Modeling the Leaching of Cobalt and Manganese from Submarine Ferromanganese Crusts by Adding Steel Scrap Using Design of Experiments and Response Surface Methodology
Abstract
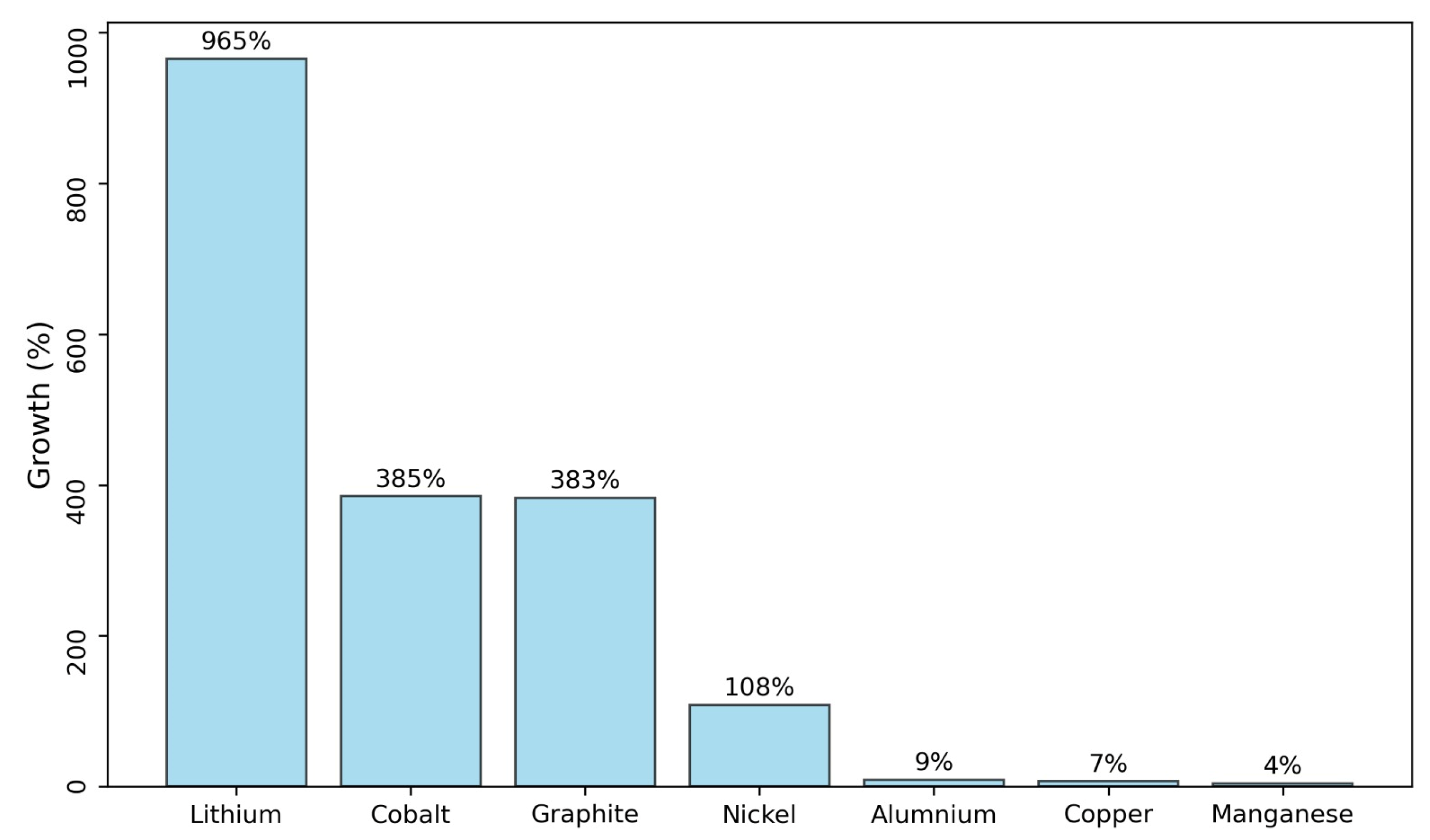
1. Introduction
2. Background
- Redox Chemistry in Ore Processing: A dual-benefit strategy harnesses the redox properties of Cu and Co ores, enabling simultaneous extraction without the need for additional oxidizing or reducing agents. This approach achieves high recovery efficiencies of 99.67% for copper and 98.20% for cobalt by capitalizing on the oxidizing properties of Co3+ in oxide ores and the reducibility of sulfide ores [34].
- Neural Networks and Response Surface Methodology: This approach has been implemented to forecast and improve the leaching characteristics of Cu-Co ores. The ANN employs a backpropagation algorithm to model the process, while RSM utilizes the Box-Behnken design for optimization. The method achieves leaching yields of 93.46% for Cu and 89.43% for Co, with a strong correlation between predicted and experimental values [20].
- Ammonium Sulfate Roasting: An innovative method involves ammonium sulphate roasting for the selective extraction of metals from oceanic Co-rich crusts. This process converts metal oxides into sulphates, achieving high leaching efficiencies for Co, Ni, and Cu, while minimizing Fe leaching. The method is efficient, environmentally friendly, and economically advantageous compared to traditional techniques [35,36].
- Metal Melt Extraction: A method involving metal melting entails heating a Cu-Co alloy with a metal extraction medium under an inert atmosphere, effectively separating Cu from the alloy. The extracted Cu can then be utilized as a high-quality raw material, while the remaining alloy is suitable for further processing, such as acid leaching [37].
- Pressure Leaching and Electrodeposition: The extraction of cathode Cu from Co concentrate involves pressure leaching, followed by Cu recovery and electrodeposition. This process incorporates steps for the removal and recycling of extractants, improving the efficiency of the extraction process and enhancing the quality of the final Cu product [38,39].
- Continuous Fluid Separation: A production method for extracting Co from Cu extraction tail liquid utilizes a continuous fluid separation system with chelate resin. This approach streamlines the process, reduces costs, and enhances cobalt purification efficiency by eliminating the need for traditional precipitation steps [40].
- Taguchi Method and ANOVA: The Taguchi method and analysis of variance (ANOVA) are employed to optimize heap leaching conditions for Cu and Co recovery from tailings. This statistical approach identifies optimal leaching parameters, including particle size, acidity, and flow rate, leading to significant metal recovery rates [41].
- Microfluidic Extraction: A procedure is employed to separate Cu and Co ions in a microchannel by optimizing parameters such as pH, flow rate, and extractant concentration. This method achieves a high Cu extraction rate while minimizing Co co-extraction, providing a more efficient and effective separation process [42].
