Impact of Professional Hygiene Instruments on the Roughness of Implant Surfaces: An In Vitro Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Working Protocol
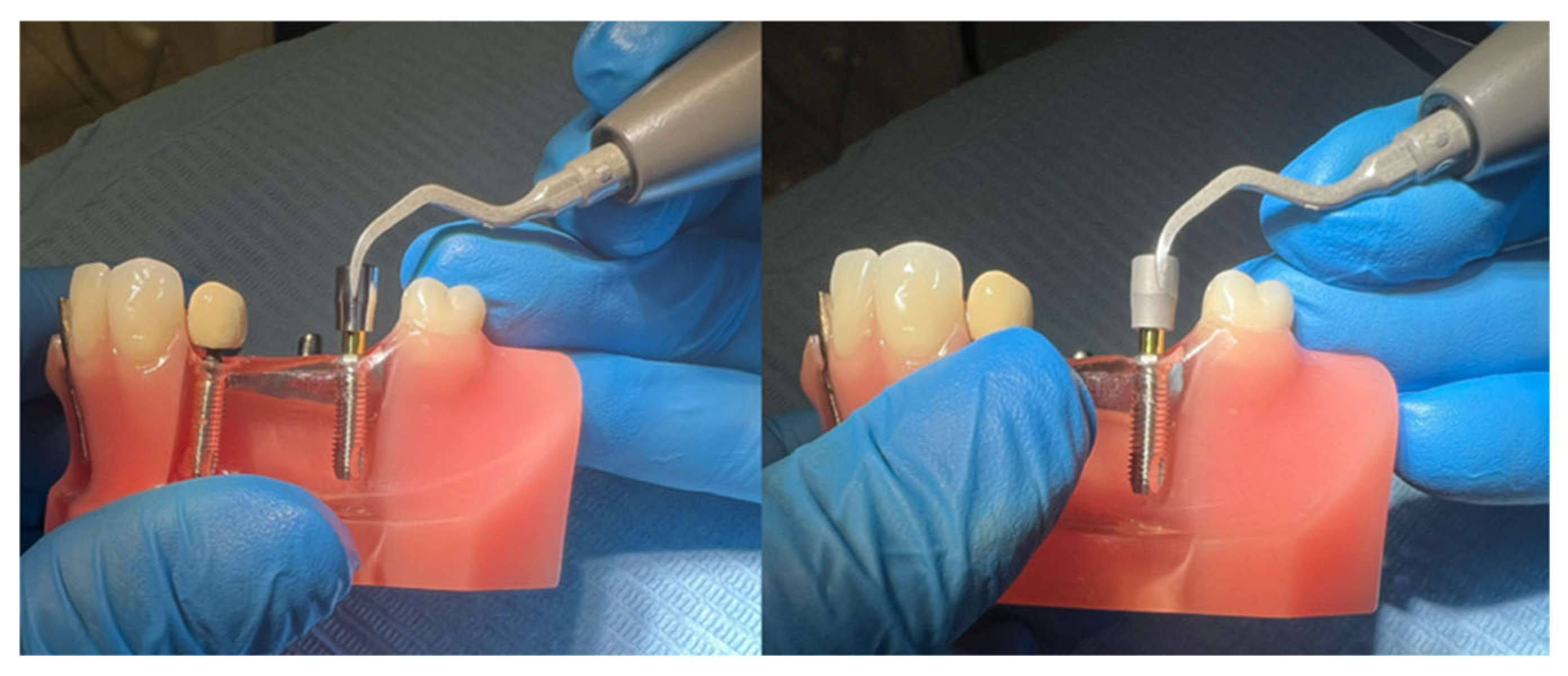
- Screwing of the healing abutment (HA) onto a model reproducing a lower dental arch (Typodont);
- Continuous circumferential instrumentation for 1 min over the entire surface of the HA (two HAs with each insert, first the machined and then the etched). The ultrasonic device was set to perio-scaler mode, power 3, irrigation 3 (Figure 6).
- Immediate, individual, and manipulation-free packaging of all instrumented HAs;
- Shipping to the DIME (Department of Mechanical, Energy, Management, and Transport Engineering) of the University of Genoa.
- Sa (arithmetic mean roughness);
- Sq (mean square roughness);
- Sp (maximum peak height);
- Sv (maximum valley depth);
- St (Sp + Sv);
- SSk: Asymmetry of the density function with respect to the mean line, a parameter related to the height distribution according to the following values:
- -
- SSk = 0 (symmetry with respect to the midline, i.e., the same density of valleys and peaks);
- -
- SSk > 0 (deviation below the midline, i.e., a more open profile with a greater density of valleys);
- -
- SSk < 0 (deviation above the midline, i.e., a fuller profile with a greater density of peaks);
- Sku: Kurtosis of the roughness profile, a parameter related to the sharpest/obtuse shape of the peaks and valleys according to the following values:
- -
- Sku = 3 (normal distribution);
- -
- Sku > 3 (acute height distribution);
- -
- Sku < 3 (obtuse height distribution);
- Sz (maximum roughness averaged over 5 peaks and 5 valleys).
Data Analysis
- Type of surface treatment of the healing abutments;
- Type of insert used.
