Investigating the Potential Molecular Mechanisms of Mogroside V on Glucose Homeostasis by Transcriptome Profiling of Adult Mouse Hypothalamic Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Cellular Tests
2.3. Isolation of RNA, Preparation of Libraries, and Sequencing
2.4. Bioinformatic Analysis
2.5. qPCR Validation
3. Results
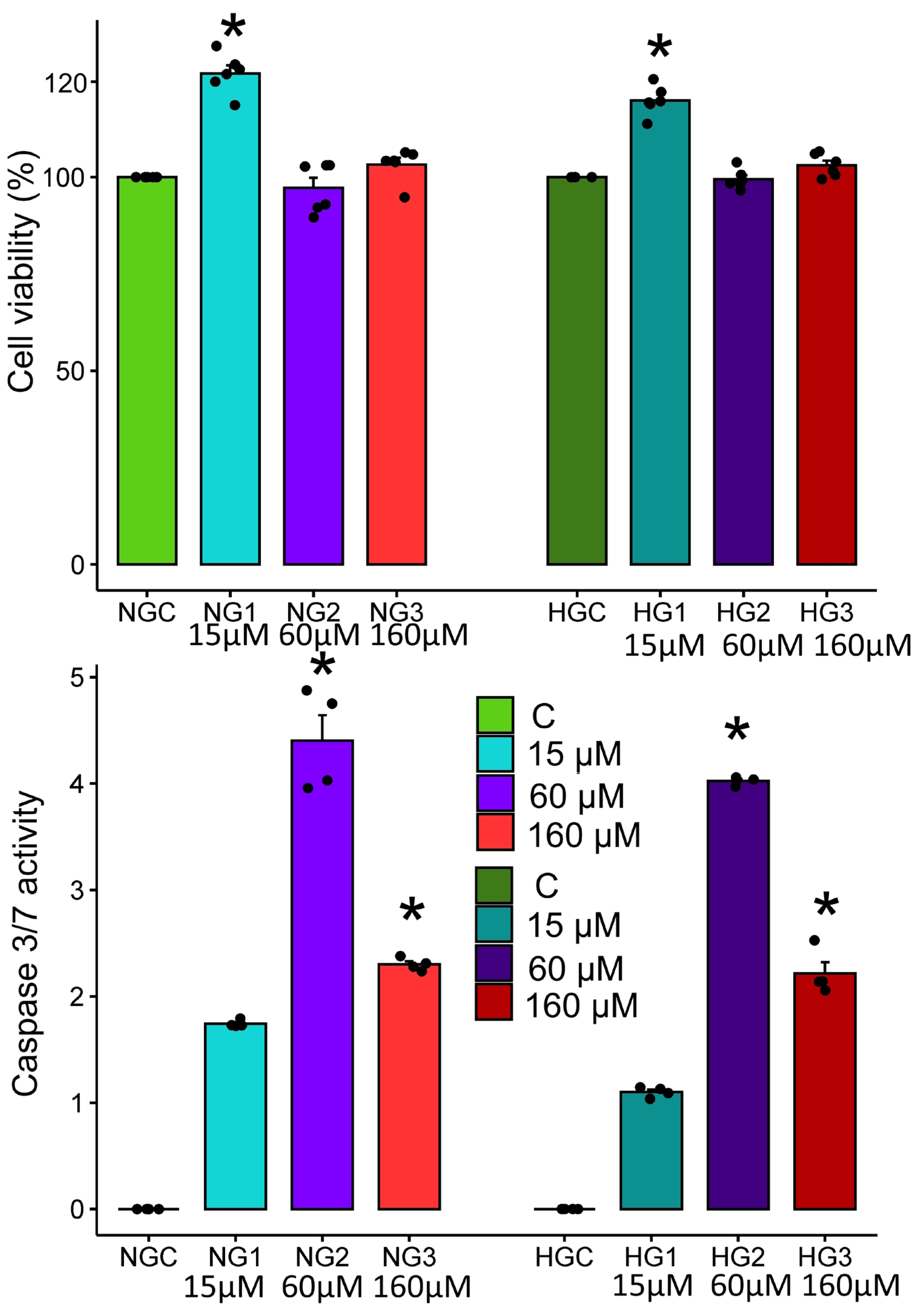
3.1. Effect of MV on mHypoA-2/12 Cell Viability and Apoptosis
3.2. Sequencing Read Statistics
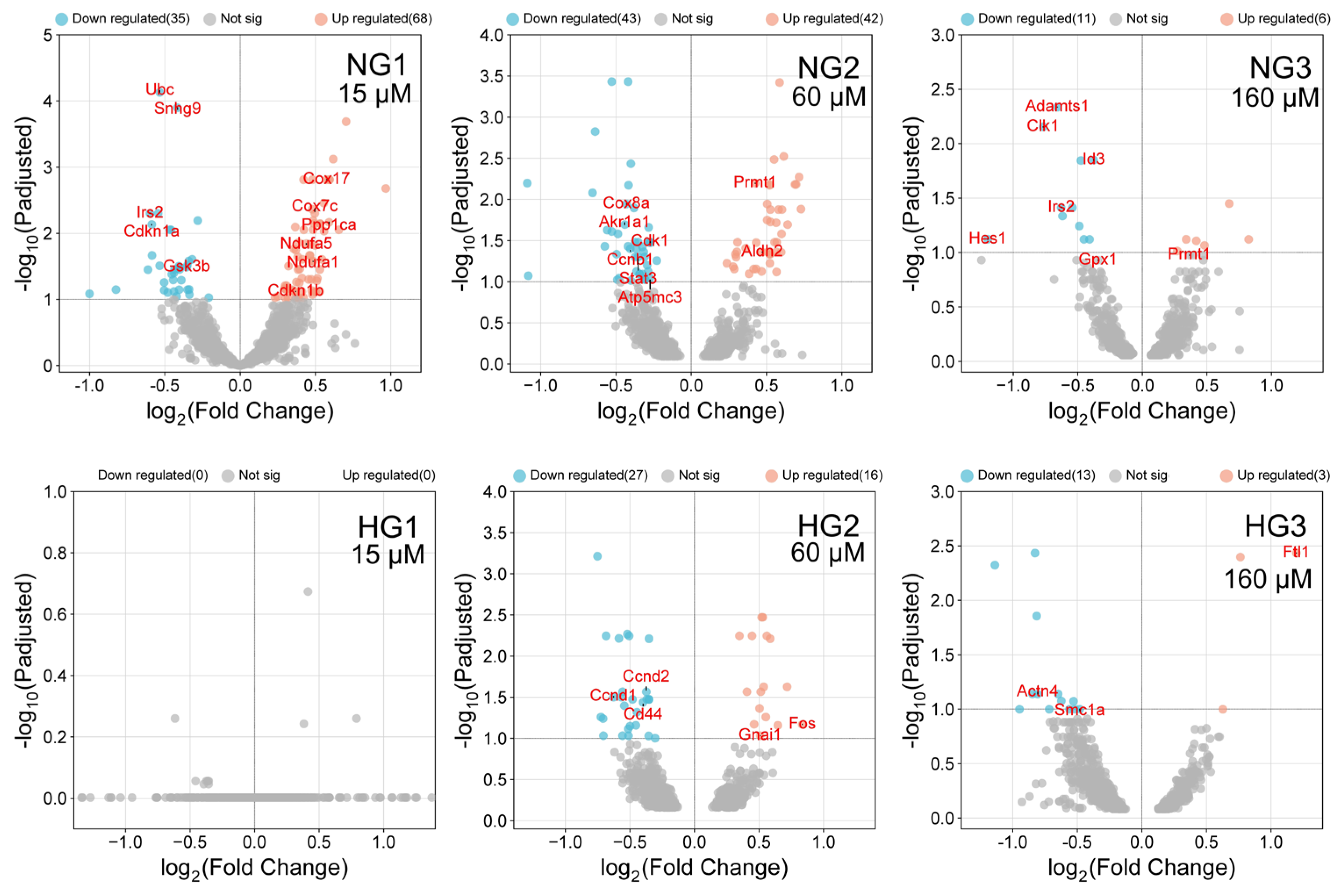
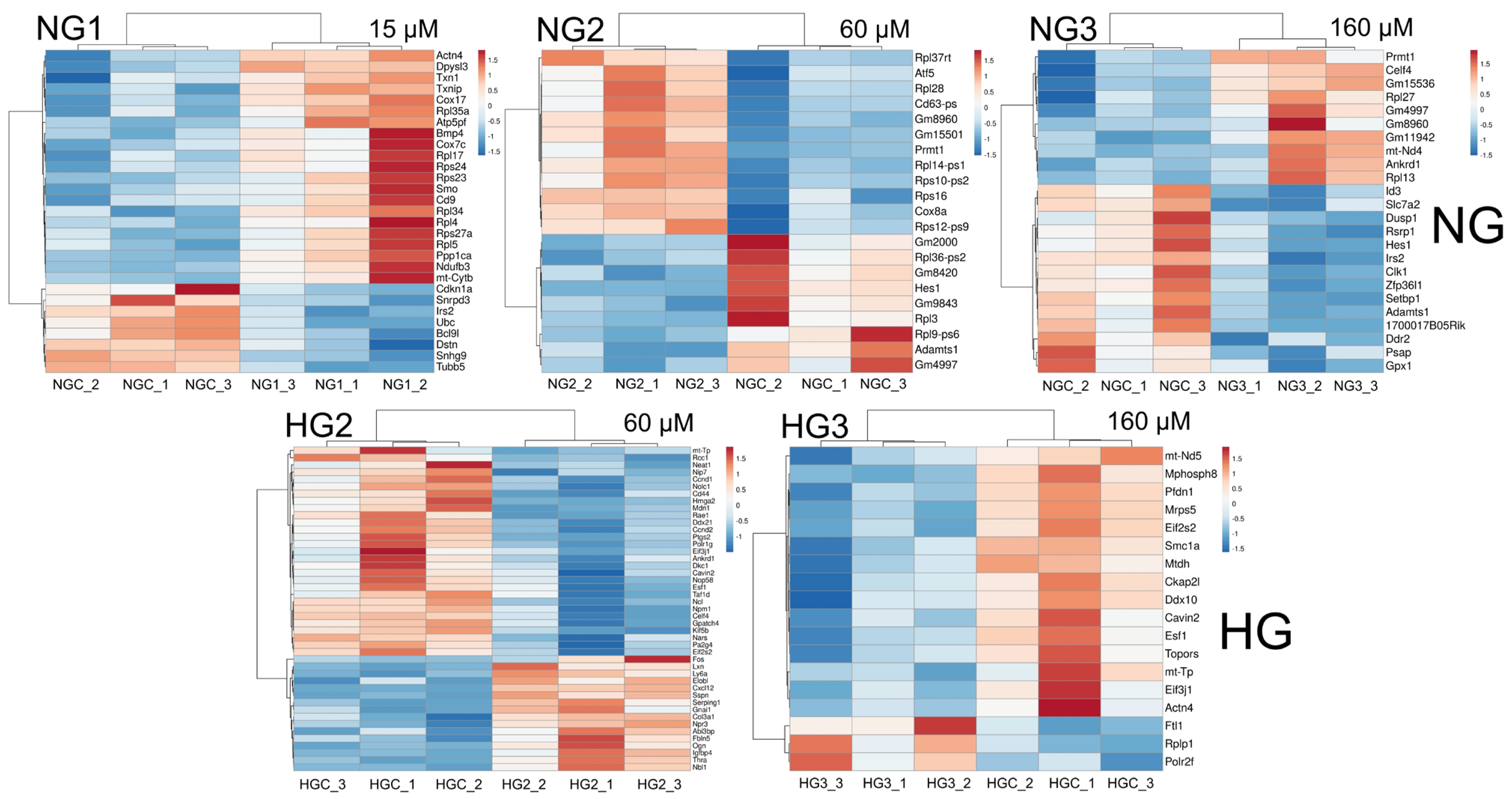
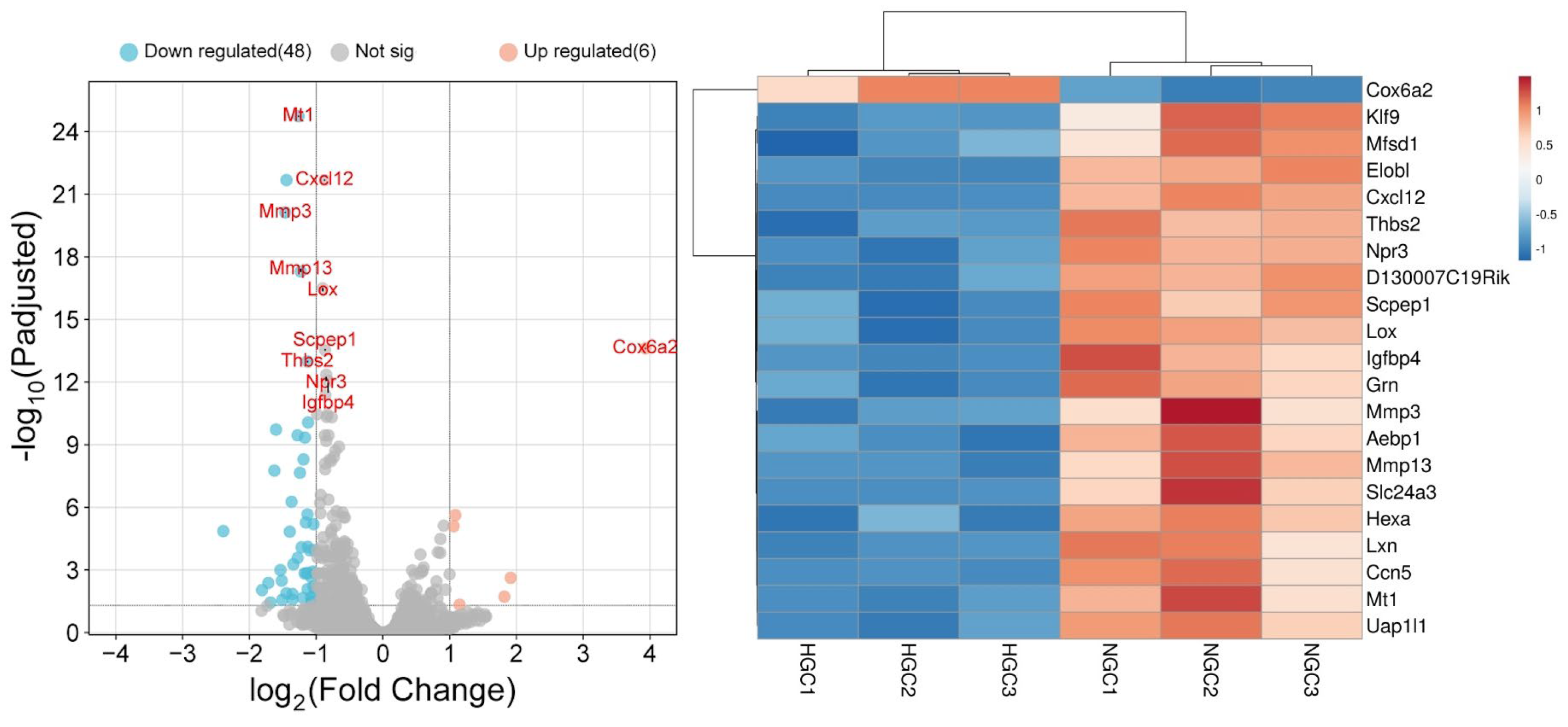
3.3. The Effect of MV Supplementation on Transcriptome
3.4. Results of qPCR Validation
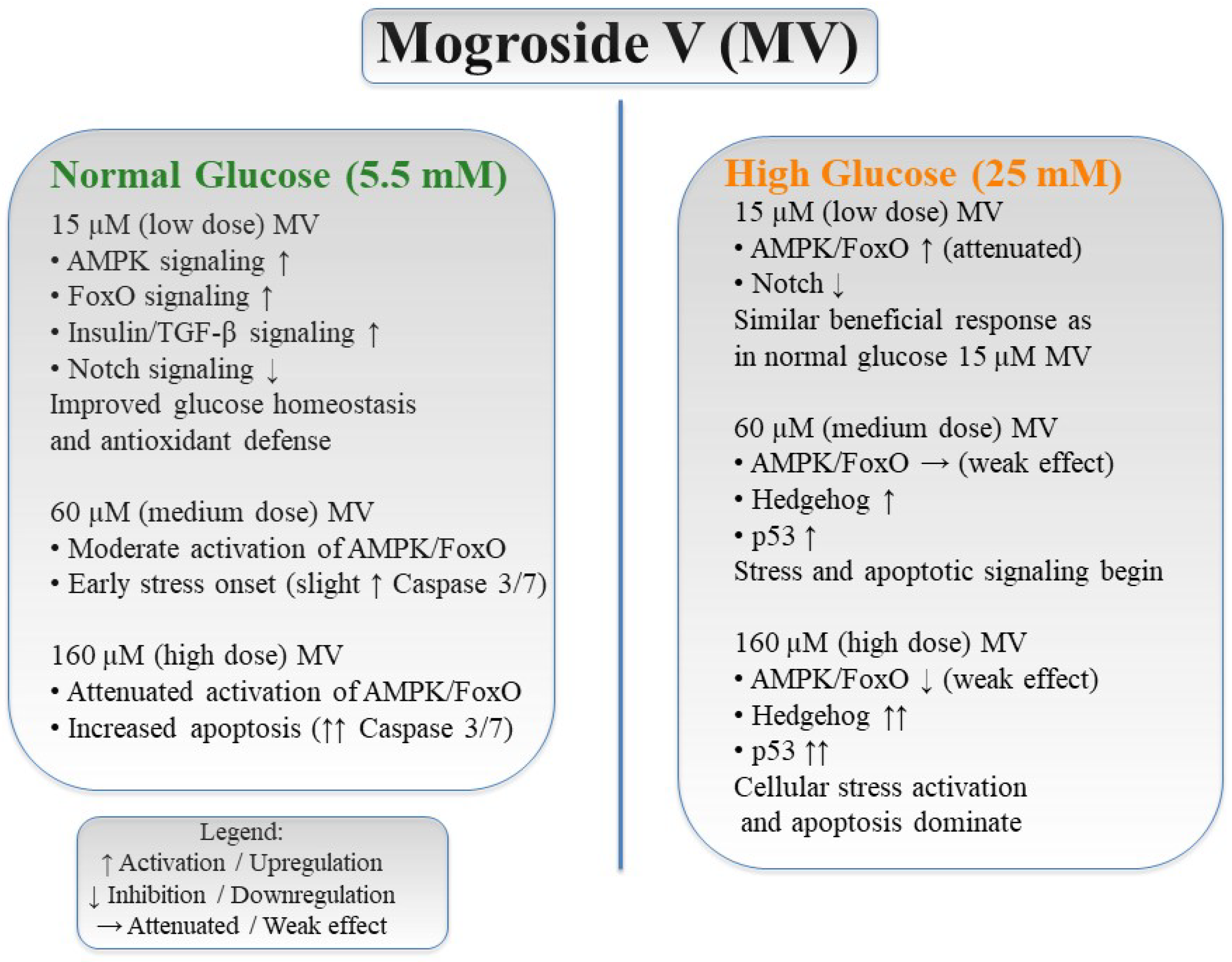
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, S.; Cui, K.; Liu, J.; Hu, J.; Yan, K.; Xiao, P.; Lu, Y.; Yang, X.; Liang, X. Mogroside-rich extract from Siraitia grosvenorii fruits ameliorates high-fat diet-induced obesity associated with the modulation of gut microbiota in mice. Frontiers 2022, 9, 870394. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Chen, Y.; Wang, H.; Liu, Z. Mogroside V: A Review of Its Structure, Synthesis, Pharmacokinetics, and Toxicity. Arch. Pharm. 2025, 358, e2400398. [Google Scholar] [CrossRef]
- Wu, J.; Jian, Y.; Wang, H.; Huang, H.; Gong, L.; Liu, G.; Yang, Y.; Wang, W. A review of the phytochemistry and pharmacology of the fruit of Siraitia grosvenorii (Swingle): A traditional Chinese medicinal food. Molecules 2022, 27, 6618. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, P.S.; Nazarians-Armavil, A.; Tung, S.; Belsham, D.D. Immortalized neurons for the study of hypothalamic function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 1030–1052. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, P.S.; Loganathan, N.; McIlwraith, E.K.; Tran, A.; Belsham, D.D. Chapter 2—Hypothalamic Cell Models, 2nd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 27–77. [Google Scholar]
- Nie, J.; Sui, L.; Zhang, H.; Zhang, H.; Yan, K.; Yang, X.; Lu, S.; Lu, K.; Liang, X. Mogroside V protects porcine oocytes from in vitro ageing by reducing oxidative stress through SIRT1 upregulation. Aging 2019, 11, 8362–8373. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Peng, C.; Xu, X.; Peng, Y.; Shi, F.; Li, Q.; Dong, J.; Chen, M. The Protective Effects of Mogroside V Against Neuronal Damages by Attenuating Mitochondrial Dysfunction via Upregulating Sirtuin3. Mol. Neurobiol. 2022, 59, 2068–2084. [Google Scholar] [CrossRef]
- Tang, Q.; Qiu, R.; Guo, M.; Wang, L.; Zhang, Y.; Chen, Y.; Cheng, Y. Mogroside V and mogrol: Unveiling the neuroprotective and metabolic regulatory roles of Siraitia grosvenorii in Parkinson’s disease. Front. Pharmacol. 2024, 15, 1413520. [Google Scholar] [CrossRef]
- Sullivan, K.A.; Hayes, J.M.; Wiggin, T.D.; Backus, C.; Oh, S.S.; Lentz, S.I.; Brosius, F.; Feldman, E.L. Mouse models of diabetic neuropathy. Neurobiol. Dis. 2007, 28, 276–285. [Google Scholar] [CrossRef]