3. Materials and Methods
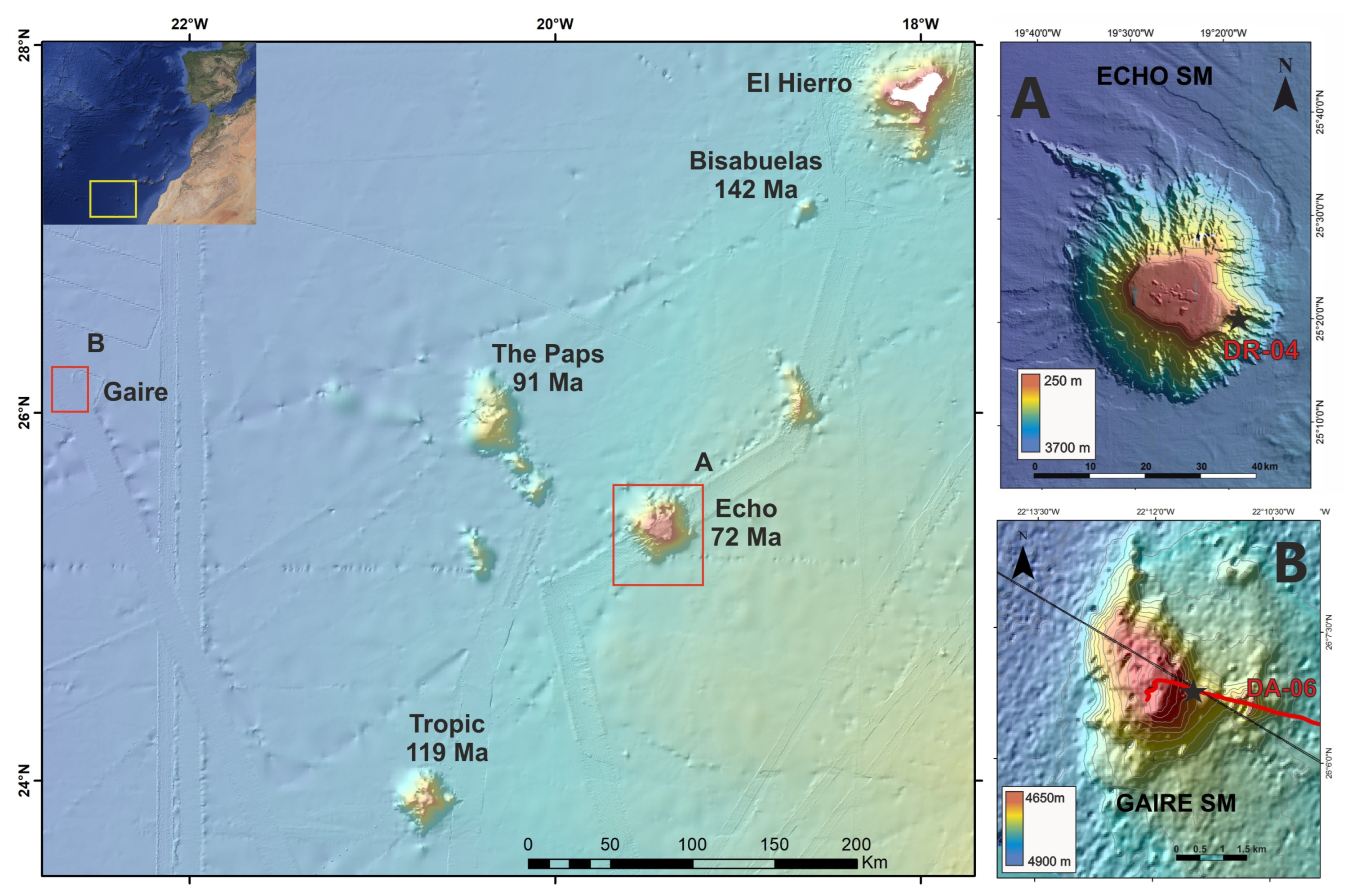
3.1. Ferromanganese Crusts
3.2. Iron Residue
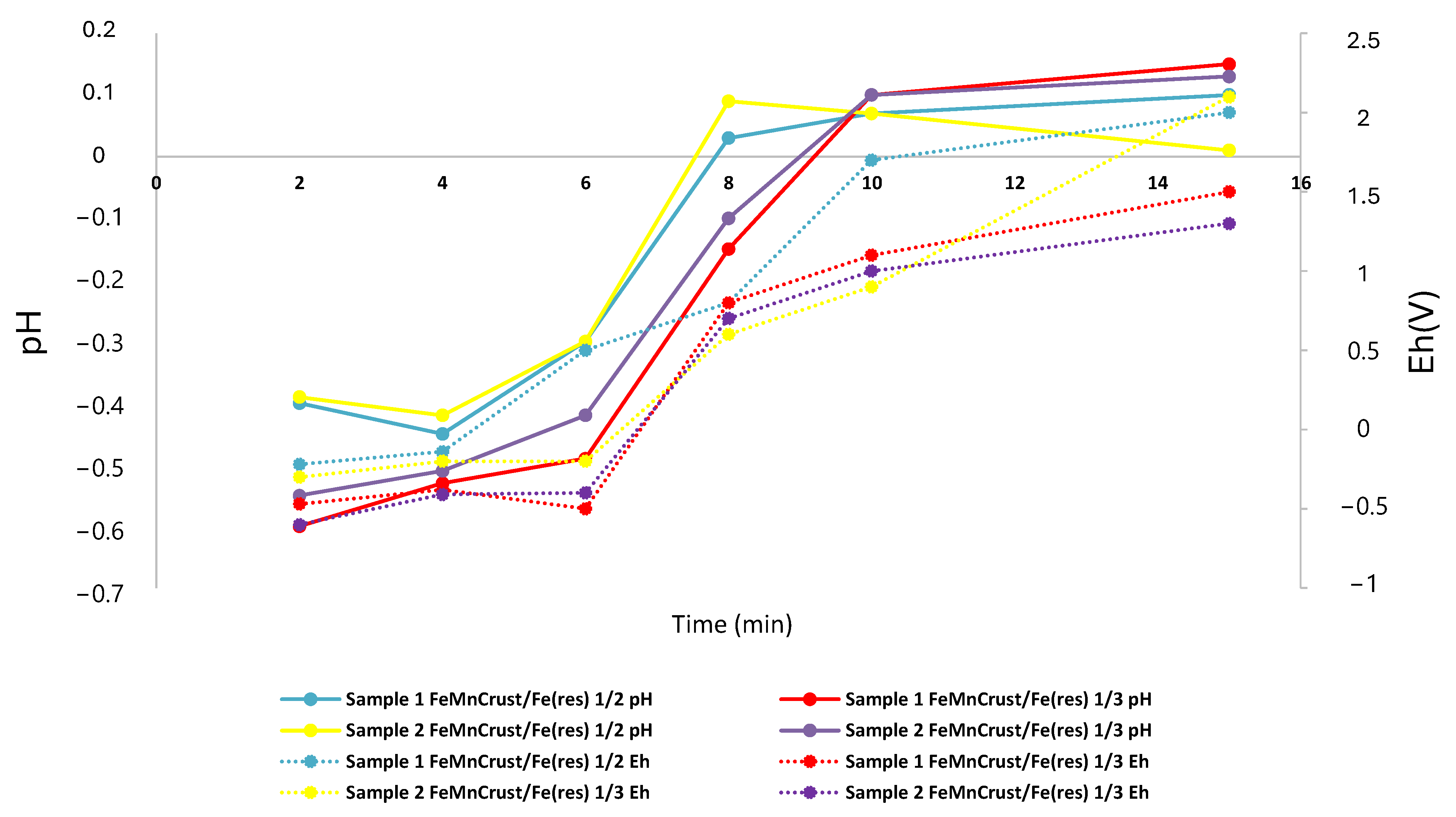
3.3. Leaching Test
3.4. Estimation of Factorial DOE
4. Results and Discussion