- Two levels for the surface treatment: machined and etched;
- Five levels for the insert:
- -
- Level 1: Non-instrumented HA (used as a control level);
- -
- Level 2: HA instrumented with Satelec perioSoft;
- -
- Level 3: HA instrumented with EMS PI;
- -
- Level 4: HA instrumented with a Mectron IC1;
- -
- Level 5: HA instrumented with a Cavitron sofTip.
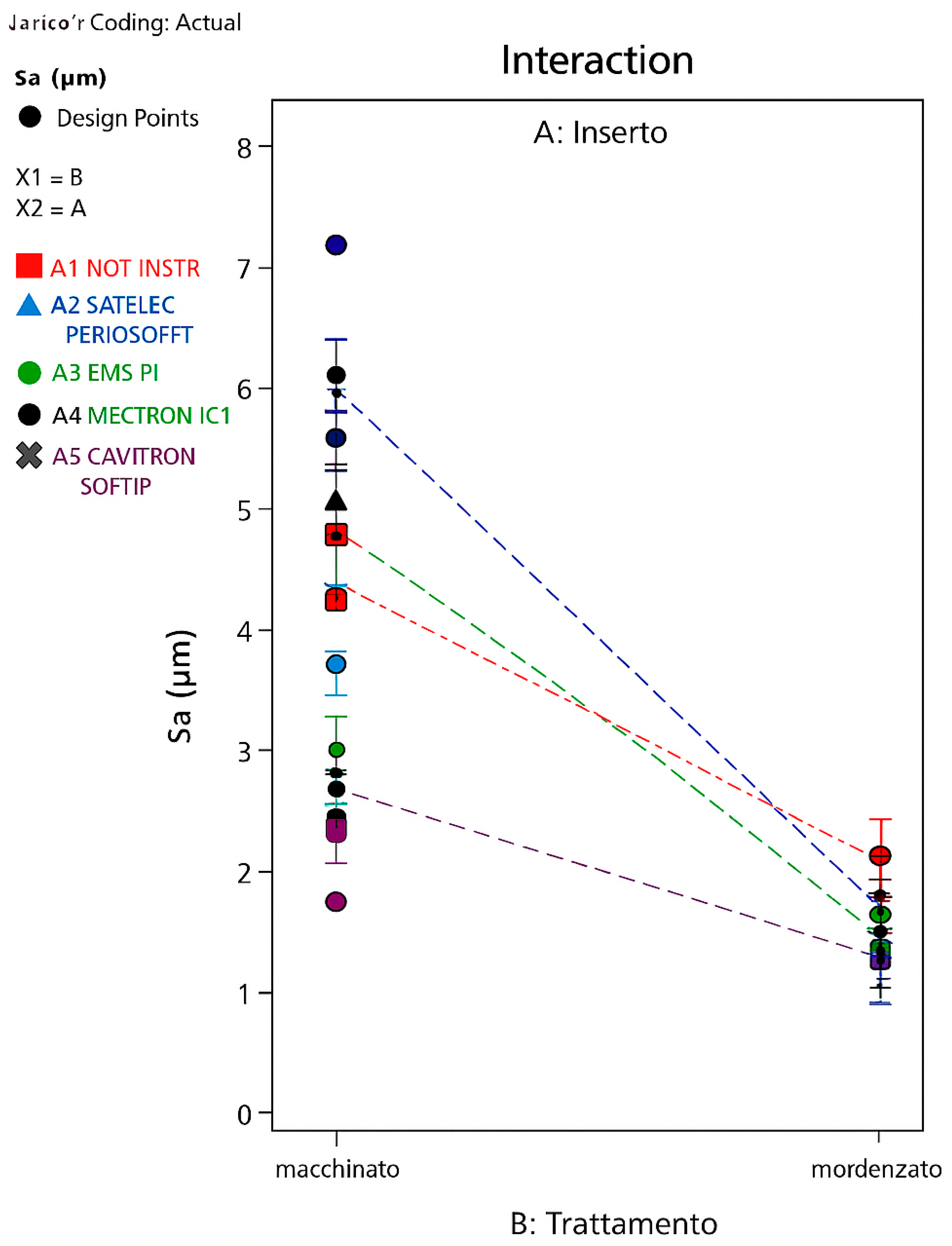
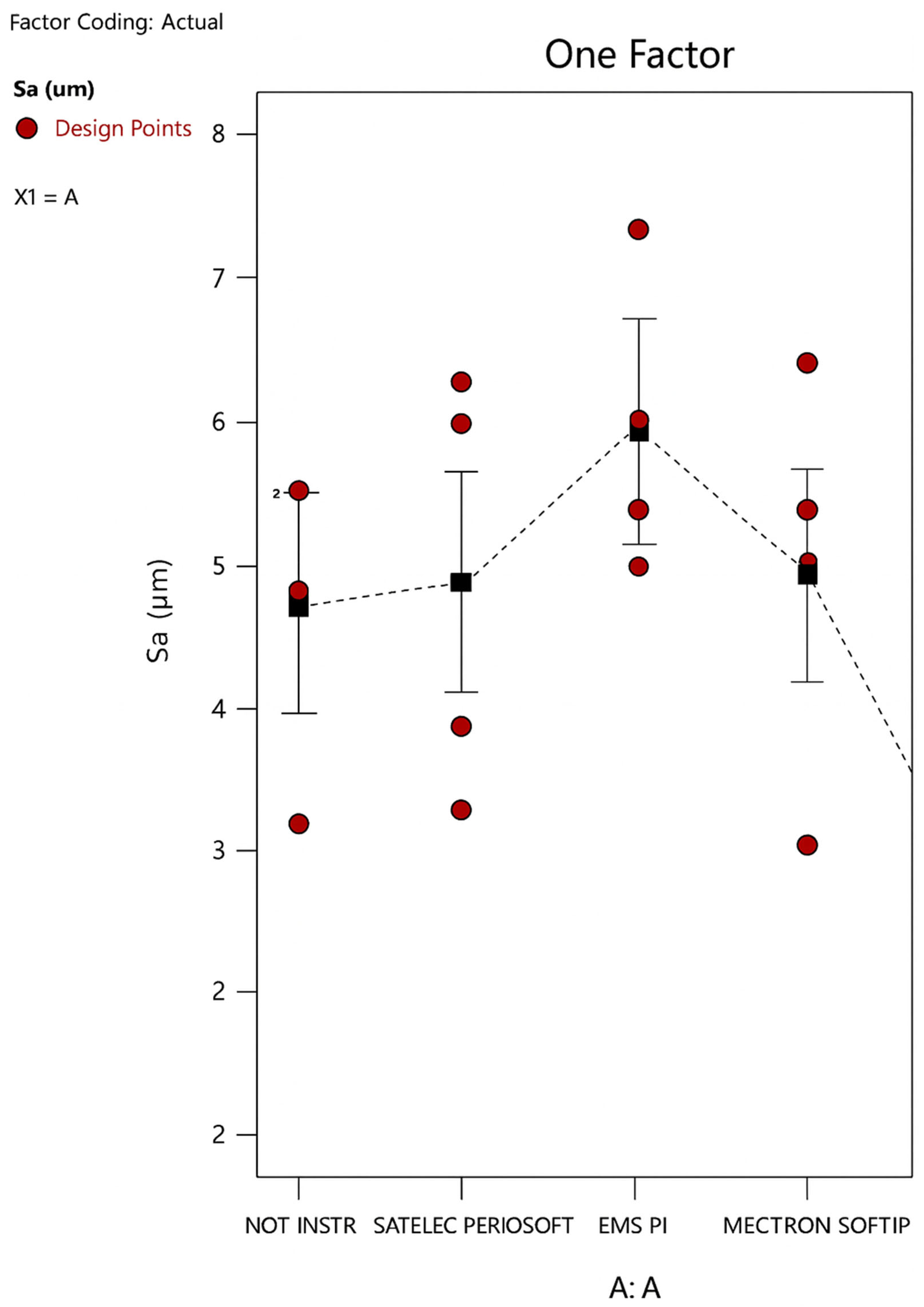
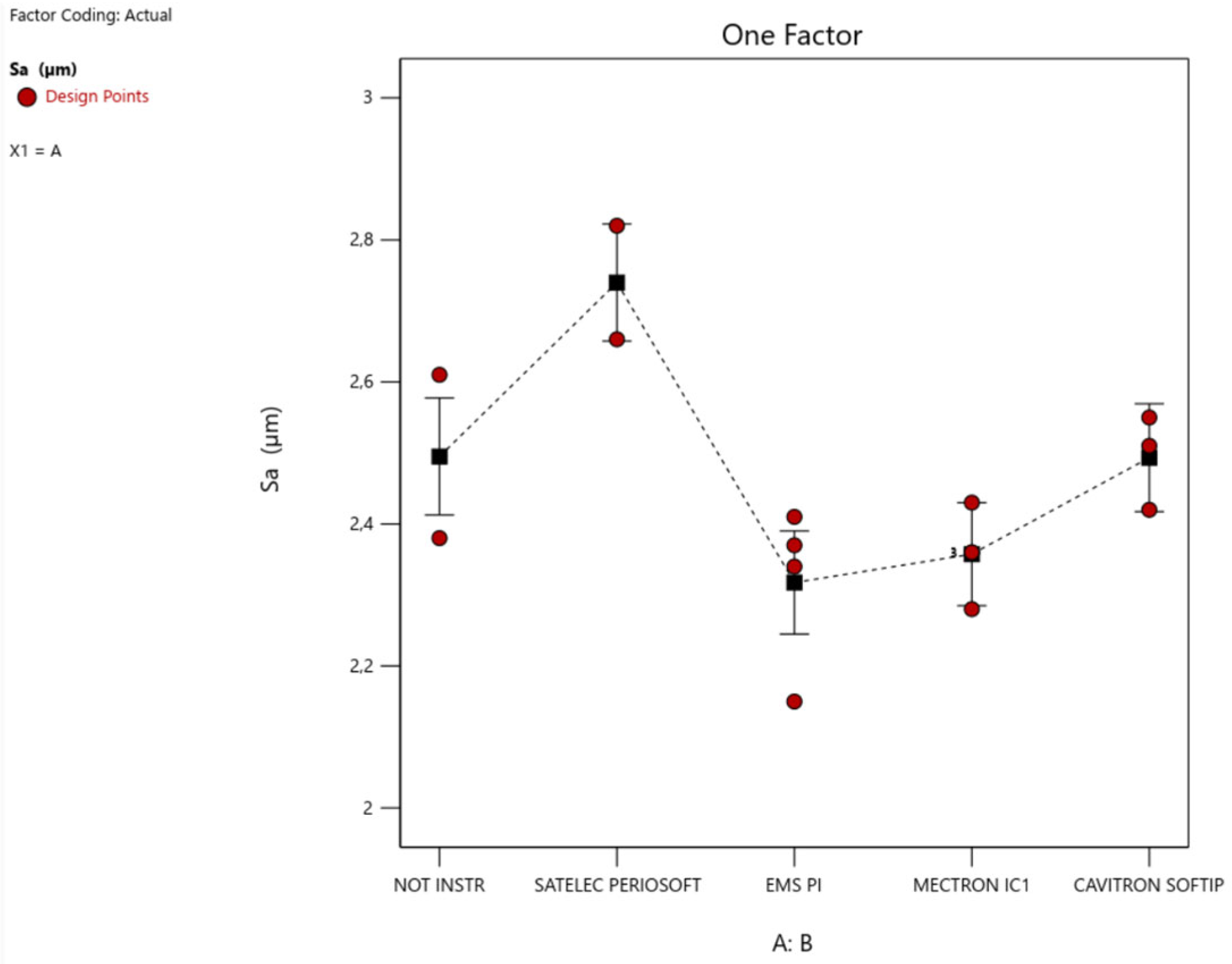
3. Results
- A greater significance of the Satelec level compared to the others, with an increase in Sa; that is, the Satelec perioSoft insert would appear to determine an increase in the surface roughness of an etched HA;
- A low but still significant significance of the EMS level, with a decrease in Sa; that is, the EMS PI insert would appear to determine a decrease in the surface roughness of an etched HA.
4. Discussion
- Most significantly, the Cavitron sofTip insert on a machined surface, bringing the Sa parameter to a level comparable to that of an etched surface, which is essentially significantly lower than the machined ones;
- Less significantly, but still significantly, the EMS PI insert on an etched surface.
5. Conclusions
- (1)
- Surface treatment (machined or etched) affects the variability of the average surface roughness (arithmetic and quadratic) to a much greater extent than the type of insert used for instrumentation.
- (2)
- Etching the surface after turning significantly decreases the average surface roughness, resulting in an average roughness of the etched surface half that of the machined surface, regardless of instrumentation, with the exception of instrumentation with the Cavitron sofTip, which makes the roughness of the machined surface comparable to that of the etched surface.
