Left Atrial Blood Flow Dynamics: Preliminary Observations Using HyperDoppler
Abstract
1. Introduction
2. Methods
2.1. HyperDoppler Technique
2.2. Description of Study Subjects
2.3. Echocardiography
2.3.1. Image Acquisition
2.3.2. HyperDoppler Image Analysis
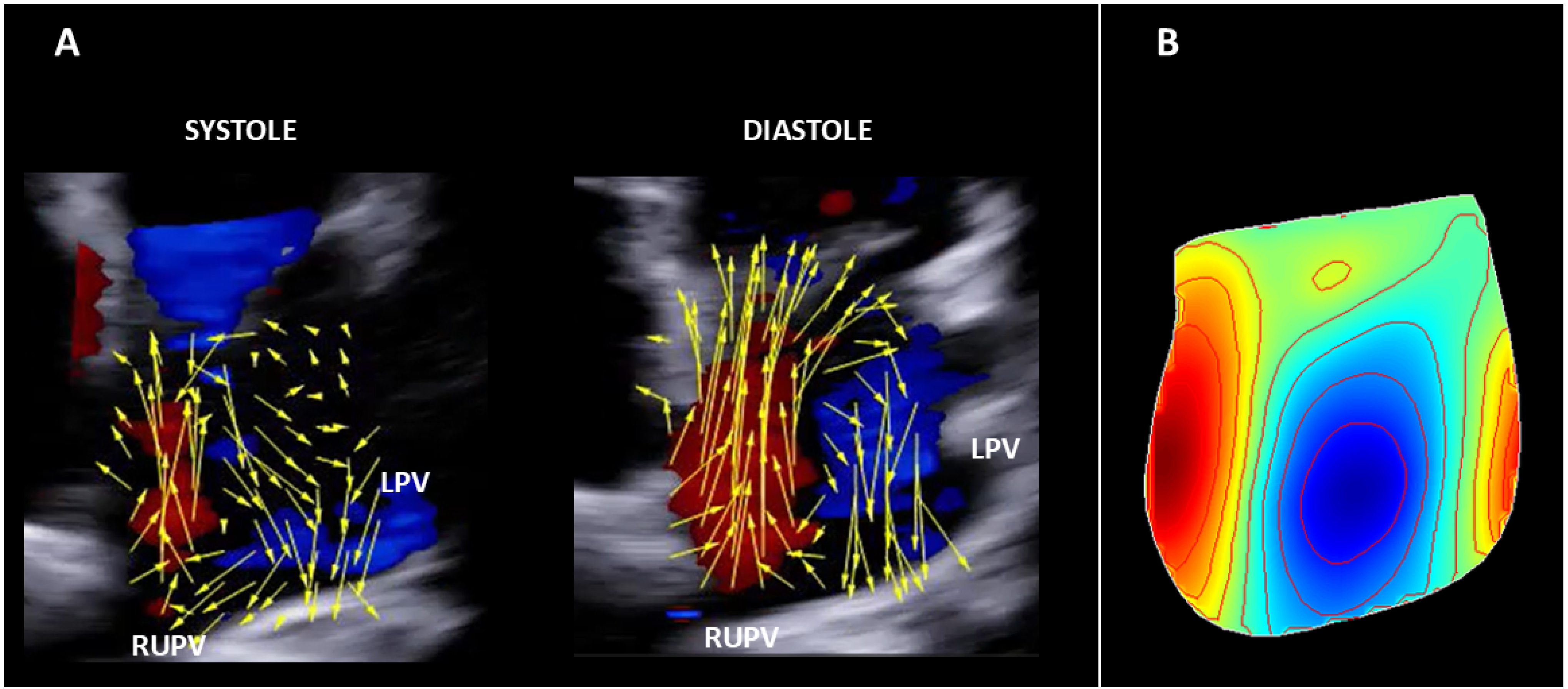
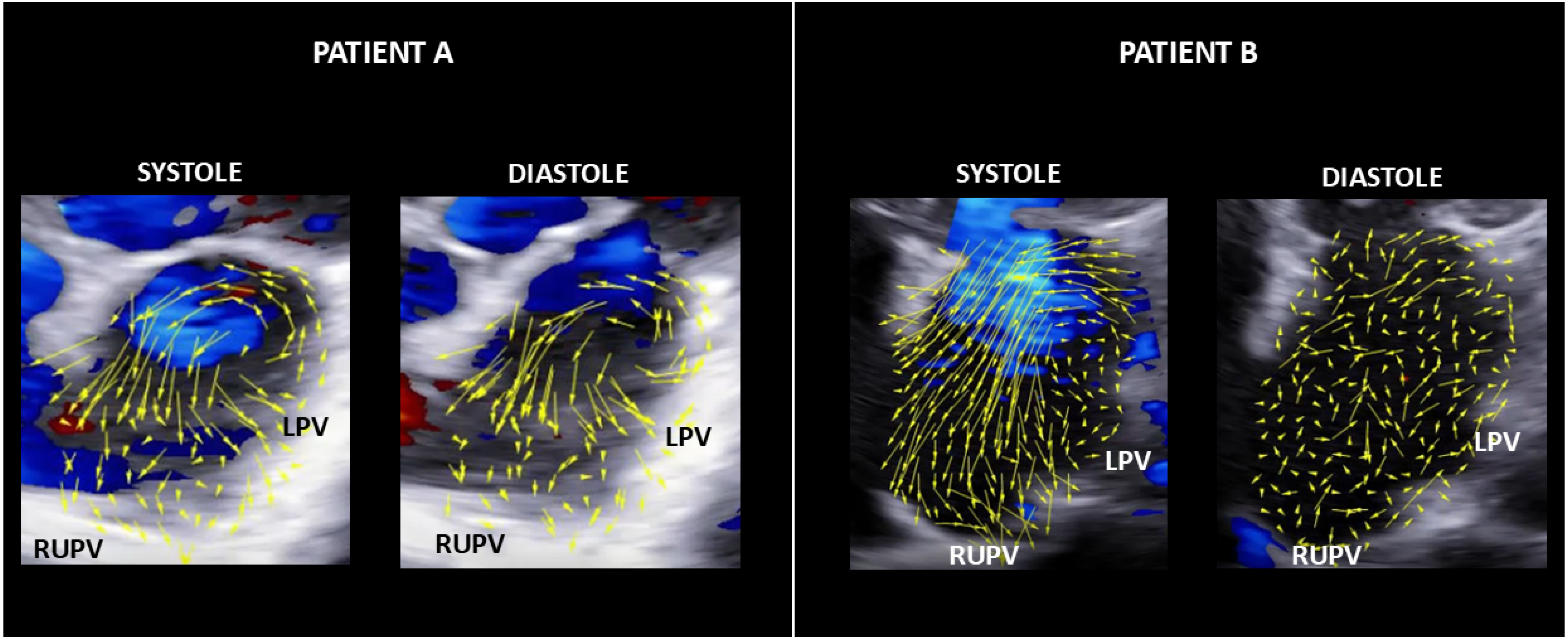
3. Results
3.1. HyperDoppler Findings
3.1.1. Healthy Subject
3.1.2. AF Patients
4. Discussion
4.1. Computational Models
4.2. 4D-Flow MRI Studies
4.3. Ultrasound Evaluations
4.4. Current Observations
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| LA | left atrium |
| LPV | left pulmonary vein |
| LV | left ventricle |
| MRI | magnetic resonance imaging |
| RUPV | right upper pulmonary vein |
References
- Sekine, T.; Nakaza, M.; Matsumoto, M.; Ando, T.; Inoue, T.; Sakamoto, S.-I.; Maruyama, M.; Obara, M.; Leonowicz, O.; Usuda, J.; et al. 4D flow MR imaging of the left atrium: What is non-physiological blood flow in the cardiac system? Magn. Reson. Med. Sci. 2022, 21, 293–308. [Google Scholar] [CrossRef]
- Watson, T.; Shantsila, E.; Lip, G.Y. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet 2009, 373, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J.G.M. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on Atrial Fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Melgaard, L.; Gorst-Rasmussen, A.; Lane, D.A.; Rasmussen, L.H.; Larsen, T.B.; Lip, G.Y.H. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA 2015, 314, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Mele, D.; Smarrazzo, V.; Pedrizzetti, G.; Capasso, F.; Pepe, M.; Severino, S.; Luisi, G.A.; Maglione, M.; Ferrari, R. Intracardiac Flow Analysis: Techniques and Potential Clinical Applications. J. Am. Soc. Echocardiogr. 2019, 32, 319–332. [Google Scholar] [CrossRef]
- Serio, L.; Beccari, R.; Nistri, S.; Cecchetto, A.; Pedrizzetti, G.; Mele, D. Intracardiac flow dynamics in mitral regurgitation: State of the art. Eur. Heart J. Imaging Methods Pract. 2025, 3, qyaf022. [Google Scholar] [CrossRef]
- Shibata, M.; Yambe, T.; Funamoto, K.; Hayase, T. Intra-left Atrial Flow Changes During the Start of Atrial Fibrillation. Heart Lung Circ. 2013, 22, S184. [Google Scholar] [CrossRef]
