Optimization of Microwave-Assisted Extraction Process for Production of Polyphenol-Rich Crude Extract from Cinnamomum iners Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Material Preparation
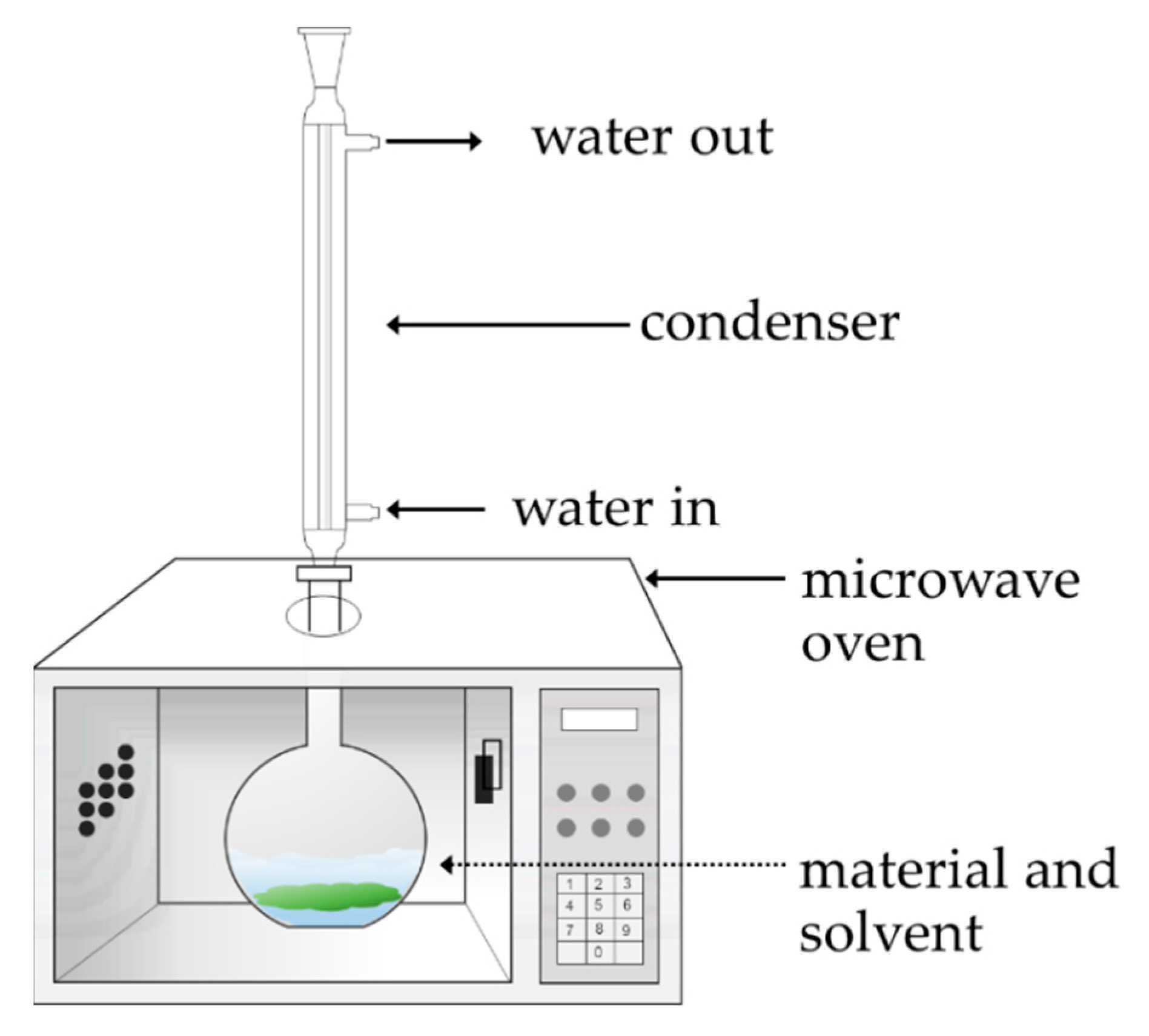
2.2. MAE Process for Polyphenol-Rich Crude Extract Production
2.3. Single-Factor Design
2.4. Response Design and Optimization of MAE Variables
2.5. Determination of Total Phenolic Content
2.6. Determination of Total Flavonoid Content
2.7. DPPH Radical Scavenging Activity Assessment
2.8. Comparison of MAE and Conventional HRE
3. Results
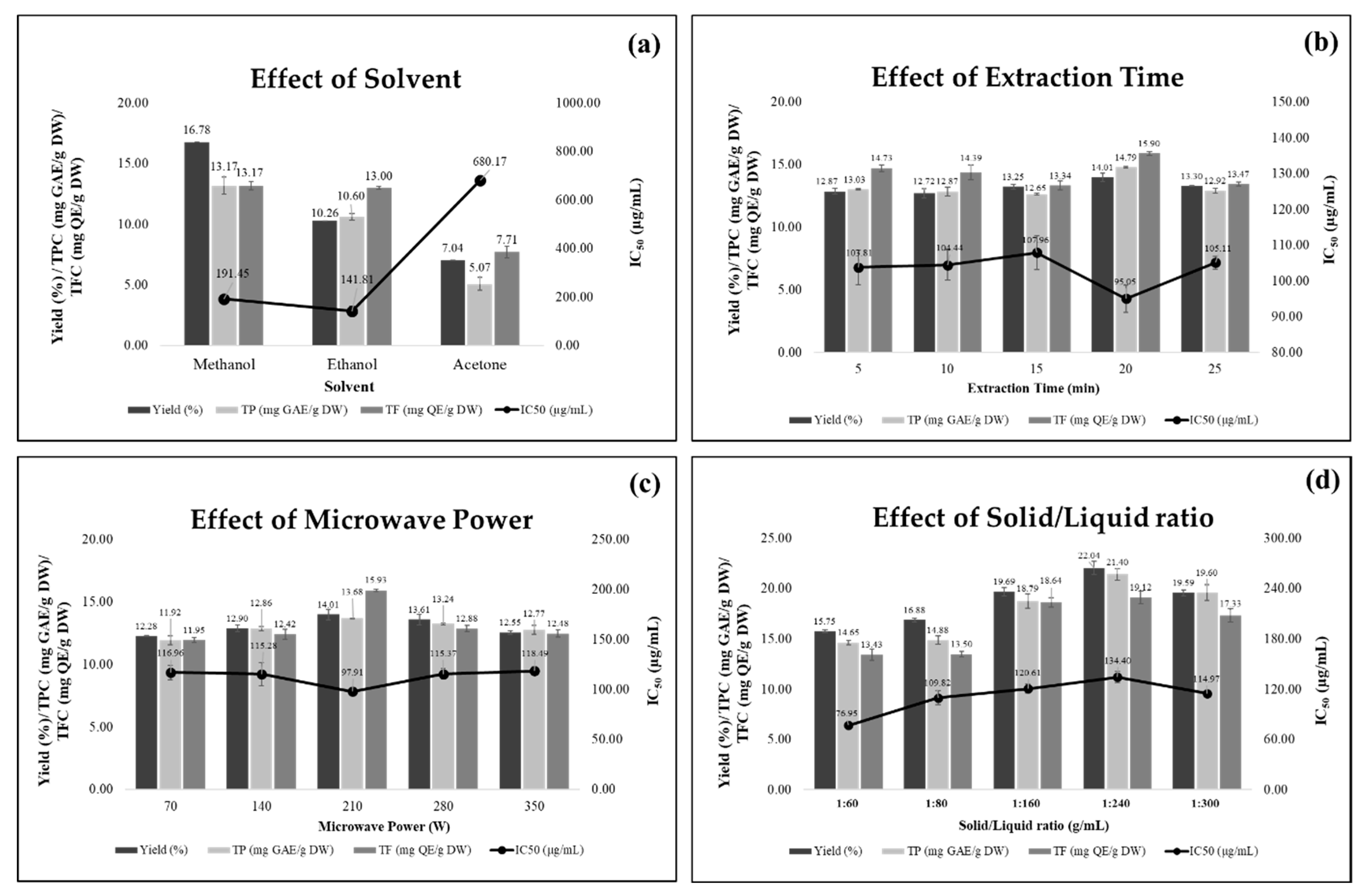
3.1. Impact of Single Factors on Antioxidant Compounds and Activity
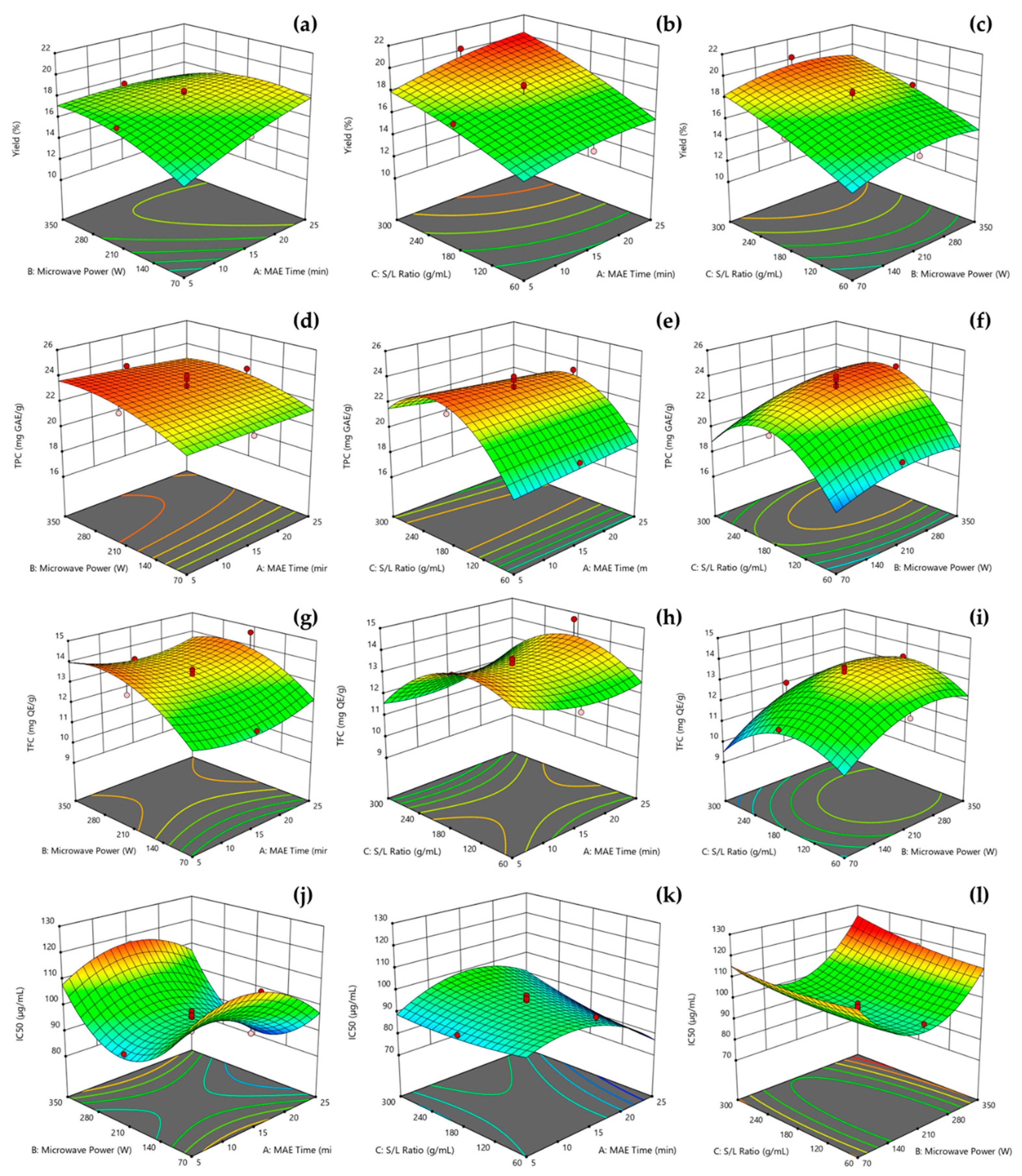
3.2. Model Fitting
+ 3.030X1X2 + 4.330X1X3 + 2.50X2X3
3.3. Optimization of MAE and Verification of Predictive Model
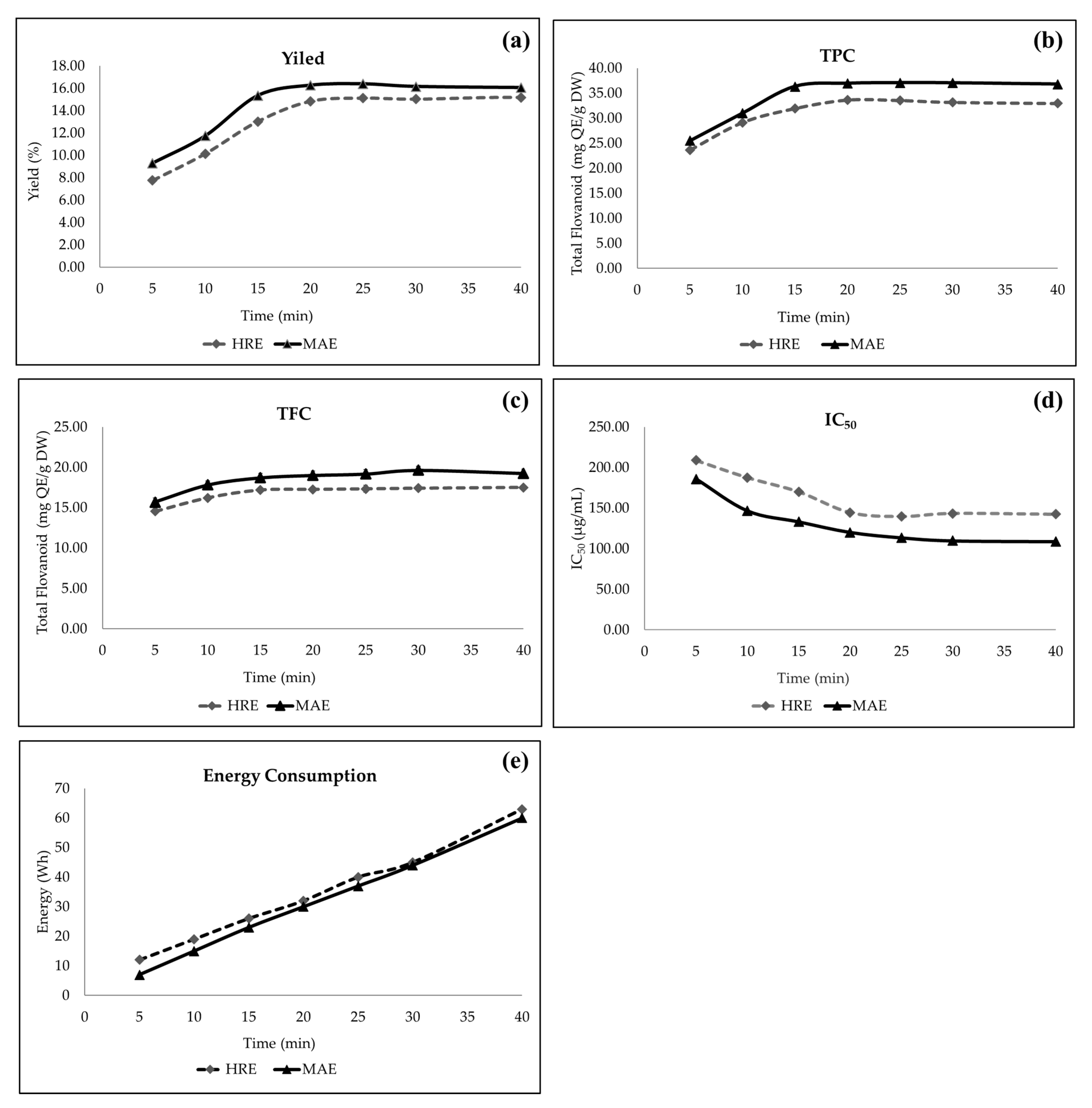
3.4. Comparison of MAE and HRE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anis, Z.; Sulaiman, O.; Hashim, R.; Mehdi, H.S.; Ghalib, M.R. Radical Scavenging Activity, Total Phenol Content and Antifungal Activity of Cinnamomum iners Wood. Iran. J. Energy Environ. 2012, 3, 74–78. [Google Scholar] [CrossRef]
- Mustaffa, F.; Indurkar, J.; Shah, M.; Ismail, S.; Mansor, S.M. Review on Pharmacological Activities of Cinnamomum iners Reinw. ex Blume. Nat. Prod. Res. 2013, 27, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Hussain, A.I.; Chatha, S.A.; Khosa, M.K.K.; Kamal, G.M.; Kamal, M.A.; Zhang, X.; Liu, M. Optimization Protocol for the Extraction of Antioxidant Components from Origanum vulgare Leaves Using Response Surface Methodology. Saudi J. Biol. Sci. 2016, 23, 389–396. [Google Scholar] [CrossRef] [PubMed]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ocampo, M.L.A.; Jiménez-Aparicio, A.R. Microwave-Assisted Extraction of Functional Compounds from Plants: A Review. Bioresources 2023, 18, 6614. [Google Scholar] [CrossRef]