- (3)
- During instrumentation, the machined surface is more susceptible to modifications in terms of Sa/Sq parameters than the etched surface.
- (4)
- Instrumentation on machined HA significantly changes the average surface roughness only when using EMS PI inserts, which increases the surface roughness, and Cavitron SofTip, which decreases it, bringing it to values similar to those of an etched surface.
- (5)
- Instrumentation on etched HA significantly changes the average surface roughness only when using EMS PI inserts, which decreases the surface roughness, and Satelec perioSoft, which increases it.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butterworth, C.; McCaul, L.; Barclay, C. Restorative dentistry and oral rehabilitation: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S41–S44. [Google Scholar] [CrossRef]
- Sanderson, W.C.; Scherbov, S. Prospective Longevity: A New Vision of Population Aging; Harvard University Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Chapple, I.L.; Van der Weijden, F.; Doerfer, C.; Herrera, D.; Shapira, L.; Polak, D.; Madianos, P.; Louropoulou, A.; Machtei, E.; Donos, N.; et al. Primary prevention of periodontitis: Managing gingivitis. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S71–S76. [Google Scholar] [CrossRef] [PubMed]
- Rajwani, A.R.; Hawes, S.N.D.; To, A.; Quaranta, A.; Rincon Aguilar, J.C. Effectiveness of Manual Toothbrushing Techniques on Plaque and Gingivitis: A Systematic Review. Oral Health Prev. Dent. 2020, 18, 843–854. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sälzer, S.; Graetz, C.; Dörfer, C.E.; Slot, D.E.; Van der Weijden, F.A. Contemporary practices for mechanical oral hygiene to prevent periodontal disease. Periodontology 2020, 84, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Baldi, D.; De Giorgis, L.; Menini, M.; Motta, F.; Colombo, J. Efficacy of Instruments for Professional Oral Hygiene on Dental Implants: A Systematic Review. Appl. Sci. 2022, 12, 26. [Google Scholar] [CrossRef]
