Layer-Charge Density. Clays Clay Miner. 1993, 41, 731–737. [Google Scholar] [CrossRef]
- Panda, H.S.; Srivastava, R.; Bahadur, D. Intercalation of Hexacyanoferrate(III) Ions in Layered Double Hydroxides: A Novel Precursor to Form Ferri-/Antiferromagnetic Exchange Coupled Oxides and Monodisperse Nanograin Spinel Ferrites. J. Phys. Chem. C 2009, 113, 9560–9567. [Google Scholar] [CrossRef]
- Meng, W.; Li, F.; Evans, D.G.; Duan, X. Preparation and thermal decomposition of magnesium/iron(III) layered double hydroxide intercalated by hexacyanoferrate(III) ions. J. Mater. Sci. 2004, 39, 4655–4657. [Google Scholar] [CrossRef]
- Yao, K.; Taniguchi, M.; Nakata, M.; Shimazu, K.; Takahashi, M.; Yamagishi, A. Mass transport on an anionic clay-modified electrode as studied by a quartz crystal microbalance. J. Electroanal. Chem. 1998, 457, 119–128. [Google Scholar] [CrossRef]
- Fernández, J.M.; Ulibarri, M.A.; Labajos, F.M.; Rives, V. The effect of iron on the crystalline phases formed upon thermal decomposition of Mg-Al-Fe hydrotalcites. J. Mater. Chem. 1998, 8, 2507–2514. [Google Scholar] [CrossRef]
- Gordon, B.M.; Williams, L.L.; Sutin, N. The Kinetics of the Oxidation of Iron(II) Ions and of Coördination Complexes1a. J. Amer. Chem. Soc. 1961, 83, 2061–2064. [Google Scholar] [CrossRef]
- Pelizzetti, E.; Mentasti, E.; Baiocchi, C. Kinetics and mechanism of oxidation of quinols by hexachloroiridate(IV) in aqueous acidic perchlorate media. J. Phys. Chem. 1976, 80, 2979–2982. [Google Scholar] [CrossRef]
- Jones, L.H. Nature of Bonding in Metal Cyanide Complexes as Related to Intensity and Frequency of Infrared Absorption Spectra. Inorg. Chem. 1963, 2, 777–780. [Google Scholar] [CrossRef]
- Braterman, P.S.; Tan, C.; Zhao, J. Orientational effects in the infrared spectrum of the double layer material, magnesium aluminum hydroxide ferrocyanide. Mater. Res. Bull. 1994, 29, 1217–1221. [Google Scholar] [CrossRef]
- Boclair, J.W.; Braterman, P.S.; Brister, B.D.; Wang, Z.; Yarberry, F. Physical and Chemical Interactions between Mg:Al Layered Double Hydroxide and Hexacyanoferrate. J. Solid State Chem. 2001, 161, 249–258. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Weier, M.; Crespo, I.; Ulibarri, M.A.; Barriga, C.; Rives, V.; Martens, W.N.; Frost, R.L. Intercalation of iron hexacyano complexes in Zn,Al hydrotalcite. Part 2. A mid-infrared and Raman spectroscopic study. J. Solid State Chem. 2004, 177, 1382–1387. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).