- Suttiarporn, P.; Choommongkol, V. Microwave-Assisted Improved Extraction and Purification of Anticancer Nimbolide from Azadirachta indica (Neem) Leaves. Molecules 2020, 25, 2913. [Google Scholar] [CrossRef]
- Shang, A.; Luo, M.; Gan, R.-Y.; Xu, X.-Y.; Xia, Y.; Guo, H.; Liu, Y.; Li, H.-B. Effects of Microwave-Assisted Extraction Conditions on Antioxidant Capacity of Sweet Tea (Lithocarpus polystachyus Rehd.). Antioxidants 2020, 9, 678. [Google Scholar] [CrossRef]
- Choommongkol, V.; Punturee, K.; Klumphu, P.; Rattanaburi, P.; Meepowpan, P.; Suttiarporn, P. Microwave-Assisted Extraction of Anticancer Flavonoid, 2′,4′-Dihydroxy-6′-Methoxy-3′,5′-Dimethyl Chalcone (DMC), Rich Extract from Syzygium nervosum Fruits. Molecules 2022, 27, 1397. [Google Scholar] [CrossRef]
- Weremfo, A.; Abassah-Oppong, S.; Adulley, F.; Dabie, K.; Seidu-Larry, S. Response Surface Methodology as a Tool to Optimize the Extraction of Bioactive Compounds from Plant Sources. J. Sci. Food Agric. 2023, 103, 26–36. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Adarkwah-Yiadom, M. Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. J. Anal. Methods Chem. 2020, 2020, 7541927. [Google Scholar] [CrossRef]
- Tomasi, I.T.; Santos, S.C.; Boaventura, R.A.; Botelho, C.M. Optimization of Microwave-Assisted Extraction of Phenolic Compounds from Chestnut Processing Waste Using Response Surface Methodology. J. Clean. Prod. 2023, 395, 136452. [Google Scholar] [CrossRef]