- Abushahba, F.; Algahawi, A.; Areid, N.; Vallittu, P.K.; Närhi, T. Efficacy of biofilm decontamination methods of dental implant surfaces: A systematic review of in vitro studies. Eur. J. Oral Sci. 2025, 133, e70005. [Google Scholar] [CrossRef] [PubMed]
- Baldi, D.; Colombo, J.; Verardi, S.; Rebaudi, A.; Rebaudi, F.; Makary, C. Clinical osseointegration of bone level implants with conical shape and textured surface with low primary stability. Minerva Stomatol. 2020, 69, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.; Liu, X. Soft tissue integration around dental implants: A pressing priority. Biomaterials 2026, 324, 123491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiorellini, J.P.; Mojaver, S.; Sarmiento, H.; Aghaloo, T. Clinical Translation of the 2024 AO/AAP Consensus on Prevention and Management of Peri-implant Diseases and Conditions. Int. J. Periodontics Restor. Dent. 2025, 45, S1–S23. [Google Scholar] [CrossRef] [PubMed]
- Baldi, D.; Colombo, J.; Gavoglio, P.; De Giorgis, L.; Motta, F.; Lugas, A.; Lertora, E.; Schierano, G. Roughness and SEM Analysis of Manual and Ultrasonic Instrumentation over Different Crown Materials for Dental Implants Restorations. Materials 2022, 15, 1159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, Y.J.; Song, Y.W.; Park, S.Y.; Cha, J.K.; Lee, H.J.; Yang, S.M.; Park, J.B.; Koo, K.T. Current understanding of the etiology, diagnosis, treatment, and management of peri-implant diseases: A narrative review for the consensus report of the Korean Academy of Periodontology. J. Periodontal