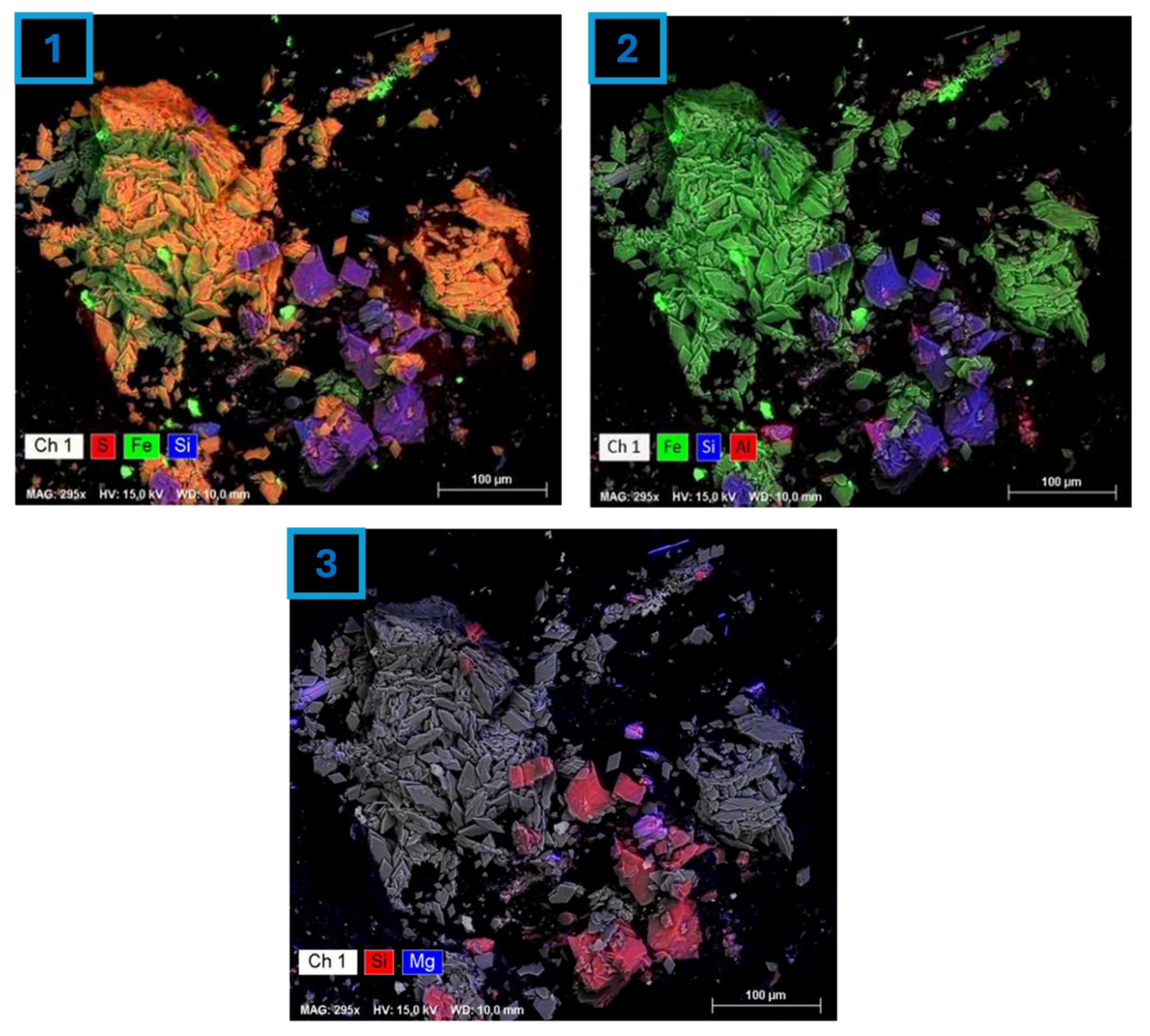
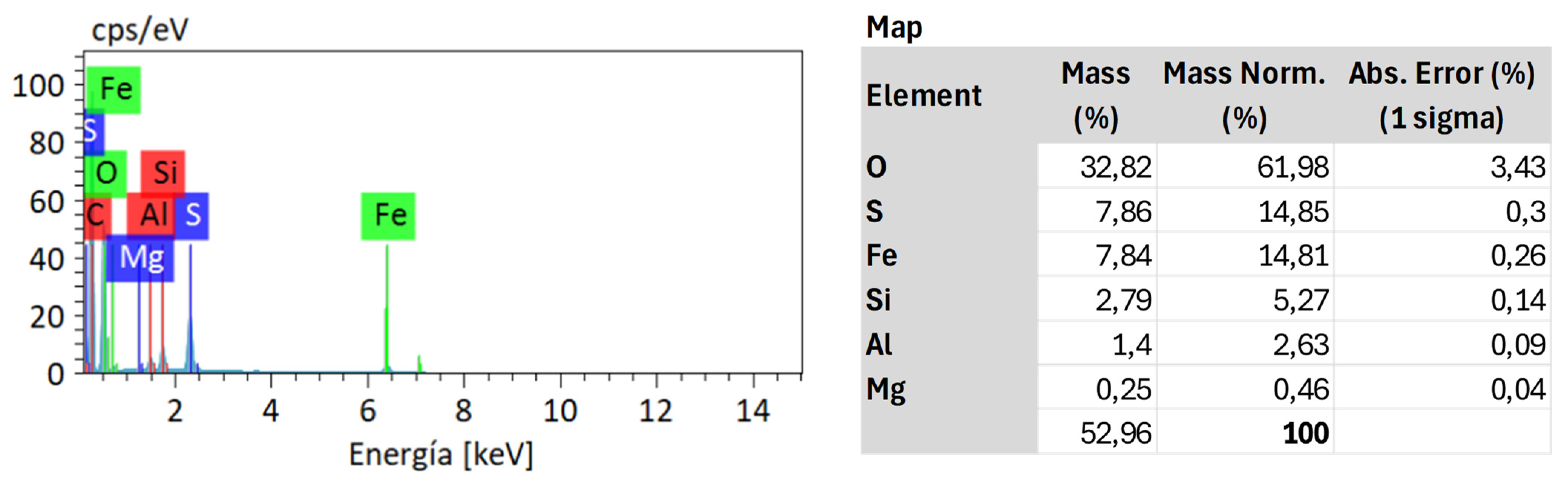
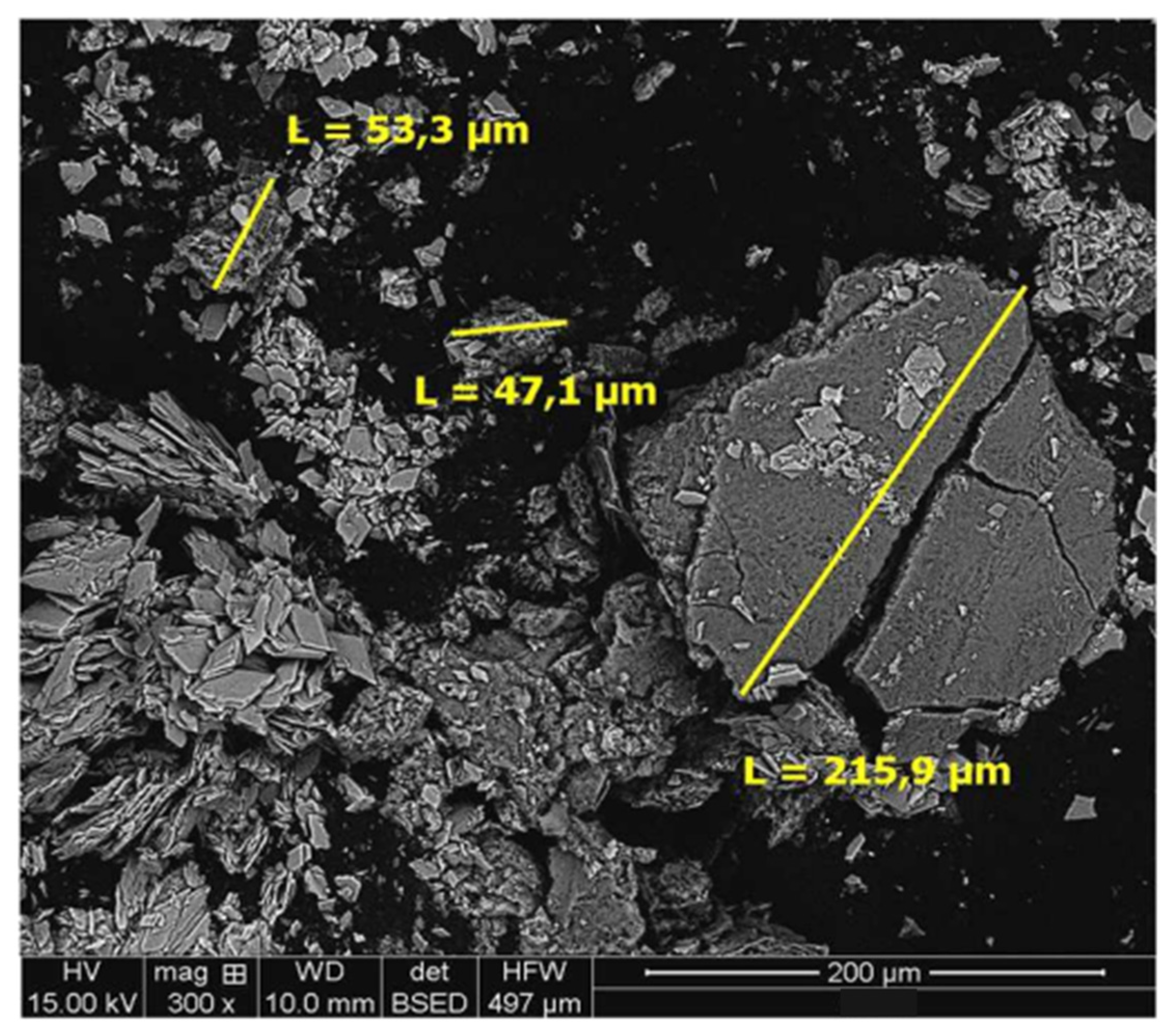
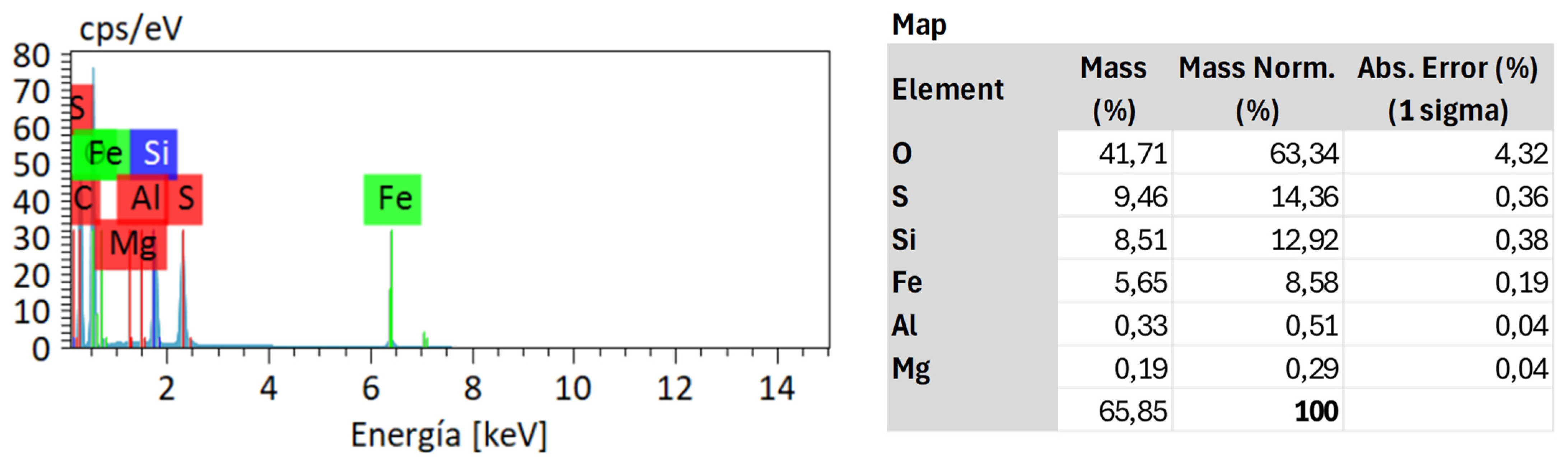
4.1. Sample Characterization
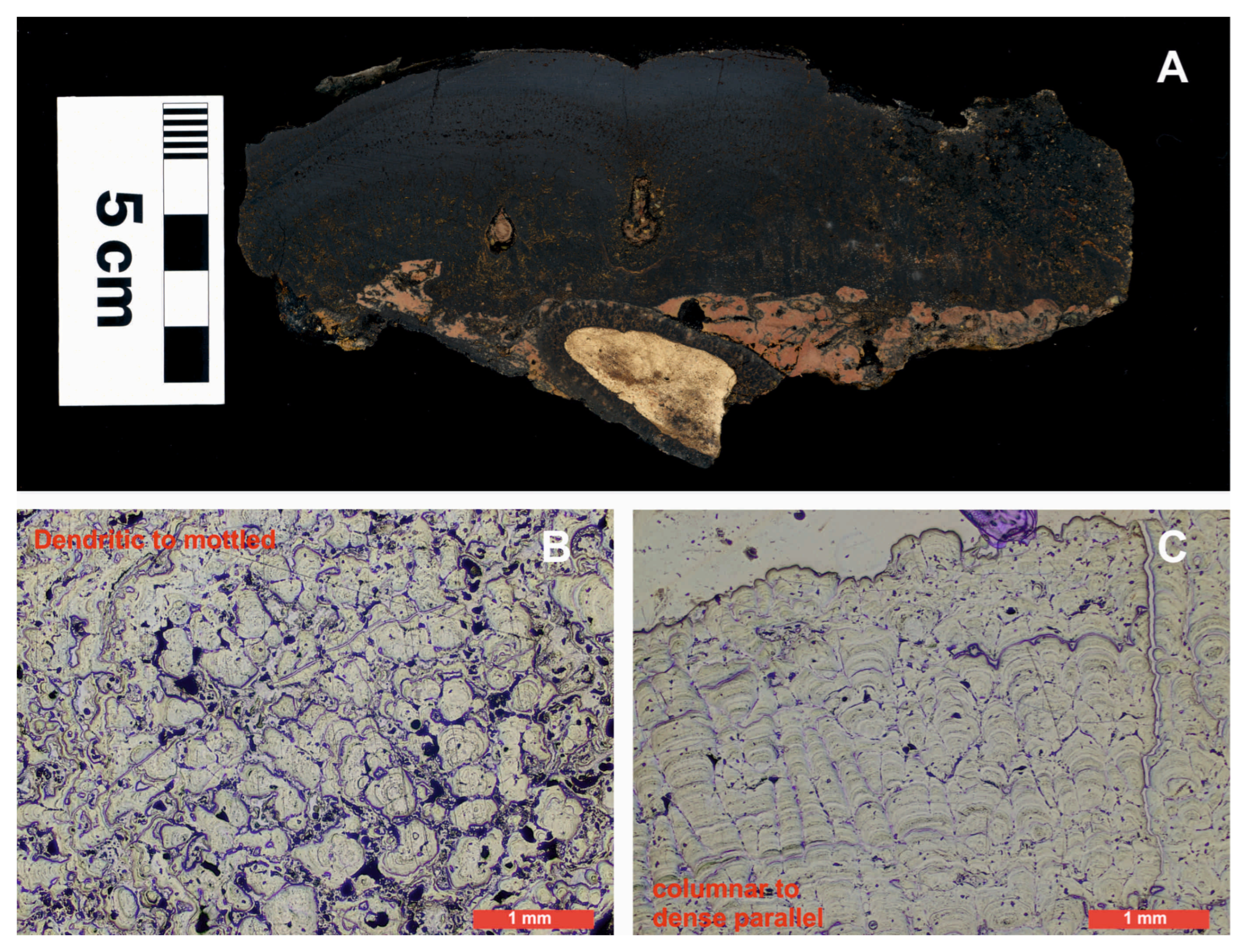
4.2. Effect of Variables