- Crouch, S.P.M.; Kozlowski, R.; Slater, K.J.; Fletcher, J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 1993, 160, 81–88. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, version 2024, 4.3.2.; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Dodt, M.; Roehr, J.T.; Ahmed, R.; Dieterich, C. FLEXBAR-Flexible barcode and adapter processing for next-generation sequencing platforms. Biology 2012, 1, 895–905. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Hu, J.; Liu, X.; Yu, L.; Hu, J.; Jiang, H.; Liu, S.; Li, M.; He, J.; Yang, X.; et al. Mogroside-rich extract from Siraitia grosvenorii fruits protects against heat stress-induced intestinal damage by ameliorating oxidative stress and inflammation in mice. Food Funct. 2023, 14, 1238–1247. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qi, X.; Zou, J.; Sun, Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 2018, 55, 1880–1888. [Google Scholar] [CrossRef]
- Qi, X.Y.; Chen, W.J.; Zhang, L.Q.; Xie, B.J. Mogrosides extract from Siraitia grosvenori scavenges free radicals in vitro and lowers oxidative stress, serum glucose, and lipid levels in alloxan-induced diabetic mice. Nutr. Res. 2008, 28, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, Y.; Ebersole, J.; Huang, C.F. Insulin secretion stimulating effects of Mogroside V and fruit extract of luo han kuo (Siraitia grosvenori Swingle) fruit extract. Yao Xue Xue Bao 2009, 44, 1252–1257. [Google Scholar]
- Eom, Y.S.; Gwon, A.R.; Kwak, K.M.; Youn, J.Y.; Park, H.; Kim, K.W.; Kim, B.J. Notch1 has an important role in β-cell mass determination and development of diabetes. Diabetes Metab. J. 2021, 45, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Pajvani, U.B.; Shawber, C.J.; Samuel, V.T.; Birkenfeld, A.L.; Shulman, G.I.; Kitajewski, J.; Accili, D. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat. Med. 2011, 17, 961–967. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, S.Y.; Deng, L.D.; Feng, L.P.; Huang, L.Z.; Yu, R.R. Antioxidant effect of mogrosides against oxidative stress induced by palmitic acid in mouse insulinoma NIT-1 cells. Braz. J. Med. Biol. Res. 2013, 46, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, W.; Wang, C.; Qi, J.; Li, H. Mogroside V protects against hepatic steatosis in mice on a high-fat diet and LO2 cells treated with free fatty acids via AMPK activation. Evid. Based Complement Altern. Med. 2020, 2020, 7826874. [Google Scholar] [CrossRef]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing glucose as well as cellular energy status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef]
- Griffeth, R.J.; Carretero, J.; Burks, D.J. Insulin receptor substrate 2 is required for testicular development. PLoS ONE 2013, 8, e62103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhalla, K.; Liu, W.J.; Thompson, K.; Anders, L.; Devarakonda, S.; Dewi, R.; Buckley, S.; Hwang, B.J.; Polster, B.; Dorsey, S.G.; et al. Cyclin D1 represses gluconeogenesis via inhibition of the transcriptional coactivator PGC1α. Diabetes 2014, 63, 3266–3278. [Google Scholar] [CrossRef]
- Salih, D.A.; Brunet, A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 2008, 20, 126–136. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.G. The interplay between TGF-β signaling and cell metabolism. Front. Cell Dev. Biol. 2022, 10, 846723. [Google Scholar] [CrossRef]