Implant. Sci. 2024, 54, 377–392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romito, G.A.; Hassan, M.A.; do Amaral, G.C.L.S.; Villar, C.C. Decision-making on peri-implant mucositis management and treatment approaches. Br. Dent. J. 2024, 236, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Maiorani, C.; Butera, A.; Pérez-Albacete Martínez, C.; Pascadopoli, M.; Sabatini, S.; Nardi, G.M.; Scribante, A. Effectiveness of Erythritol-Based Air Polishing and Ultrasonic Instrumentation with PEEK Inserts in Peri-Implant Maintenance: A Randomized Clinical Trial Including Different Prosthetic Materials. Dent. J. 2025, 13, 235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iommiello, A.M.; Pesce, P.; Sanavia, C.; Arata, F.; Caponio, V.C.; Baldi, D.; Migliorati, M.; Menini, M. The use of water flosser in implant-supported fixed prosthesis: A narrative review and the opinion of Italian dental hygienists. Minerva Dent. Oral Sci. 2025, 74, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Delucchi, F.; Ingegnieros, L.; Pesce, P.; Baldi, D.; Canullo, L.; Bagnasco, F.; Zunino, P.; Menini, M. Efficacy and safety of erythritol air-polishing in implant dentistry: A systematic review. Int. J. Dent. Hyg. 2024, 23, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Taymour, N.; Abd El-Fattah, A.; Kandil, S.; Fahmy, A.E.; Al-Qahtani, N.H.; Khaled, A.; Al-Dulaijan, Y.A.; Gepreel, M.A.-H. Revolutionizing Dental Polymers: The Versatility and Future Potential of Polyetheretherketone in Restorative Dentistry. Polymers 2024, 17, 80. [Google Scholar] [CrossRef]
- Ozgu, I.; Ustun, K. Effects of Mechanical Methods Used in Peri-implantitis Treatment on Implant Surface Decontamination and Roughness. J. Vis. Exp. 2025, 217, e67778. [Google Scholar] [CrossRef]