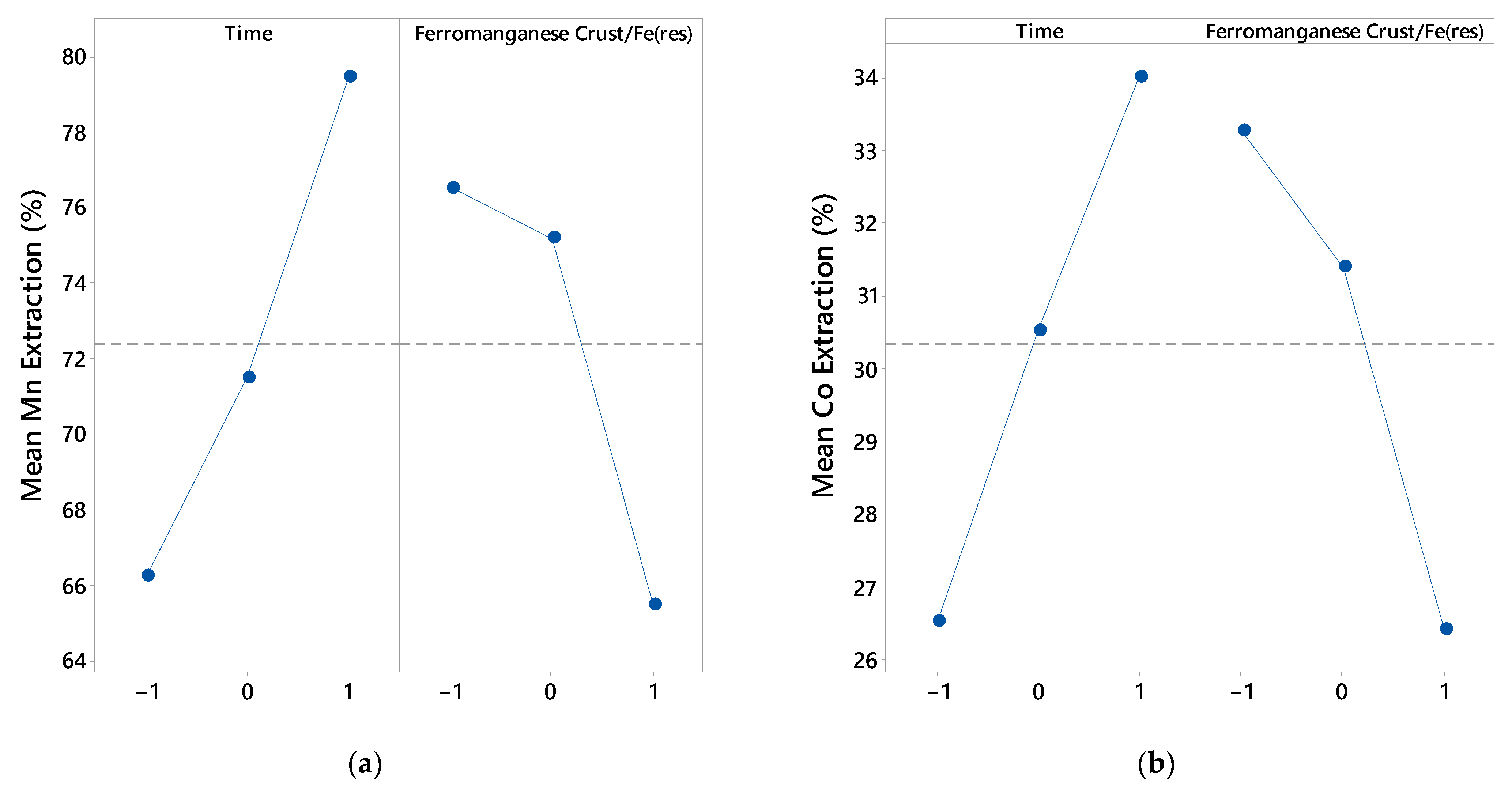

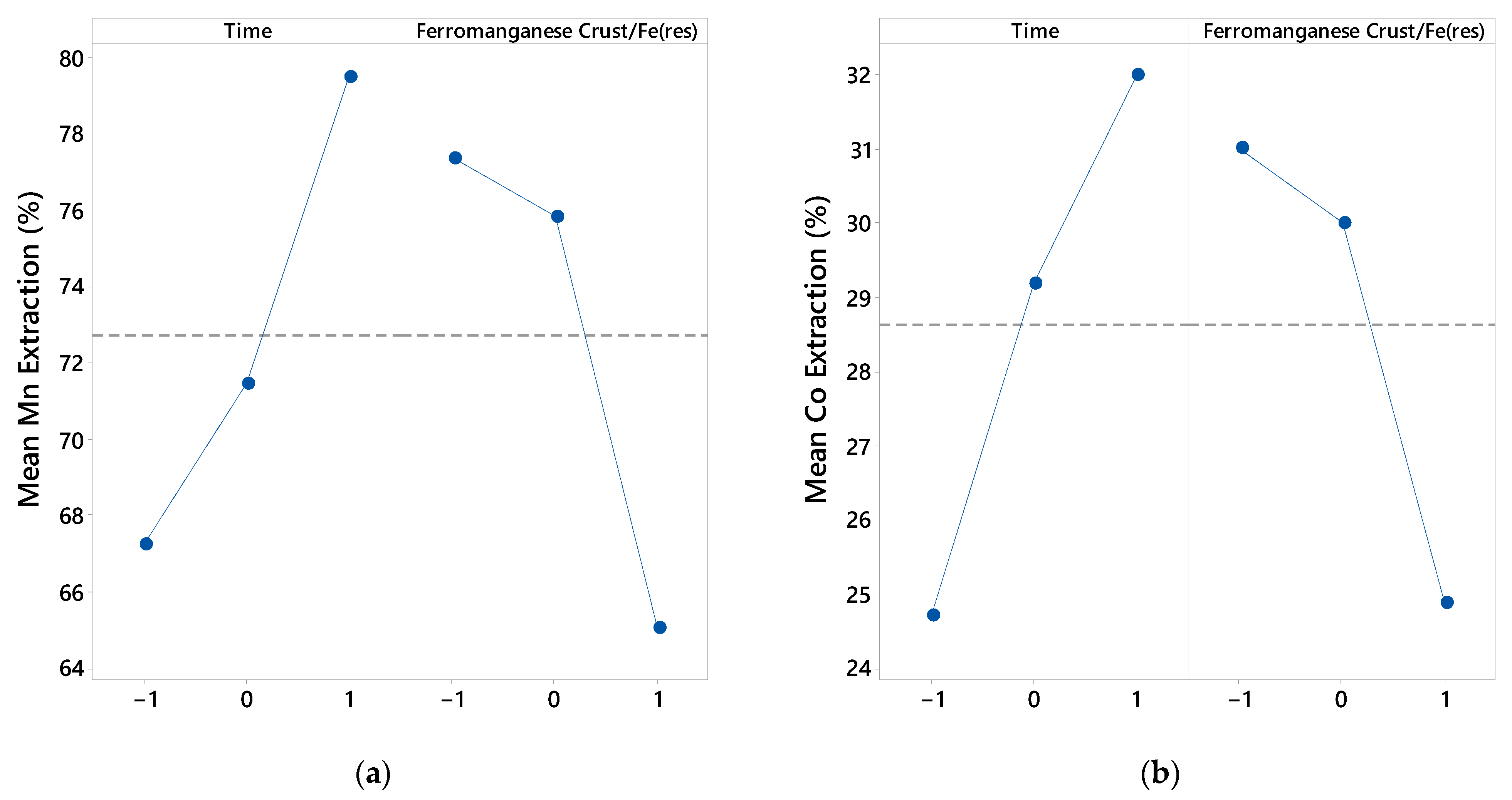
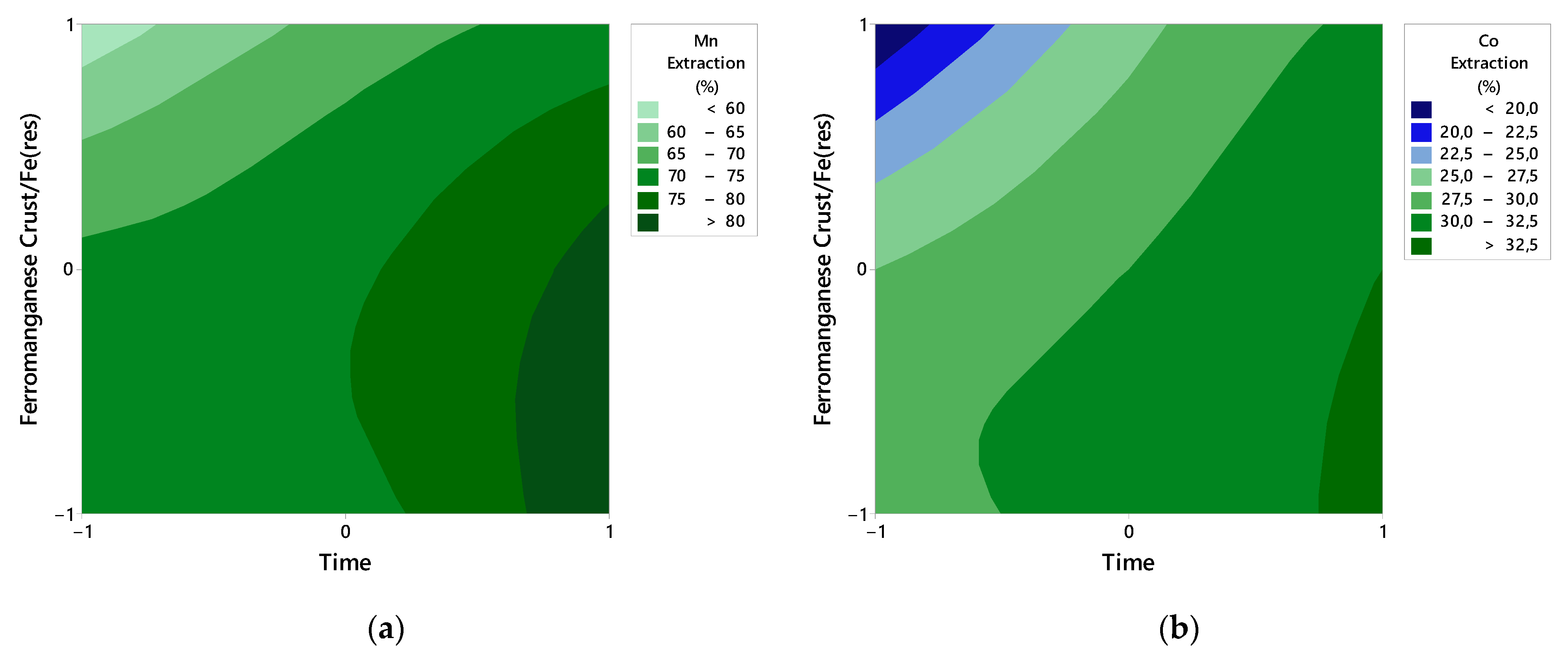
- The p-values of the models (p < 0.05) confirm their statistical significance.
- The R2 statistic demonstrates that a substantial proportion of the total variability is explained by the models.
- The F-test results (Table 8) show the models’ significance, as the F-values from the regressions are significantly greater than the critical F-values for all fitted models.