- Febriani, H.; Lubis, M.F.; Sumaiyah, S.; Hasibuan, P.A.Z.; Syahputra, R.A.; Astyka, R.; Juwita, N.A. Optimization of Microwave-Assisted Extraction to Obtain a Polyphenol-Rich Crude Extract from Duku (Lansium domesticum Corr.) Leaf and the Correlation with Antioxidant and Cytotoxic Activities. Kuwait J. Sci. 2025, 52, 100315. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A Review of Chemical Constituents and Health-Promoting Effects of Citrus Peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef] [PubMed]
- Safer, A.M.; Afzal, M.; Nomani, M.; Mousa, S.A. Chapter 76—Green Tea Extract in the Management of Hepatic Fibrosis. In Tea in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 903–909. [Google Scholar]
- Ibrahim, F.M.; Abdelsalam, E.; Mohammed, R.S.; Ashour, W.E.S.; Vilas-Boas, A.A.; Pintado, M.; El Habbasha, E.S. Polyphenol-Rich Extracts and Essential Oil from Egyptian Grapefruit Peel as Potential Antioxidant, Antimicrobial, and Anti-Inflammatory Food Additives. Appl. Sci. 2024, 14, 2776. [Google Scholar] [CrossRef]
- Phantu, S.; Suttiarporn, P. CMMSE: A Comparison of Model Fitting Using Different Response Surface Designs for Optimizing Microwave-Assisted Simultaneous Distillation and Extraction in Khlu Herbal Tea Essential Oil Production. J. Math. Chem. 2024, 1–18. [Google Scholar] [CrossRef]
- Bunmusik, W.; Suttiarporn, P.; Phankaew, T.; Thitisut, P.; Seangwattana, T. The Effects of Solvent-Based Ultrasonic-Assisted Extraction of Bioactive Compounds and Antioxidant Activities from Pigmented Rice Bran. Mater. Today Proc. 2023, 77, 1073–1078. [Google Scholar] [CrossRef]
- Tanruean, K.; Poolprasert, P.; Suwannarach, N.; Kumla, J.; Lumyong, S. Phytochemical Analysis and Evaluation of Antioxidant and Biological Activities of Extracts from Three Clauseneae Plants in Northern Thailand. Plants 2021, 10, 117. [Google Scholar] [CrossRef]