- Hempton, T.; Lancaster, D.; Pechter, J. Implant Maintenance; Technique and tools for effective debridement of artificial anatomy. Dimens. Dent. Hyg. 2011, 9, 58–61. [Google Scholar]
- Pattison, A.M.; Sumi, J.Y. Post-surgical implant care. Dimens. Dent. Hyg. 2015, 13, 24–29. [Google Scholar]
- Salvi, G.E.; Persson, G.R.; Heitz-Mayfield, L.J.; Frei, M.; Lang, N.P. Adjunctive local antibiotic therapy in the treatment of peri-implantitis II: Clinical and radiographic outcomes. Clin. Oral Implant. Res. 2007, 18, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Baldi, D.; Menini, M.; Pera, F.; Ravera, G.; Pera, P. Plaque accumulation on exposed titanium surfaces and peri-implant tissue behavior. A preliminary 1-year clinical study. Int. J. Prosthodont. 2009, 22, 447–455. [Google Scholar] [PubMed]
- Blasi, A.; Iorio-Siciliano, V.; Pacenza, C.; Pomingi, F.; Matarasso, S.; Rasperini, G. Biofilm removal from implants supported restoration using different instruments: A 6-month comparative multicenter clinical study. Clin. Oral Implant. Res. 2014, 27, e68–e73. [Google Scholar] [CrossRef] [PubMed]






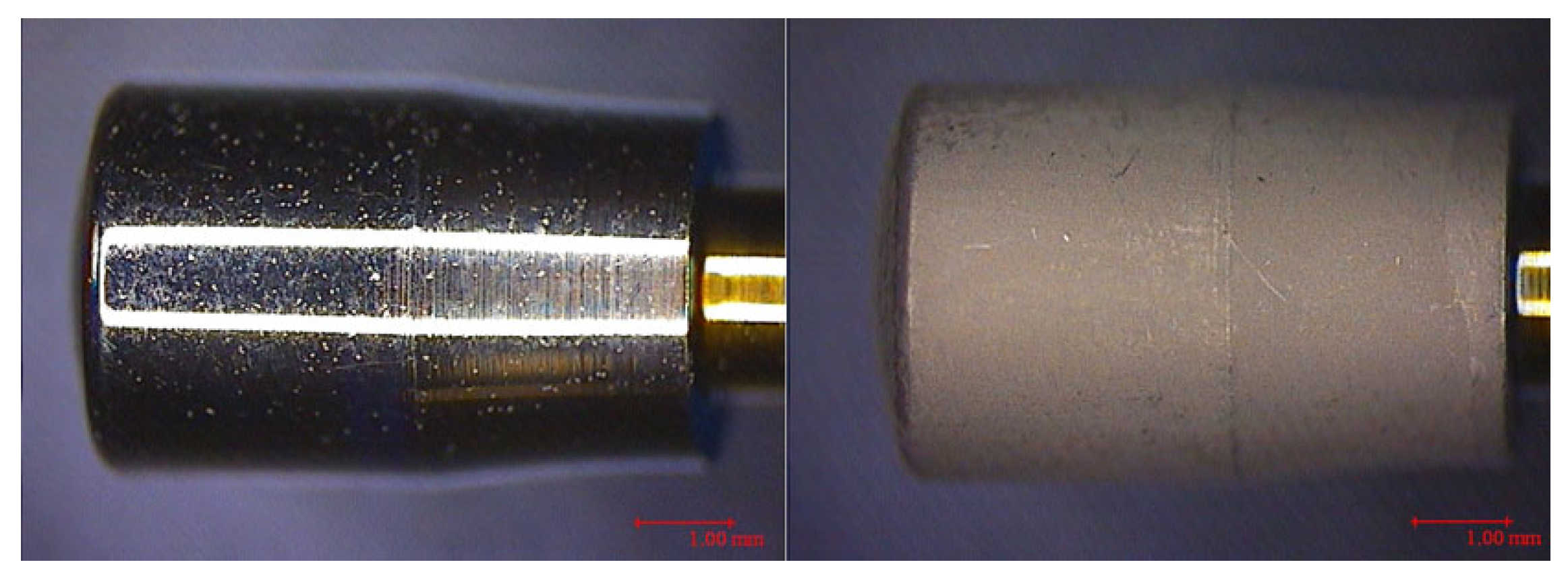


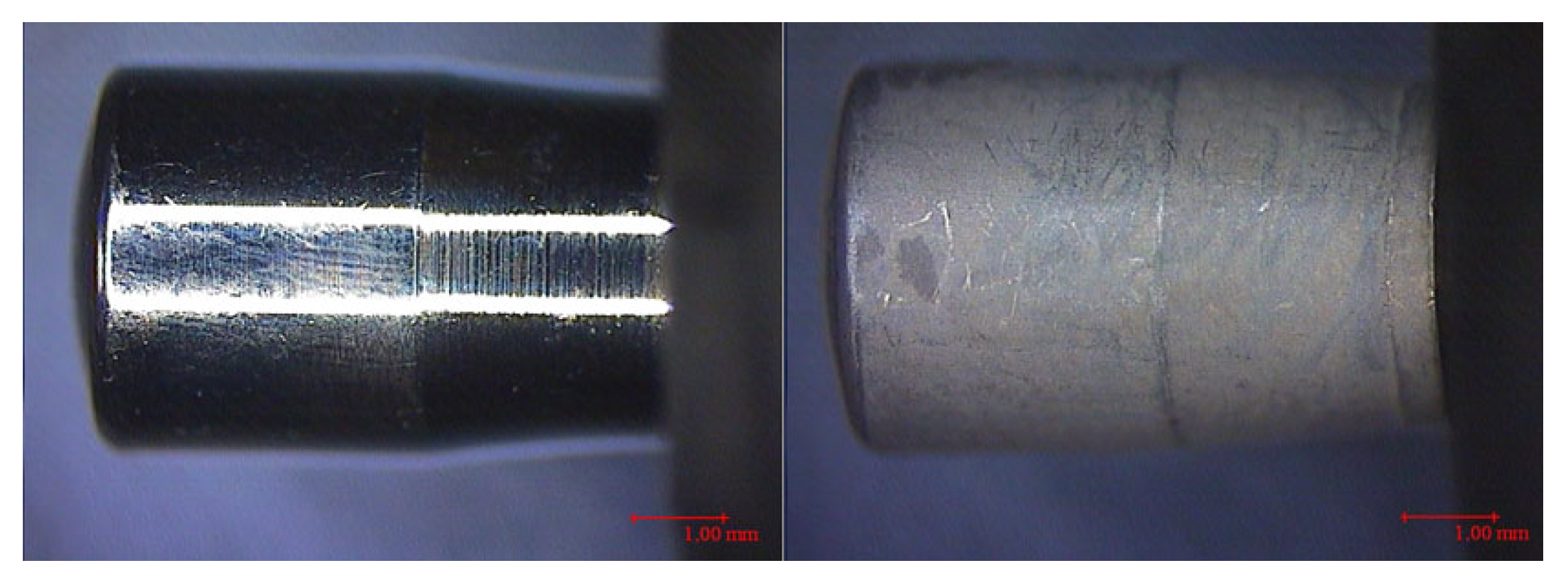
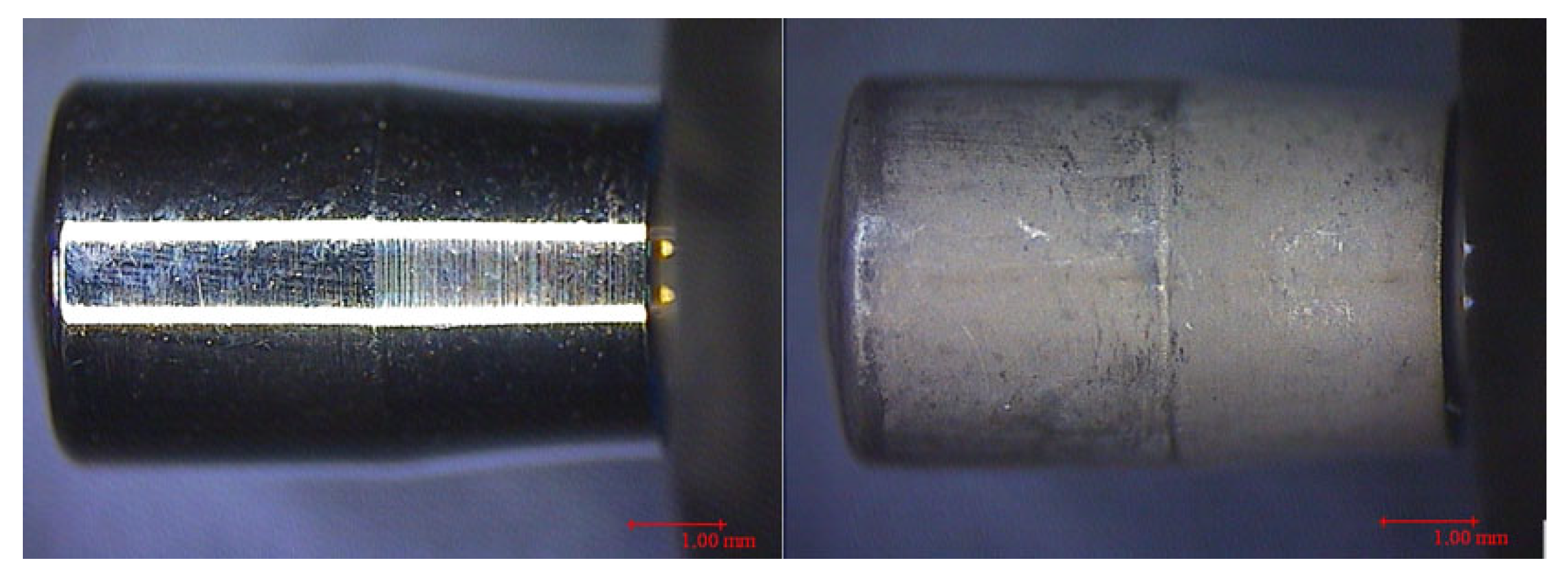


| Healing Abutment (HA) | CODE | SURFACES | PARAMETERS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sa (µm) | Sq (µm) | Sp (µm) | Sv (µm) | St (µm) | SSk | Sku | Sz (µm) | |||
| HA machined not instrumented | 1 | 5.66 | 7.52 | 15.2 | 24.4 | 39.6 | −1.1 | 3.99 | 37.9 | |
| 2 | 5.05 | 6.84 | 19.7 | 20.7 | 40.5 | −0.273 | 3.69 | 39.2 | ||
| 3 | 5.68 | 7.67 | 22 | 23.5 | 45.5 | −0.179 | 3.48 | 43.7 | ||
| 4 | 3.29 | 4.49 | 12 | 16.4 | 28.4 | −0.658 | 4.01 | 27.4 | ||
| HA etched not instrumented | 1 | 2.61 | 3.3 | 13.9 | 14.3 | 28.2 | 0.0385 | 3.2 | 25.8 | |