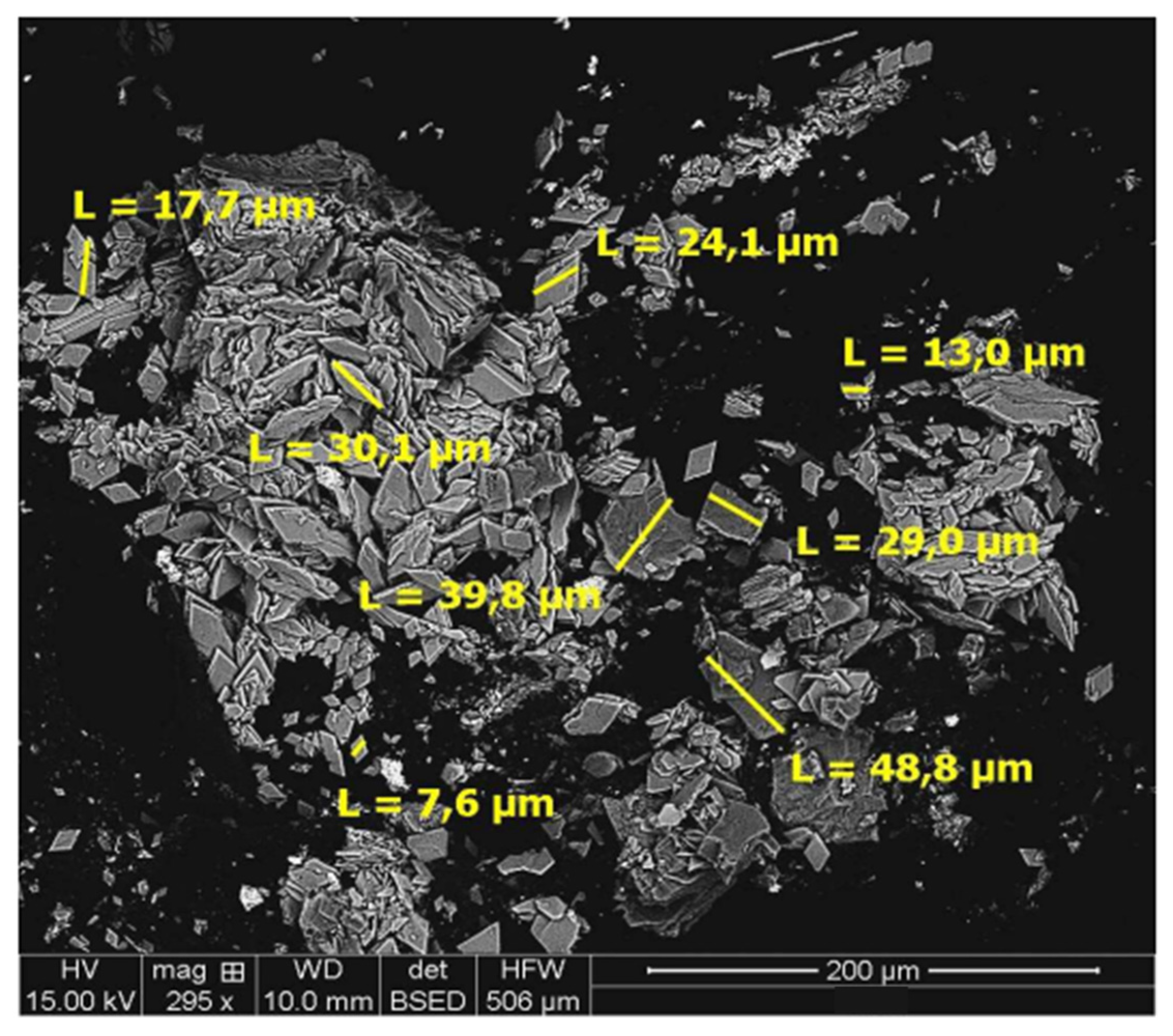
4.3. Residue Analysis
4.3.1. DRAGO 0511/DR04-15
4.3.2. SUBVENT 1/DA06-4
5. Conclusions
- Mn and Co extraction can be effectively modeled using multiple regression techniques, with strong goodness-of-fit indicators.
- Optimal Mn and Co extraction is achieved at longer durations (30 min) and lower ferromanganese crust/Fe(res) ratios (1/3) for both samples, S1 and S2.
- Gradient analysis shows that Mn and Co extraction for both samples is directly proportional to time and inversely proportional to the ferromanganese crust/Fe(res) ratio in most cases, except for Co extraction at higher time levels and lower ferromanganese crust/Fe(res) ratios.
- No precipitation of Co or Mn species was observed in the studied residues.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toro Villarroel, N.R.; Torres Albornoz, D.A. La Fuerza del Litio; Universidad Arturo Prat: Iquique, Chile, 2023; ISBN 9789564164717. [Google Scholar]
- Koschinsky, A.; Heinrich, L.; Boehnke, K.; Cohrs, J.C.; Markus, T.; Shani, M.; Singh, P.; Smith Stegen, K.; Werner, W. Deep-Sea Mining: Interdisciplinary Research on Potential Environmental, Legal, Economic, and Societal Implications. Integr. Environ. Assess. Manag. 2018, 14, 672–691. [Google Scholar] [CrossRef]
- Mohammadi, A.; Fazeli, A. Future Market and Challenges of Lithium/Sulfur Batteries. In Nanostructured Materials for Lithium/Sulfur Batteries; Springer International Publishing: Cham, Switzerland, 2024; pp. 697–721. [Google Scholar]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Naseri, T.; Pourhossein, F.; Mousavi, S.M.; Kaksonen, A.H.; Kuchta, K. Manganese Bioleaching: An Emerging Approach for Manganese Recovery from Spent Batteries. Rev. Environ. Sci. Biotechnol. 2022, 21, 447–468. [Google Scholar] [CrossRef]
- Habib, K.; Hansdóttir, S.T.; Habib, H. Critical Metals for Electromobility: Global Demand Scenarios for Passenger Vehicles, 2015–2050. Resour. Conserv. Recycl. 2020, 154, 104603. [Google Scholar] [CrossRef]
- Toro, N.; Gálvez, E.; Saldaña, M.; Jeldres, R.I. Submarine Mineral Resources: A Potential Solution to Political Conflicts and Global Warming. Min. Eng. 2022, 179, 107441. [Google Scholar] [CrossRef]
- Gulley, A.L. The Development of China’s Monopoly over Cobalt Battery Materials. Miner. Econ. 2024, 37, 619–631. [Google Scholar] [CrossRef]
- Müller, D.; Groves, D.I.; Santosh, M.; Yang, C.-X. Critical Metals: Their Applications with Emphasis on the Clean Energy Transition. Geosyst. Geoenviron. 2024, 4, 100310. [Google Scholar] [CrossRef]
- Pérez, K.; Toro, N.; Robles, P.; Gallegos, S.; Gálvez, E.; González, F.J.; Marino, E.; Hernández, P.C. Cobalt and Manganese Extraction from Ocean Nodules by Co-Processing with Steel Metallurgical Slag. Metals 2023, 13, 1079. [Google Scholar] [CrossRef]
- Torres, D.; Pérez, K.; Galleguillos Madrid, F.M.; Leiva, W.H.; Gálvez, E.; Salinas-Rodríguez, E.; Gallegos, S.; Jamett, I.; Castillo, J.; Saldana, M.; et al. Salar de Atacama Lithium and Potassium Productive Process. Metals 2024, 14, 1095. [Google Scholar] [CrossRef]
- Pérez, K.; Toro, N.; Robles, P.; Galleguillos Madrid, F.M.; Gálvez, E.; González, F.J.; Marino, E.; Castillo, J.; Jamett, I.; Hernández, P.C. Extraction of Cobalt and Manganese from Ferromanganese Crusts Using Industrial Metal Waste through Leaching. Metals 2024, 14, 1044. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-Ocean Mineral Deposits as a Source of Critical Metals for High- and Green-Technology Applications: Comparison with Land-Based Resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Halbach, P.; Marchig, V.; Scherhag, C. Regional Variations in Mn, Ni, Cu, and Co of Ferromanganese Nodules from a Basin in the Southeast Pacific. Mar. Geol. 1980, 38, M1–M9. [Google Scholar] [CrossRef]
- Lusty, P.A.J.; Hein, J.R.; Josso, P. Formation and Occurrence of Ferromanganese Crusts: Earth’s Storehouse for Critical Metals. Elements 2018, 14, 313–318. [Google Scholar] [CrossRef]
- Mohwinkel, D.; Kleint, C.; Koschinsky, A. Phase Associations and Potential Selective Extraction Methods for Selected High-Tech Metals from Ferromanganese Nodules and Crusts with Siderophores. Appl. Geochem. 2014, 43, 13–21. [Google Scholar] [CrossRef]
- Konstantinova, N.; Son, V.T.; Thang, L.A.; Trung, T.T.; Giang, V.T.; Dung, N.T.T.; Vanshtein, B.; Cherkashov, G. Ferromanganese Crusts of the Vietnam Margin, Central South China Sea: Composition and Genesis. Mar. Geol. 2022, 453, 106911. [Google Scholar] [CrossRef]
- Mwanat, M.H.M.; Kasongo, K.B. Cobalt Dissolution from Concentrate in Sulfuric Acid—Ferrous Sulfate System: Process Parameters Optimization by Response Surface Methodology (RSM). J. Sustain. Metall. 2021, 7, 1838–1851. [Google Scholar] [CrossRef]
- Muthalib, N.; Abdullah, N.; Ismail, S. Reductive leaching of low grade manganese ore (lgmo) using glucose in sulphuric acid: Optimization condition using response surface methodology. ASEAN Eng. J. 2018, 8, 64–73. [Google Scholar] [CrossRef]
- Mbuya, B.; Mulaba-Bafubiandi, A.F. Predicting Optimized Dissolution of Selected African Copperbelt Copper-Cobalt-Bearing Ores by Means of Neural Network Prediction and Response Surface Methodology Modeling. Process Integr. Optim. Sustain. 2023, 7, 583–597. [Google Scholar] [CrossRef]
- Mathaba, M.; Banza, J.C. Application of Machine Learning Approach (Artificial Neural Network) and Shrinking Core Model in Cobalt (II) and Copper (II) Leaching Process. J. Environ. Sci. Health Part A 2024, 59, 25–32. [Google Scholar] [CrossRef]
- Yu, B.; Zhan, D.; Liu, J.; Wang, H.; Yang, S.; Hu, X. Response Surface Method Optimization to Improve Copper Extraction from Refractory Copper Oxide Ore. Min. Met. Explor. 2022, 39, 2221–2228. [Google Scholar] [CrossRef]
- Salehi, S.; Noaparast, M.; Shafaei, S.Z. Response Surface Methodology (RSM) for Optimization of Chalcopyrite Concentrate Leaching with Silver-Coated Pyrite. Physicochem. Probl. Miner. Process. 2016, 52, 1023–1035. [Google Scholar] [CrossRef]