- Liu, X.; Dong, M.; Chen, X.; Jiang, M.; Lv, X.; Yan, G. Antioxidant Activity and Phenolics of an Endophytic Xylaria sp. from Ginkgo biloba. Food Chem. 2007, 105, 548–554. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Effects of Extraction Solvent on Wheat Bran Antioxidant Activity Estimation. LWT Food Sci. Technol. 2004, 37, 717–721. [Google Scholar] [CrossRef]
- Ismail-Suhaimy, N.W.; Gani, S.S.A.; Zaidan, U.H.; Halmi, M.I.E.; Bawon, P. Optimizing Conditions for Microwave-Assisted Extraction of Polyphenolic Content and Antioxidant Activity of Barleria lupulina Lindl. Plants 2021, 10, 682. [Google Scholar] [CrossRef]
- Hihat, S.; Touati, N.; Sellal, A.; Madani, K. Response Surface Methodology: An Optimal Design for Maximising the Efficiency of Microwave-Assisted Extraction of Total Phenolic Compounds from Coriandrum sativum Leaves. Processes 2024, 12, 1031. [Google Scholar] [CrossRef]
- Wang, D.; Lv, J.; Fu, Y.; Shang, Y.; Liu, J.; Lyu, Y.; Yu, X. Optimization of Microwave-Assisted Extraction Process of Total Flavonoids from Salicornia Bigelovii Torr. and Its Hepatoprotective Effect on Alcoholic Liver Injury Mice. Foods 2024, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Zhang, J.J.; Li, Y.; Meng, X.; Li, H.B. Microwave-Assisted Extraction of Phenolic Compounds from Melastoma sanguineum Fruit: Optimization and Identification. Molecules 2018, 23, 2498. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Emam-Djomeh, Z.; Mirzaee, H.A.; Khomeiri, M.; Mahoonak, A.S.; Aydani, E. Optimization of Microwave-Assisted Extraction (MAE) and Soxhlet Extraction of Phenolic Compound from Licorice Root. J. Food Sci. Technol. 2015, 52, 3242–3253. [Google Scholar] [CrossRef] [PubMed]