- Park, K.H.; Son, J.W.; Park, W.J.; Lee, S.H.; Kim, U.; Park, J.S.; Shin, D.G.; Kim, Y.J.; Choi, J.H.; Houle, H.; et al. Characterization of the left atrial vortex flow by two-dimensional transesophageal contrast echocardiography using particle image velocimetry. Ultrasound Med. Biol. 2013, 39, 62–71. [Google Scholar] [CrossRef]
- Fiorencis, A.; Pepe, M.; Smarrazzo, V.; Martini, M.; Severino, S.; Pergola, V.; Evangelista, M.; Incarnato, P.; Previtero, M.; Maglione, M.; et al. Noninvasive Evaluation of Intraventricular Flow Dynamics by the HyperDoppler Technique: First Application to Normal Subjects, Athletes, and Patients with Heart Failure. J. Clin. Med. 2022, 11, 2216. [Google Scholar] [CrossRef]
- Hong, G.R.; Pedrizzetti, G.; Tonti, G.; Li, P.; Wei, Z.; Kim, J.K.; Bawela, A.; Liu, S.; Chung, N.; Houle, H.; et al. Characterization and Quantification of Vortex Flow in the Human Left Ventricle by Contrast Echocardiography Using Vector Particle Image Velocimetry. JACC Cardiov Imaging 2008, 1, 705–717. [Google Scholar] [CrossRef]
- Kheradvar, A.; Houle, H.; Pedrizzetti, G.; Tonti, G.; Belcik, T.; Ashraf, M.; Lindner, J.; Gharib, M.; Sahn, D. Echographic Particle Image Velocimetry: A novel technique for quantification of left ventricular blood vorticity pattern. J. Am. Soc. Echocardiogr. 2010, 23, 86–94. [Google Scholar] [CrossRef]
- Cimino, S.; Pedrizzetti, G.; Tonti, G.; Canali, E.; Petronilli, V.; De Luca, L.; Iacoboni, C.; Agati, L. In vivo analysis of intraventricular fluid dynamics in healthy hearts. Eur. J. Mech. B/Fluids 2012, 35, 40–46. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Martiniello, A.R.; Bianchi, V.; D'Onofrio, A.; Caso, P.; Tonti, G. Changes in electrical activation modify the direction of left ventricular flow momentum: Novel observations using Echocardiographic Particle Image Velocimetry. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Uejima, T.; Koike, A.; Sawada, H.; Aizawa, T.; Ohtsuki, S.; Tanaka, M.; Furukawa, T.; Fraser, A.G. A new echocardiographic method for identifying vortex flow in the left ventricle: Numerical validation. Ultrasound Med. Biol. 2010, 36, 772–788. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Vedula, V.; George, R.; Younes, L.; Mittal, R. Hemodynamics in the left atrium and its effect on ventricular flow patterns. J. Biomech. Eng. 2015, 137, 111003. [Google Scholar] [CrossRef]