- Ayodele, T.J.; Daniyan, A.A.; Adeleke, A.A.; Ola-Omole, O.O. Optimization of Leaching Parameters for the Extraction of Copper from Hematite-Dominated Copper Ore Using Response Surface Methodology (RSM). Niger. J. Technol. 2023, 42, 353–363. [Google Scholar] [CrossRef]
- Asl, M.D.; Salahshoori, I.; Seyfaee, A.; Hatami, A.; Golbarari, A.A. Experimental Results and Optimization via the Design of Experiment (DOE) of the Copper Ion Recovery from Aqueous Solutions Using Emulsion Liquid Membrane (ELM) Method. Desalination Water Treat. 2020, 204, 238–256. [Google Scholar] [CrossRef]
- Rajendran, S.; Vakamalla, T.R.; Mangadoddy, N. Numerical Methods in Mineral Processing: An Overview. Miner. Process. Benef. Oper. Process Optim. Through Model. 2023, 251–285. [Google Scholar] [CrossRef]
- Cisternas, L.A.; Lucay, F.A.; Botero, Y.L. Trends in Modeling, Design, and Optimization of Multiphase Systems in Minerals Processing. Minerals 2019, 10, 22. [Google Scholar] [CrossRef]
- Castillo-Villagra, R.; Icarte, G.; Thoben, K.D. Modelling the Make Process of the Mineral Supply Chain Upstream Segment. Resources 2023, 12, 132. [Google Scholar] [CrossRef]
- Noome, Z.M.; le Roux, J.D.; Padhi, R. Optimal Control of Mineral Processing Plants Using Constrained Model Predictive Static Programming. J. Process Control 2023, 129, 103067. [Google Scholar] [CrossRef]
- Bourgeois, F.; Julcour-Lebigue, C.; Cassayre, L.; Bailly, F.; Cyr, M.; Touzé, S. Guiding Mineralization Process Development with Geochemical Modelling. In Proceedings of the GHGT 2018—14th International Conference on Greenhouse Gas Control Technologies, Melbourne, Australia, 21–26 October 2018. [Google Scholar] [CrossRef]
- Yakovlev, B.V.; Nikiforova, L.V. Mathematical Modeling of a Particle Motion in Mineral Processing Devices. AIP Conf. Proc. 2021, 2328, 030004. [Google Scholar] [CrossRef]
- Parian, M.; Lamberg, P.; Rosenkranz, J. Process Simulations in Mineralogy-Based Geometallurgy of Iron Ores. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2021, 130, 25–30. [Google Scholar] [CrossRef]
- Straka, M.; Saderova, J.; Bindzar, P. Simulation and Modelling of Transport Processes for the Needs of Mineral Resources Delivery Support. In Proceedings of the 4th EAI International Conference on Management of Manufacturing Systems, Krynica-Zdrój, Poland, 8–10 October 2019; EAI/Springer Innovations in Communication and Computing; Springer International Publishing: Cham, Switzerland, 2020; pp. 93–101. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, W.; Che, J.; Feng, S.; Chen, Y.; Wang, C. Efficient Extraction of Cobalt and Copper: Leveraging Redox Chemistry in Oxide and Sulfide Copper-Cobalt Ores. Sep. Purif. Technol. 2025, 354, 128671. [Google Scholar] [CrossRef]
- Ju, J.; Feng, Y.; Li, H.; Ma, R.; Li, Y.; Zhao, H.; Wang, H.; Jiang, S. An Innovative Method for the Efficient and Selective Extraction of Co, Ni, Cu, and Mn from Oceanic Cobalt-Rich Crusts by Ammonium Sulfate Roasting: Behavior, Roasting Kinetics and Mechanism. Min. Eng. 2024, 207, 108543. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, G.; Zhao, Y.; Feng, X. A Study of Recovery of Copper and Cobalt from Copper–Cobalt Oxide Ores by Ammonium Salt Roasting. Hydrometallurgy 2012, 129–130, 140–144. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, C.; Guo, X.; Tian, Q.; Cui, F.; Gan, X. Method for Extracting and Treating Copper-Cobalt Alloy by Utilizing Metal Melt. Chinese Patent No. CN111172399A, 19 May 2020. [Google Scholar]
- da Silveira Leite, D.; Carvalho, P.L.G.; de Lemos, L.R.; Mageste, A.B.; Rodrigues, G.D. Hydrometallurgical Separation of Copper and Cobalt from Lithium-Ion Batteries Using Aqueous Two-Phase Systems. Hydrometallurgy 2017, 169, 245–252. [Google Scholar] [CrossRef]
- Xu, K.; Deng, Q.; Su, T.; Tan, T.; Wang, C.; Chen, L. The Extracting Method of Tough Cathode is Extracted in a Kind of Cobalt Concentrate. Chinese Patent No. CN110318072A, 11 October 2019. [Google Scholar]
- Yang, J.; Xu, W.; Zhang, S.; Chen, J.; Sun, H. Production Method for Extracting Cobalt from Copper Extraction Tail Liquid. Chinese Patent No. CN111286607A, 16 June 2020. [Google Scholar]
- Bampole, D.L.; Mulamba, E.L. Mathematical Modeling for Enhancement Heap Leaching of D.M.S Tailings for the Recovering Copper and Cobalt: Using Taguchi Method and Analysis Variance. IOSR J. Appl. Chem. 2017, 10, 50–57. [Google Scholar] [CrossRef]
- Xiao, B.Q.; Jiang, F.; Peng, J.H.; Ju, S.H.; Zhang, L.H.; Yin, S.H.; Zhang, L.B.; Xu, L.; Tian, S.H. Optimization Study of Operation Parameters for Extracting Cu2+ from Sulfuric Solution Containing Co2+ with LIX984N in a Laminar Microchip. Arab. J. Sci. Eng. 2018, 43, 2145–2153. [Google Scholar] [CrossRef]
- Sobhi Amjad, R.; Asadollahzadeh, M.; Torkaman, R.; Torab-Mostaedi, M. Optimizing the Extraction of Cobalt Ions under Response Surface Methodology and without Organic Solutions. Can. J. Chem. Eng. 2023, 101, 3532–3540. [Google Scholar] [CrossRef]
- Movahhedi, H.; Mohammad Beygian, A.; Keshavarz Alamdari, E.; Moradkhani, D. Developing of a Counter-Current Copper Leaching Process Using Response Surface Methodology. Miner. Process. Extr. Metall. Rev. 2024, 45, 1–11. [Google Scholar] [CrossRef]
- Mbuya, B.; Meta-Mvita, J.; Mulaba-Bafubiandi, A.F. Bayesian Statistics Study of a Sustainable Dissolution of Cobalt-Bearing Minerals from Cu-Co Ores. Can. J. Chem. Eng. 2023, 101, 5832–5843. [Google Scholar] [CrossRef]
- Lasheen, T.; Elenein, S.A.A.; Saleh, W.A.; Orabi, A.H.; Ismaiel, D.A. Reductive Leaching Kinetics of Low Grade Manganese Deposits in H2SO4 Solution Using Malonic Acid as Reducing Agent. Int. J. Sci. Basic Appl. Res. (IJSBAR) 2014, 15, 151–163. [Google Scholar]
- van den Bogaard, P. The Origin of the Canary Island Seamount Province—New Ages of Old Seamounts. Sci. Rep. 2013, 3, 2107. [Google Scholar] [CrossRef]
- Somoza, L. Presentación Parcial de Datos e Información Sobre los Límites de la Plataforma Continental de España al Oeste de las Islas Canarias, Conforme a la Parte vi y El Anexo Ii de la Convención de las Naciones Unidas Sobre el Derecho del Mar; Instituto Geológico y Minero de España: Madrid, Spain, 2014. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: New York, NY, USA, 2012; ISBN 978-1-118-14692-7. [Google Scholar]