- Boateng, I.D.; Kumar, R.; Daubert, C.R.; Flint-Garcia, S.; Mustapha, A.; Kuehnel, L.; Somavat, P. Sonoprocessing Improves Phenolics Profile, Antioxidant Capacity, Structure, and Product Qualities of Purple Corn Pericarp Extract. Ultrason. Sonochem. 2023, 95, 106418. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, H.; Farhat, A.; Romdhane, M.; Bouajila, J. Enhanced Solvent-Free Microwave Extraction of Foeniculum vulgare Mill. Essential Oil Seeds Using Double Walled Reactor. Arab. J. Chem. 2019, 12, 3863–3870. [Google Scholar] [CrossRef]
- Farhat, A.; Benmoussa, H.; Bachoual, R.; Nasfi, Z.; Elfalleh, W.; Romdhane, M.; Bouajila, J. Efficiency of the Optimized Microwave-Assisted Extractions on the Yield, Chemical Composition, and Biological Activities of Tunisian Rosmarinus officinalis L. Essential Oil. Food Bioprod. Process. 2017, 105, 224–233. [Google Scholar] [CrossRef]
- Chan, C.H.; Yeoh, H.K.; Yusoff, R.; Ngoh, G.C. A First-Principles Model for Plant Cell Rupture in Microwave-Assisted Extraction of Bioactive Compounds. J. Food Eng. 2016, 188, 98–107. [Google Scholar] [CrossRef]
- González-de-Peredo, V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, F.; Palma, M.; Carrera, C. Optimization of a Microwave-Assisted Extraction Method for Maximum Flavonols and Antioxidant Activity of Onion Extracts. Antioxidants 2022, 11, 2393. [Google Scholar] [CrossRef]
- Nour, A.H.; Oluwaseun, A.R.; Nour, A.H.; Omer, M.S.; Ahmed, N. Microwave-Assisted Extraction of Bioactive Compounds. In Microwave Heating: Electromagnetic Fields Causing Thermal and Non-Thermal Effects; IntechOpen: London, UK, 2021; pp. 1–31. [Google Scholar]
- Esparza, I.; Cimminelli, M.J.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Stability of Phenolic Compounds in Grape Stem Extracts. Antioxidants 2020, 9, 720. [Google Scholar] [CrossRef]