| 2 | 3.08 | 3.94 | 24.3 | 14.9 | 39.2 | 0.905 | 5.1 | 23.9 | ||
| 3 | 2.97 | 4.07 | 28.1 | 13.3 | 41.4 | 0.899 | 7.37 | 28.6 | ||
| 4 | 2.38 | 2.95 | 9.64 | 12.3 | 21.9 | −0.164 | 2.87 | 21.1 | ||
| HA machined SATELEC PERIOSOFT | AL | 1 | 6.46 | 8.86 | 25.8 | 31.4 | 57.1 | −0.899 | 4.48 | 55.9 |
| 2 | 3.56 | 4.69 | 16.1 | 16.2 | 32.3 | −0.144 | 3.46 | 29.6 | ||
| 3 | 6.17 | 7.75 | 19.8 | 25.2 | 45 | 0.222 | 2.73 | 40.1 | ||
| 4 | 4.11 | 5.28 | 12.6 | 17.4 | 30 | −0.78 | 3.38 | 28.9 | ||
| HA etched SATELEC PERIOSOFT | AM | 1 | 2.01 | 2.57 | 11.2 | 11.7 | 22.8 | 0.0959 | 3.43 | 20.3 |
| 2 | 2.66 | 3.45 | 22.2 | 13.7 | 35.8 | 0.305 | 4.45 | 30.8 | ||
| 3 | 5.73 | 6.71 | 21.2 | 18.9 | 40.1 | −0.118 | 2.07 | 35.5 | ||
| 4 | 2.82 | 3.59 | 17.9 | 16 | 33.9 | 0.13 | 3.42 | 29.4 | ||
| HA machined EMS PI | BL | 1 | 5.24 | 7.12 | 13.6 | 25 | 38.5 | −1.29 | 4.58 | 37 |
| 2 | 7.43 | 10.3 | 24.9 | 34.2 | 59.1 | −0.927 | 4.24 | 54.8 | ||
| 3 | 5.6 | 7.35 | 13.5 | 25.9 | 39.4 | −1.18 | 4.36 | 37.2 | ||
| 4 | 6.19 | 8.64 | 22.3 | 32.2 | 54.4 | −0.687 | 4.41 | 50.4 | ||
| HA etched EMS PI | BM | 1 | 2.41 | 3.02 | 10.6 | 14.5 | 25.1 | −0.00148 | 3.04 | 22.8 |
| 2 | 2.34 | 2.97 | 13.3 | 12.1 | 25.4 | 0.162 | 3.26 | 23.2 | ||
| 3 | 2.15 | 2.73 | 10.9 | 14.1 | 24.9 | 0.0139 | 3.32 | 21.9 | ||
| 4 | 2.37 | 3.01 | 11.9 | 12 | 23.9 | −0.195 | 3.17 | 22 | ||
| HA machined MECTRON IC1 | CL | 1 | 5.24 | 7.25 | 19 | 25.2 | 44.1 | −1.01 | 4.53 | 40 |
| 2 | 5.6 | 7.18 | 15.7 | 37.8 | 53.4 | −0.721 | 3.26 | 34.4 | ||
| 3 | 6.6 | 8.4 | 29.4 | 25.4 | 54.8 | −0.258 | 2.85 | 48.6 | ||
| 4 | 3.09 | 3.97 | 19.2 | 15.7 | 34.9 | −0.00288 | 3.52 | 32 | ||
| HA etched MECTRON IC1 | CM | 1 | 2.36 | 2.96 | 14.4 | 13.6 | 28.1 | 0.0849 | 3.1 | 23.2 |
| 2 | 2.28 | 2.84 | 12.3 | 10.4 | 22.7 | 0.0555 | 3.04 | 20.1 | ||