- Mo, Q.; Fu, H.; Zhao, D.; Zhang, J.; Wang, C.; Wang, D.; Li, M. Protective effects of Mogroside V on oxidative stress induced by H2O2 in skin fibroblasts. Drug Des. Dev. Ther. 2021, 15, 4901–4909. [Google Scholar] [CrossRef]
- Saxena, M.; Yeretssian, G. NOD-like receptors: Master regulators of inflammation and cancer. Front. Immunol. 2014, 14, 327. [Google Scholar] [CrossRef]
- Chen, G.; Shaw, M.H.; Kim, Y.G.; Nuñez, G. NOD-like receptors: Role in innate immunity and inflammatory disease. Annu. Rev. Pathol. 2009, 4, 365–398. [Google Scholar] [CrossRef]
- Elder, D.A.; D’Alessio, D.A.; Eyal, O.; Mueller, R.; Smith, F.O.; Kansra, A.R.; Rose, S.R. Abnormalities in glucose tolerance are common in children with Fanconi anemia and associated with impaired insulin secretion. Pediatr. Blood Cancer 2008, 51, 256–260. [Google Scholar] [CrossRef]
- Noda, T.; Takahashi, A.; Kondo, N.; Mori, E.; Okamoto, N.; Nakagawa, Y.; Ohnishi, K.; Zdzienicka, M.Z.; Thompson, L.H.; Helleday, T.; et al. Repair pathways independent of the Fanconi anemia nuclear core complex play a predominant role in mitigating formaldehyde-induced DNA damage. Biochem. Biophys. Res. Commun. 2011, 404, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sipple, J.; Maynard, S.; Mehta, P.A.; Rose, S.R.; Davies, S.M.; Pang, Q. Fanconi anemia links reactive oxygen species to insulin resistance and obesity. Antioxid. Redox Signal. 2012, 17, 1083–1098. [Google Scholar] [CrossRef]
- Benchoula, K.; Parhar, I.S.; Wong, E.H. The crosstalk of hedgehog, PI3K and Wnt pathways in diabetes. Arch. Biochem. Biophys. 2021, 15, 108743. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.P.; Murphy, M.E. The role of the p53 tumor suppressor in metabolism and diabetes. J. Endocrinol. 2016, 231, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