- Garrido, T.; Gizdavic-Nikolaidis, M.; Leceta, I.; Urdanpilleta, M.; Guerrero, P.; de la Caba, K.; Kilmartin, P.A. Optimizing the Extraction Process of Natural Antioxidants from Chardonnay Grape Marc Using Microwave-Assisted Extraction. Waste Manag. 2019, 88, 110–117. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Rácz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.L.; Thong, W.L.; How, S.E. Cinnamomum iners as Mitogen-Activated Protein Kinase Kinase Inhibitor. Int. J. Eng. Technol. 2009, 1, 310–313. [Google Scholar] [CrossRef]
- Mustaffa, F.; Indurkar, J.; Ismail, S.; Mordi, M.N.; Ramanathan, S.; Mansor, S.M. Antioxidant Capacity and Toxicity Screening of Cinnamomum iners Standardized Leaves Methanolic Extract. Int. J. Pharmacol. 2010, 6, 888–895. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Dong, X.; Jiang, G.; Zhang, H.; Xie, H.; Jiang, Y. Flavonoid Contents and Antioxidant Activities from Cinnamomum Species. Innov. Food Sci. Emerg. Technol. 2009, 10, 627–632. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, P.K.J. Phenolic Antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
| Independent Variable | Code | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| MAE time (min) | X1 | 5 | 15 | 25 |
| Microwave power (W) | X2 | 70 | 210 | 350 |
| Plant leaf-to-solvent ratio (g/mL) | X3 | 1:60 | 1:180 | 1:300 |
| Run | X1 | X2 | X3 | Y1 (%) | Y2 (mg GAE/g) | Y3 (mg QE/g) | Y4 (µg/mL) |
|---|---|---|---|---|---|---|---|
| 6 | 1 (25) | −1 (70) | 1 (1:300) | 0.69 | 0.02 | 0.18 | 0.45 |
| 17 | 0 (15) | 0 (210) | 0 (1:180) | 0.86 | 0.94 | 0.35 | 12.16 |
| 14 | 0 (15) | 0 (210) | 1 (1:300) | 0.52 | 0.28 | 0.88 | 11.24 |
| 16 | 0 (15) | 0 (210) | 0 (1:180) | 0.35 | 0.23 | 0.25 | 0.10 |
| 5 | −1 (5) | −1 (70) | 1 (1:300) | 0.25 | 0.12 | 0.46 | 1.48 |
| 13 | 0 (15) | 0 (210) | −1 (1:60) | 0.65 | 0.21 | 0.92 | 9.31 |
| 4 | 1 (25) | 1 (350) | −1 (1:60) | 0.37 | 0.49 | 0.71 | 10.80 |
| 7 | −1 (5) | 1 (350) | 1 (1:300) | 0.41 | 0.84 | 0.76 | 0.60 |