| 3 | 2.36 | 2.97 | 11.8 | 13.7 | 25.5 | 0.00816 | 3.07 | 22.7 | ||
| 4 | 2.43 | 3.04 | 11.6 | 11.4 | 23 | 0.0668 | 2.95 | 21.8 | ||
| HA machined CAVITRON SOFTIP | DL | 1 | 2.48 | 3.14 | 13.2 | 17.2 | 30.4 | 0.0274 | 3.36 | 23.1 |
| 2 | 3.11 | 3.92 | 20.7 | 14.1 | 34.7 | 0.245 | 3.48 | 28.6 | ||
| 3 | 3.23 | 4.11 | 18.8 | 21.2 | 40 | −0.067 | 3.5 | 34.5 | ||
| 4 | 4.55 | 6.4 | 20.1 | 22.5 | 42.7 | −0.377 | 4.17 | 39.6 | ||
| HA etched CAVITRON SOFTIP | DM | 1 | 2.51 | 3.14 | 12.2 | 12.6 | 24.8 | −0.128 | 2.98 | 22.4 |
| 2 | 2.42 | 3.06 | 13.3 | 12.6 | 25.9 | 0.334 | 3.18 | 21.3 | ||
| 3 | 2.03 | 2.59 | 10.6 | 10.7 | 21.4 | 0.209 | 3.27 | 19.3 | ||
| 4 | 2.55 | 3.14 | 11.5 | 10.6 | 22.1 | 0.161 | 2.78 | 21 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, J.; Baldi, F.; Gavoglio, P.; Marchisio, G.; Baldi, D. Impact of Professional Hygiene Instruments on the Roughness of Implant Surfaces: An In Vitro Analysis. Appl. Sci. 2025, 15, 12598. https://doi.org/10.3390/app152312598
Colombo J, Baldi F, Gavoglio P, Marchisio G, Baldi D. Impact of Professional Hygiene Instruments on the Roughness of Implant Surfaces: An In Vitro Analysis. Applied Sciences. 2025; 15(23):12598. https://doi.org/10.3390/app152312598
Chicago/Turabian StyleColombo, Jacopo, Francesca Baldi, Paola Gavoglio, Giulia Marchisio, and Domenico Baldi. 2025. "Impact of Professional Hygiene Instruments on the Roughness of Implant Surfaces: An In Vitro Analysis" Applied Sciences 15, no. 23: 12598. https://doi.org/10.3390/app152312598
APA StyleColombo, J., Baldi, F., Gavoglio, P., Marchisio, G., & Baldi, D. (2025). Impact of Professional Hygiene Instruments on the Roughness of Implant Surfaces: An In Vitro Analysis. Applied Sciences, 15(23), 12598. https://doi.org/10.3390/app152312598