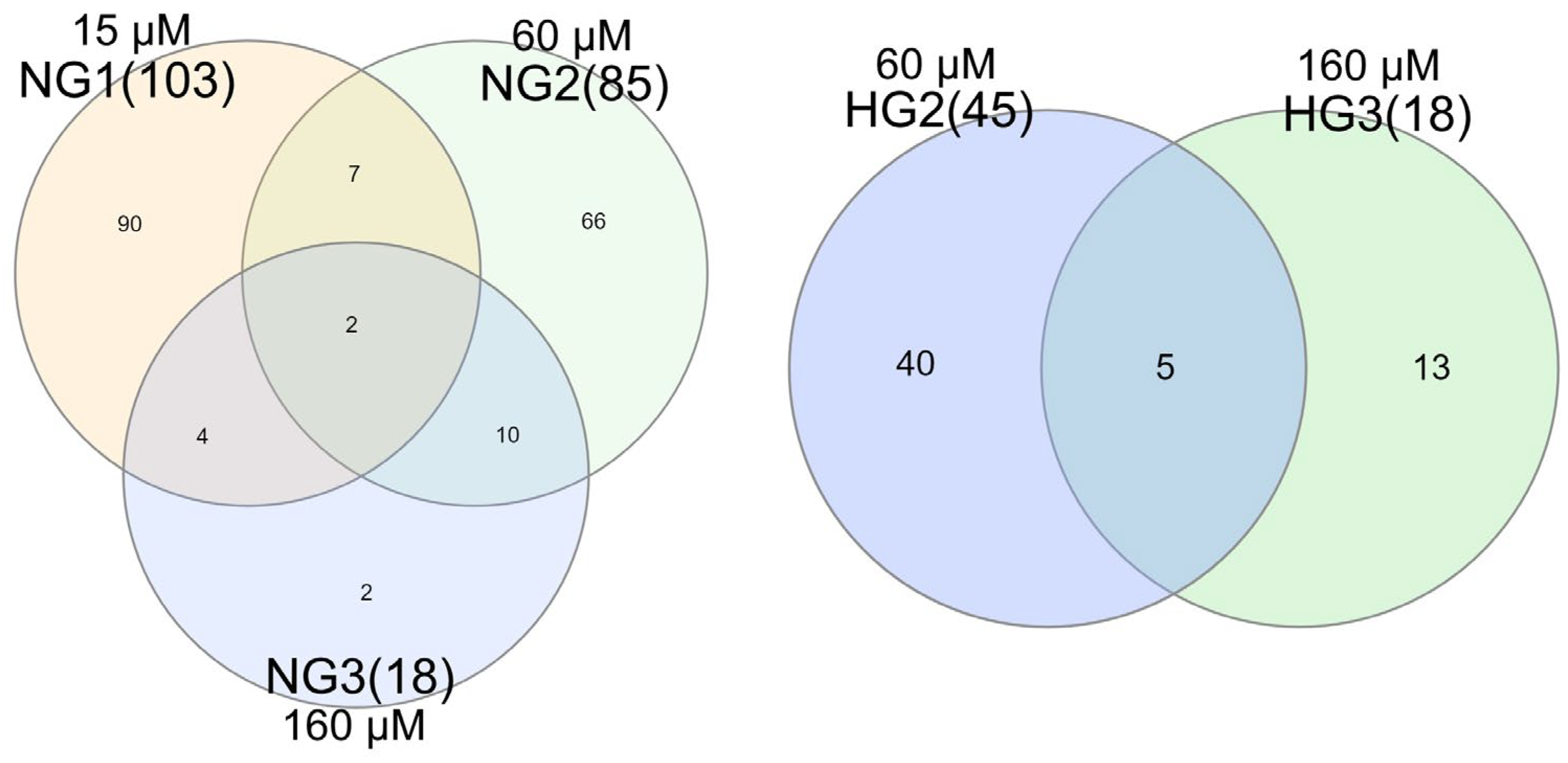
| Sample | Number of Raw Reads | Number of Filtered Reads | Number of Uniquely Mapped Reads | Percent of Uniquely Mapped Reads | Number of Assigned Reads to the Gene Annotation File | Percent Assigned Reads to Gene Annotation File |
|---|---|---|---|---|---|---|
| NGC_1 | 7,024,194 | 6,819,606 | 5,101,214 | 74.80 | 3,786,271 | 74.22 |
| NGC_2 | 8,064,356 | 7,829,472 | 5,763,145 | 73.60 | 4,222,354 | 73.26 |
| NGC_3 | 6,610,456 | 6,417,918 | 4,721,760 | 73.57 | 3,508,021 | 74.29 |
| NG1_1 | 6,876,912 | 6,676,614 | 5,061,389 | 75.80 | 3,677,097 | 72.64 |
| NG1_2 | 6,924,626 | 6,722,938 | 5,076,605 | 75.51 | 3,876,931 | 76.36 |
| NG1_3 | 6,990,753 | 6,787,139 | 4,886,019 | 71.98 | 3,646,909 | 74.63 |
| NG2_1 | 6,754,984 | 6,558,237 | 4,753,471 | 72.48 | 3,396,249 | 71.44 |
| NG2_2 | 7,561,127 | 7,340,900 | 5,355,406 | 72.95 | 3,915,059 | 73.10 |
| NG2_3 | 6,702,721 | 6,507,496 | 4,524,972 | 69.53 | 3,272,671 | 72.32 |
| NG3_1 | 7,640,773 | 7,418,226 | 5,270,040 | 71.04 | 3,852,720 | 73.10 |
| NG3_2 | 6,626,976 | 6,433,957 | 4,821,765 | 74.94 | 3,636,609 | 75.42 |
| NG3_3 | 6,938,489 | 6,736,397 | 4,954,267 | 73.54 | 3,660,057 | 73.87 |
| HGC_1 | 9,654,290 | 9,373,097 | 7,044,551 | 75.15 | 5,210,198 | 73.96 |
| HGC_2 | 7,772,986 | 7,546,588 | 5,702,379 | 75.56 | 4,206,607 | 73.76 |
| HGC_3 | 8,127,220 | 7,890,505 | 5,740,302 | 72.74 | 4,221,399 | 73.53 |
| HG1_1 | 6,986,642 | 6,783,148 | 5,061,894 | 74.62 | 3,757,010 | 74.22 |