- Ghosh, M.K.; Barik, S.P.; Anand, S. Sulphuric Acid Leaching Of Polymetallic Nodules Using Paper As A Reductant. Trans. Indian. Inst. Met. 2008, 61, 477–481. [Google Scholar] [CrossRef]
- Mitić, M.; Tošić, S.; Pavlović, A.; Mašković, P.; Kostić, D.; Mitić, J.; Stevanović, V. Optimization of the Extraction Process of Minerals from Salvia officinalis L. Using Factorial Design Methodology. Microchem. J. 2019, 145, 1224–1230. [Google Scholar] [CrossRef]
- Mathews, P.G. Design of Experiments with MINITAB; Tony, W.A., Ed.; ASQ Quality Press: Milwaukee, MI, USA, 2005; ISBN 0873896378. [Google Scholar]
- Ebrahimzade, H.; Khayati, G.R.; Schaffie, M. A Novel Predictive Model for Estimation of Cobalt Leaching from Waste Li-Ion Batteries: Application of Genetic Programming for Design. J. Environ. Chem. Eng. 2018, 6, 3999–4007. [Google Scholar] [CrossRef]

| Reaction | ΔG° (kJ) | Reaction No. |
|---|---|---|
| Fe3O4 (s) + 4H2SO4 (l) FeSO4 (aq) + Fe2(SO4)3 (s) + 4H2O (l) | −264.27 | (1) |
| 2FeSO4 (aq) + 2H2SO4 (aq) + MnO2 (s) Fe2(SO4)3 (s) + 2H2O (l) + MnSO4 (aq) | −221.44 | (2) |
| CoO + H2SO4 CoSO4 + H2O | −115.43 | (3) |
| Co3O4 + 4H2SO4 + 2FeSO4 3CoSO4 + 4H2O + Fe2(SO4)3 | −354.18 | (4) |
| Element | Mass (wt. %) |
|---|---|
| Fe3O4 | 82.65 |
| Fe2O3 | 13.03 |
| Fe0 | 0.27 |
| CaO | 0.75 |
| SiO2 | 1.5 |
| Al2O3 | 0.3 |
| MgO | 0.2 |
| SO3 | 0.08 |
| P2O5 | 0.2 |
| K2O | 0.015 |
| MnO | 0.8 |
| C | 0.2 |
| Parameters [Variable]/Values | Low | Medium | High |
|---|---|---|---|
| ] | 10 | 20 | 30 |
| ] | 1/3 | 1/2 | 1/1 |
| Codifications | −1 | 0 | 1 |
| Sample | Drago 0511/DR04-15 [S1] | Subvent 1/DA06-4 [S2] | ||||
|---|---|---|---|---|---|---|
| Test | Time (min) | Ferromanganese Crust/Fe(res) | Mn Extraction (%) | Co Extraction (%) | Mn Extraction (%) | Co Extraction (%) |
| 1 | −1 | −1 | 74.15 | 31 | 73.95 | 29 |
| 2 | −1 | 0 | 69.5 | 28 | 71.25 | 27.5 |
| 3 | −1 | 1 | 55 | 20.55 | 56.5 | 17.6 |
| 4 | 0 | −1 | 72.45 | 32.8 | 73.45 | 31 |
| 5 | 0 | 0 | 74.21 | 31.5 | 74.21 | 30 |
| 6 | 0 | 1 | 67.8 | 27.25 | 66.7 | 26.55 |
| 7 | 1 | −1 | 83 | 35.95 | 84.7 | 33 |
| 8 | 1 | 0 | 81.85 | 34.65 | 82 | 32.5 |
| 9 | 1 | 1 | 73.55 | 31.4 | 71.8 | 30.45 |
| Sample | Main Minerals | Accessory Minerals |
|---|---|---|
| DRAGO 0511/DR04-15 | vernadite, birnessite, goethite | Quartz, calcite, CFA, clay |
| SUBVENT 1/DA06-4 | vernadite, birnessite, goethite | 10 Å Mn-oxides, quartz, feldspars, CFA, clay |
| Sample | Fe wt% | Mn wt% | Co µg/g | Ni µg/g | Cu µg/g | REE + Y µg/g |
|---|---|---|---|---|---|---|
| DRAGO 0511/DR04-15 | 19.9 | 14.5 | 4262 | 2359 | 474 | 2298 |
| SUBVENT 1/DA06-4 | 19.4 | 15.7 | 2424 | 2844 | 1342 | 2323 |
| Mean | 19.7 | 15.1 | 3343 | 2602 | 908 | 2311 |
| Sample | Regression Model | R2 (%) | Equation |
|---|---|---|---|
| S1 | 82.64 | (2) | |
| S1 | 98.86 | (3) | |
| S2 | 92.89 | (4) | |
| S2 | 90.30 | (5) |
| Equation | F-Value | p-Value | S | Critical F-Value |
|---|---|---|---|---|
| (2) | 14.28 | 0.005 | 3.95919 | 5.14 |
| (3) | 87.07 | 0.000 | 0.69439 | 19.24 |
| (4) | 21.77 | 0.003 | 2.76168 | 5.41 |
| (5) | 15.52 | 0.006 | 1.82772 | 5.41 |
| Equation | KS | Critical KS | Normality Test of Residuals (p-Value) | ||
|---|---|---|---|---|---|
| (2) | −0.02379 | 1.075 | 0.185 | 0.453 | >0.15 |
| (3) | −0.05316 | 1.098 | 0.143 | 0.453 | >0.15 |
| (4) | 0.02917 | 1.038 | 0.174 | 0.453 | >0.15 |
| (5) | −0.18040 | 1.140 | 0.097 | 0.453 | >0.15 |
| Sample S1 | ∇ Mn Extraction (%) [Equation (2)] | ∇ Co Extraction (%) [Equation (3)] | ||||||
|---|---|---|---|---|---|---|---|---|
| −1 | 0 | 1 | −1 | 0 | 1 | |||
| −1 | (6.6; −5.5) | (6.6; −5.5) | (6.6; −5.5) | −1 | (5.4; −4.9) | (6.9; −4.9) | (8.3; −4.9) | |
| 0 | (6.6; −5.5) | (6.6; −5.5) | (6.6; −5.5) | 0 | (2.3; −3.4) | (3.7; −3.4) | (5.2; −3.4) | |
| 1 | (6.6; −5.5) | (6.6; −5.5) | (6.6; −5.5) | 1 | (−0.8; −2) | (0.6; −2) | (2.1; −2) | |
| Sample S2 | ∇ Mn Extraction (%) [Equation (4)] | ∇ Co Extraction (%) [Equation (5)] | ||||||
| −1 | 0 | 1 | −1 | 0 | 1 | |||
| −1 | (6.1; 3.1) | (6.1; −6.2) | (6.1; −15.5) | −1 | (5.9; −5.3) | (3.6; −5.3) | (1.4; −5.3) | |
| 0 | (6.1; 3.1) | (6.1; −6.2) | (6.1; −15.5) | 0 | (5.9; −3.1) | (3.6; −3.1) | (1.4; −3.1) | |
| 1 | (6.1; 3.1) | (6.1; −6.2) | (6.1; −15.5) | 1 | (5.9; −0.9) | (3.6; −0.9) | (1.4; −0.9) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, K.; Toro, N.; Mura, M.; Saldana, M.; Madrid, F.M.G.; Salazar, I.; González, F.J.; Marino, E.; Castillo, J.; Castillo, I.; et al. Modeling the Leaching of Cobalt and Manganese from Submarine Ferromanganese Crusts by Adding Steel Scrap Using Design of Experiments and Response Surface Methodology. Appl. Sci. 2025, 15, 1155. https://doi.org/10.3390/app15031155
Pérez K, Toro N, Mura M, Saldana M, Madrid FMG, Salazar I, González FJ, Marino E, Castillo J, Castillo I, et al. Modeling the Leaching of Cobalt and Manganese from Submarine Ferromanganese Crusts by Adding Steel Scrap Using Design of Experiments and Response Surface Methodology. Applied Sciences. 2025; 15(3):1155. https://doi.org/10.3390/app15031155
Chicago/Turabian StylePérez, Kevin, Norman Toro, Mauricio Mura, Manuel Saldana, Felipe M. Galleguillos Madrid, Iván Salazar, Francisco Javier González, Egidio Marino, Jonathan Castillo, Ignacio Castillo, and et al. 2025. "Modeling the Leaching of Cobalt and Manganese from Submarine Ferromanganese Crusts by Adding Steel Scrap Using Design of Experiments and Response Surface Methodology" Applied Sciences 15, no. 3: 1155. https://doi.org/10.3390/app15031155
APA StylePérez, K., Toro, N., Mura, M., Saldana, M., Madrid, F. M. G., Salazar, I., González, F. J., Marino, E., Castillo, J., Castillo, I., & Hernández, P. C. (2025). Modeling the Leaching of Cobalt and Manganese from Submarine Ferromanganese Crusts by Adding Steel Scrap Using Design of Experiments and Response Surface Methodology. Applied Sciences, 15(3), 1155. https://doi.org/10.3390/app15031155