- Feng, L.; Gao, H.; Qi, N.; Danton, M.; Hill, N.A.; Luo, X. Fluid–structure interaction in a fully coupled three-dimensional mitral–atrium–pulmonary model. Biomech. Model. Mechanobiol. 2021, 20, 1267–1295. [Google Scholar] [CrossRef]
- Kilner, P.J.; Yang, G.-Z.; Wilkes, A.J.; Mohiaddin, R.H.; Firmin, D.N.; Yacoub, M.H. Asymmetric redirection of flow through the heart. Nature 2000, 404, 759–761. [Google Scholar] [CrossRef]
- Fyrenius, A.; Wigström, L.; Ebbers, T.; Karlsson, M.; Engvall, J.; Bolger, A.F. Three dimensional flow in the human left atrium. Heart 2001, 86, 448–455. [Google Scholar] [CrossRef]
- Föll, D.; Taeger, S.; Bode, C.; Jung, B.; Markl, M. Age gender, blood pressure, and ventricular geometry influence normal 3D blood flow characteristics in the left heart. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 366–373. [Google Scholar] [CrossRef]
- Suwa, K.; Saitoh, T.; Takehara, Y.; Sano, M.; Nobuhara, M.; Saotome, M.; Urushida, T.; Katoh, H.; Satoh, H.; Sugiyama, M.; et al. Characteristics of Intra-Left Atrial Flow Dynamics and Factors Affecting Formation of the Vortex Flow: Analysis with Phase-Resolved 3-Dimensional Cine Phase Contrast Magnetic Resonance Imaging. Circ. J. 2014, 79, 144–152. [Google Scholar] [CrossRef]
- Fluckiger, J.U.; Goldberger, J.J.; Lee, D.C.; Ng, J.; Lee, R.; Goyal, A.; Markl, M. Left Atrial Flow Velocity Distribution and Flow Coherence Using Four-Dimensional FLOW MRI: A Pilot Study Investigating the Impact of Age and Pre- and Postintervention Atrial Fibrillation on Atrial Hemodynamics. J. Magn. Reson. Imaging 2013, 38, 580–587. [Google Scholar] [CrossRef]
- Garcia, J.; Sheitt, H.; Bristow, M.S.; Lydell, C.; Howarth, A.G.; Heydari, B.; Prato, F.S.; Drangova, M.; Thornhill, R.E.; Nery, P.; et al. Left atrial vortex size and velocity distributions by 4D flow MRI in patients with paroxysmal atrial fibrillation: Associations with age and CHA2 DS2-VASc risk score. J. Magn. Reson. Imaging 2020, 51, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Spartera, M.; Pessoa-Amorim, G.; Stracquadanio, A.; Von Ende, A.; Fletcher, A.; Manley, P.; Neubauer, S.; Ferreira, V.M.; Casadei, B.; Hess, A.T.; et al. Left atrial 4D flow cardiovascular magnetic resonance: A reproducibility study in sinus rhythm and atrial fibrillation. Cardiovasc. Magn. Reson. 