| HG1_2 | 6,788,499 | 6,590,776 | 4,858,013 | 73.70 | 3,539,760 | 72.86 |
| HG1_3 | 8,389,540 | 8,145,184 | 5,936,049 | 72.87 | 4,216,850 | 71.03 |
| HG2_1 | 1,475,4397 | 1,432,4657 | 1,063,1005 | 74.21 | 7,773,074 | 73.11 |
| HG2_2 | 7,232,614 | 7,021,955 | 5,292,166 | 75.36 | 3,895,991 | 73.61 |
| HG2_3 | 7,055,436 | 6,849,938 | 4,994,352 | 72.91 | 3,623,593 | 72.55 |
| HG3_1 | 7,576,444 | 7,355,771 | 5,409,038 | 73.53 | 3,945,481 | 72.94 |
| HG3_2 | 7,931,327 | 7,700,317 | 5,879,749 | 76.35 | 4,310,446 | 73.31 |
| HG3_3 | 7,103,674 | 6,896,771 | 4,917,027 | 71.29 | 3,792,926 | 77.13 |
| Pathway | Common Between Analysis | Corrected p-Value |
|---|---|---|
| Type II diabetes mellitus | NG1/NG3 | 0.004 |
| Regulation of lipolysis in adipocytes | NG1/NG3 | 0.004 |
| Adipocytokine signaling pathway | NG1/NG3 | 0.004 |
| Longevity regulating pathway | NG1/NG3 | 0.004 |
| Insulin resistance | NG1/NG3 | 0.004 |
| AMPK signaling pathway | NG1/NG3 | 0.004 |
| Autophagy—animal | NG1/NG3 | 0.004 |
| FoxO signaling pathway | NG1/NG3 | 0.004 |
| Insulin signaling pathway | NG1/NG3 | 0.004 |
| Non-alcoholic fatty liver disease (NAFLD) | NG1/NG3 | 0.004 |
| cGMP-PKG signaling pathway | NG1/NG3 | 0.005 |
| MicroRNAs in cancer | NG1/NG3 | 0.007 |
| Maturity onset diabetes of the young | NG2/NG3 | 0.011 |
| Notch signaling pathway | NG2/NG3 | 0.014 |
| Fanconi anemia pathway | NG2/NG3 | 0.014 |
| TGF-beta signaling pathway | NG2/NG3 | 0.021 |
| Glucagon signaling pathway | NG2/NG3 | 0.022 |
| FoxO signaling pathway | NG2/NG3 | 0.026 |
| Signaling pathways regulating pluripotency of stem cells | NG2/NG3 | 0.027 |