| 2 | 1 (25) | −1 (70) | −1 (1:60) | 0.77 | 0.20 | 0.04 | 2.25 |
| 10 | 1 (25) | 0 (210) | 0 (1:180) | 0.37 | 0.28 | 0.72 | 3.90 |
| 9 | −1 (5) | 0 (210) | 0 (1:180) | 0.33 | 0.66 | 0.19 | 0.51 |
| 11 | 0 (15) | −1 (70) | 0 (1:180) | 0.80 | 0.71 | 0.68 | 4.73 |
| 1 | −1 (5) | −1 (70) | −1 (1:60) | 0.45 | 0.21 | 0.19 | 5.96 |
| 15 | 0 (15) | 0 (210) | 0 (1:180) | 0.81 | 0.29 | 0.11 | 6.98 |
| 3 | −1 (5) | 1 (350) | −1 (1:60) | 0.42 | 0.47 | 0.48 | 2.52 |
| 18 | 0 (15) | 0 (210) | 0 (1:180) | 0.13 | 0.09 | 13.58 0.08 | 0.42 |
| 12 | 0 (15) | 1 (350) | 0 (1:180) | 0.16 | 0.47 | 0.25 | 3.14 |
| 19 | 0 (15) | 0 (210) | 0 (1:180) | 0.52 | 0.56 | 0.29 | 0.69 |
| 20 | 0 (15) | 0 (210) | 0 (1:180) | 0.64 | 0.46 | 0.30 | 96.30 10.88 |
| 8 | 1 (25) | 1 (350) | 1 (1:300) | 0.59 | 0.23 | 0.21 | 7.25 |
| Source | Y1—Yield | Y2—TPC | Y3—TFC | Y4—IC50 | ||||
|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Model | 20.97 | 0.000 a | 20.83 | 0.000 a | 12.77 | 0.000 a | 24.72 | 0.000 a |
| X1 | 18.83 | 0.001 a | 0.87 | 0.372 NS | 0.00 | 0.950 NS | 12.20 | 0.006 a |
| X2 | 6.20 | 0.032 a | 16.05 | 0.002 a | 33.36 | 0.000 a | 5.41 | 0.042 a |
| X3 | 112.53 | 0.000 a | 23.43 | 0.001 a | 9.27 | 0.012 a | 8.01 | 0.018 a |
| X12 | 0.87 | 0.373 NS | 0.00 | 0.966 NS | 3.28 | 0.100 NS | 29.57 | 0.000 a |
| X22 | 5.96 | 0.035 a | 2.83 | 0.124 NS | 10.33 | 0.009 a | 126.00 | 0.000 a |
| X32 | 0.94 | 0.354 NS | 58.51 | 0.000 a | 21.08 | 0.001 a | 1.24 | 0.291 NS |
| X1 X2 | 22.08 | 0.001 a | 0.67 | 0.434 NS | 0.75 | 0.406 NS | 8.29 | 0.016 a |
| X1 X3 | 2.29 | 0.161 NS | 4.35 | 0.064 NS | 6.47 | 0.029 a | 16.93 | 0.002 a |
| X2 X3 | 0.51 | 0.493 NS | 1.65 | 0.228 NS | 0.63 | 0.445 NS | 5.65 | 0.039 a |
| Lack of Fit | 0.77 | 0.610 NS | 1.63 | 0.511 NS | 2.30 | 0.191 NS | 2.18 | 0.363 NS |
| R2 | 0.9497 | 0.9494 | 0.9199 | 0.9570 | ||||