2021, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Spartera, M.; Stracquadanio, A.; Pessoa-Amorim, G.; Harston, G.; Mazzucco, S.; Young, V.; Von Ende, A.; Hess, A.T.; Ferreira, V.M.; Kennedy, J.; et al. Reduced Left Atrial Rotational Flow Is Independently Associated with Embolic Brain Infarcts. JACC Cardiovasc. Imaging 2023, 16, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.N.; Taxak, A.; Kumar, S. Normal pulmonary venous anatomy and non-anomalous variations demonstrated on CT angiography: What the radiologist needs to know? Br. J. Radiol. 2020, 93, 20200595. [Google Scholar] [CrossRef]
- Mangia, M.; D’Andrea, E.; Cecchetto, A.; Beccari, R.; Mele, D.; Nistri, S. Current and Clinically Relevant Echocardiographic Parameters to Analyze Left Atrial Function. J. Cardiovasc. Dev. Dis. 2024, 11, 241. [Google Scholar] [CrossRef]
| Normal Subject | Patient A | Patient B | |
|---|---|---|---|
| Age (years) | 39 | 68 | 80 |
| Sex | Male | Male | Female |
| Heart Rate (bpm) | 75 | 70 | 90 |
| BSA (m2) | 1.90 | 1.80 | 1.97 |
| Cardiac rhythm | SR | AF | AF |
| SBP (mmHg) | 120 | 110 | 120 |
| DBP (mmHg) | 70 | 60 | 70 |
| LV EDD (mm) | 40 | 38 | 38 |
| LVMI (g/m2) | 45 | 74 | 68 |
| EDVI (mL/m2) | 49 | 42 | 45 |
| LV EF (%) | 61 | 65 | 63 |
| LAVI (mL/m2) | 20 | 30 | 56 |
| PASP (mmHg) | 23 | 30 | 36 |
| TAPSE (mm) | 27 | 21 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smarrazzo, V.; Miano, V.; Maglione, M.; Pedrizzetti, G.; Mele, D. Left Atrial Blood Flow Dynamics: Preliminary Observations Using HyperDoppler. Appl. Sci. 2025, 15, 12548. https://doi.org/10.3390/app152312548
Smarrazzo V, Miano V, Maglione M, Pedrizzetti G, Mele D. Left Atrial Blood Flow Dynamics: Preliminary Observations Using HyperDoppler. Applied Sciences. 2025; 15(23):12548. https://doi.org/10.3390/app152312548
Chicago/Turabian StyleSmarrazzo, Vittorio, Vittoria Miano, Marco Maglione, Gianni Pedrizzetti, and Donato Mele. 2025. "Left Atrial Blood Flow Dynamics: Preliminary Observations Using HyperDoppler" Applied Sciences 15, no. 23: 12548. https://doi.org/10.3390/app152312548
APA StyleSmarrazzo, V., Miano, V., Maglione, M., Pedrizzetti, G., & Mele, D. (2025). Left Atrial Blood Flow Dynamics: Preliminary Observations Using HyperDoppler. Applied Sciences, 15(23), 12548. https://doi.org/10.3390/app152312548