| Breast cancer | NG2/NG3 | 0.028 |
| Gene | Internal Control | |
|---|---|---|
| HPRT | PPIA | |
| Ndufb3 | 0.3027 | 0.4912 |
| Irs2 | 0.9405 | 0.7628 |
| Adamts1 | 0.6881 | 0.7063 |
| Serping1 | 0.6908 | 0.5787 |
| Abi3bp | 0.8514 | 0.7253 |
| Eif3j1 | 0.6943 | 0.7301 |
| Cavin2 | 0.6767 | 0.7764 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szmatoła, T.; Gurgul, A.; Ocłoń, E.; Jasielczuk, I.; Semik-Gurgul, E. Investigating the Potential Molecular Mechanisms of Mogroside V on Glucose Homeostasis by Transcriptome Profiling of Adult Mouse Hypothalamic Cells. Appl. Sci. 2025, 15, 12391. https://doi.org/10.3390/app152312391
Szmatoła T, Gurgul A, Ocłoń E, Jasielczuk I, Semik-Gurgul E. Investigating the Potential Molecular Mechanisms of Mogroside V on Glucose Homeostasis by Transcriptome Profiling of Adult Mouse Hypothalamic Cells. Applied Sciences. 2025; 15(23):12391. https://doi.org/10.3390/app152312391
Chicago/Turabian StyleSzmatoła, Tomasz, Artur Gurgul, Ewa Ocłoń, Igor Jasielczuk, and Ewelina Semik-Gurgul. 2025. "Investigating the Potential Molecular Mechanisms of Mogroside V on Glucose Homeostasis by Transcriptome Profiling of Adult Mouse Hypothalamic Cells" Applied Sciences 15, no. 23: 12391. https://doi.org/10.3390/app152312391
APA StyleSzmatoła, T., Gurgul, A., Ocłoń, E., Jasielczuk, I., & Semik-Gurgul, E. (2025). Investigating the Potential Molecular Mechanisms of Mogroside V on Glucose Homeostasis by Transcriptome Profiling of Adult Mouse Hypothalamic Cells. Applied Sciences, 15(23), 12391. https://doi.org/10.3390/app152312391