| Adj-R2 | 0.9044 | 0.9038 | 0.8479 | 0.9183 | ||||
| Pred-R2 | 0.7752 | 0.6009 | 0.5181 | 0.7673 | ||||
| Variable | Unit | Optimal Condition | Modified Optimal Condition | |
|---|---|---|---|---|
| Extraction time | min | 25.00 | 25.00 | |
| Microwave power | W | 214.24 | 210 | |
| Solid–liquid ratio | g/mL | 1:195.76 | 1:196 | |
| Response | Unit | Predicted Value | Predicted Value | Actual Value |
| Yield | % | 18.56 | 18.58 | 0.23 |
| TPC | mg GAE/g | 22.86 | 22.83 | 0.26 |
| TFC | mg QE/g | 13.89 | 13.86 | 0.07 |
| Antioxidant activity (IC50) | µg/mL | 83.30 | 83.13 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanruean, K.; Luangkamin, S.; Srisurat, T.; Bunmusik, W.; Suttiarporn, P. Optimization of Microwave-Assisted Extraction Process for Production of Polyphenol-Rich Crude Extract from Cinnamomum iners Leaves. Appl. Sci. 2025, 15, 1265. https://doi.org/10.3390/app15031265
Tanruean K, Luangkamin S, Srisurat T, Bunmusik W, Suttiarporn P. Optimization of Microwave-Assisted Extraction Process for Production of Polyphenol-Rich Crude Extract from Cinnamomum iners Leaves. Applied Sciences. 2025; 15(3):1265. https://doi.org/10.3390/app15031265
Chicago/Turabian StyleTanruean, Keerati, Suwaporn Luangkamin, Thanarak Srisurat, Wasitthi Bunmusik, and Panawan Suttiarporn. 2025. "Optimization of Microwave-Assisted Extraction Process for Production of Polyphenol-Rich Crude Extract from Cinnamomum iners Leaves" Applied Sciences 15, no. 3: 1265. https://doi.org/10.3390/app15031265
APA StyleTanruean, K., Luangkamin, S., Srisurat, T., Bunmusik, W., & Suttiarporn, P. (2025). Optimization of Microwave-Assisted Extraction Process for Production of Polyphenol-Rich Crude Extract from Cinnamomum iners Leaves. Applied Sciences, 15(3), 1265. https://doi.org/10.3390/app15031265







