AI-Based Image Time-Series Analysis of the Niacin Skin Flush Test in Schizophrenia and Bipolar Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria for Study Participants
2.2. Characteristics of Study Participants
2.3. Measurement of Skin Reaction Measurement and Image Processing in the Niacin Skin Flush Test
2.4. Deep Learning Classification Using Transfer Learning
- ResNet50 and ResNet101 (Microsoft Research)—classic, deep residual networks known for their stable training even with a large number of layers. They are often used as a benchmark in many classification tasks.
- EfficientNetB0 (Google AI)—a modern, lightweight architecture optimized for high computational efficiency, making it particularly attractive in clinical applications where both accuracy and model speed are essential.
- InceptionV3 (Google Brain)—thanks to its design, it is characterized by the ability to capture features at various scales (both local and distributed), making it useful in the context of medical image analysis.
- DenseNet121 (Facebook AI Research)—can effectively use information between layers through dense connections, which can translate into better representation of subtle differences in diagnostic images.
2.5. Measures for Evaluating the Quality of Classifiers
- 1.
- Accuracy is one of the most commonly used classification metrics. It measures the percentage of correctly classified cases relative to the total number of cases in the test set:
- TP (True Positives)—the number of true positives (correctly classified as positive),
- TN (True Negatives)—the number of true negatives (correctly classified as negative),
- FP (False Positives)—the number of false positives (incorrectly classified as positive),
- FN (False Negatives)—the number of false negatives (incorrectly classified as negative).
- 2.
- Precision—measures the accuracy of the model’s positive predictions. It determines what proportion of cases classified as positive are actually positive:
- 3.
- Recall/Sensitivity—measures the model’s ability to detect true positives. It determines the proportion of true positives that the model correctly recognizes:
- 4.
- F1-Score is the harmonic mean of precision and sensitivity. As a harmonized measure, it is particularly useful for imbalanced classes where precision or sensitivity can be misleading when considered individually:
- 5.
- Area Under the Curve (AUC) is a measure of classifier performance that considers all possible classification threshold values. It is calculated by plotting the Receiver Operating Characteristic (ROC) curve based on the sensitivity (TP rate) and specificity (FP rate) values for various decision thresholds.
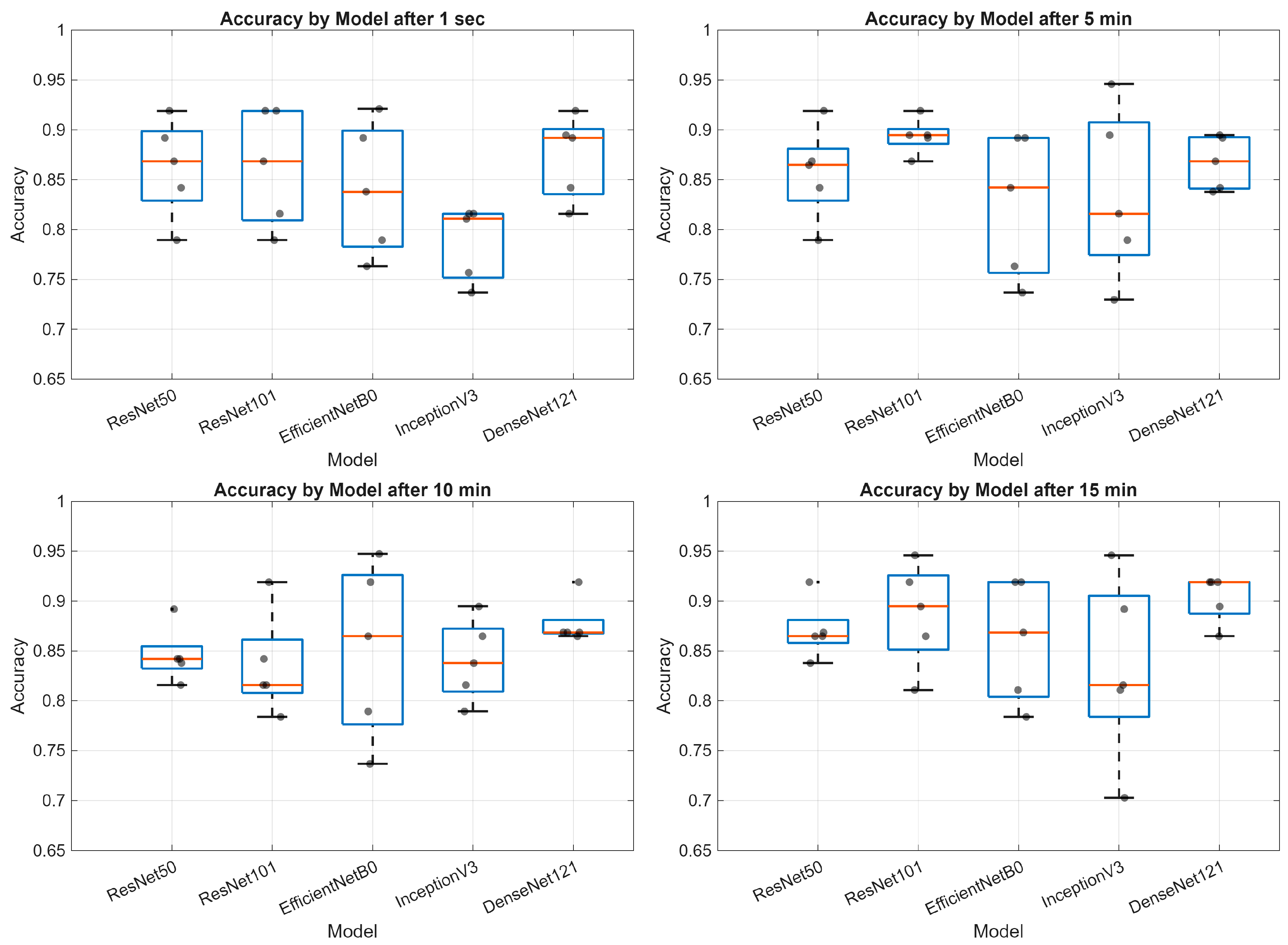
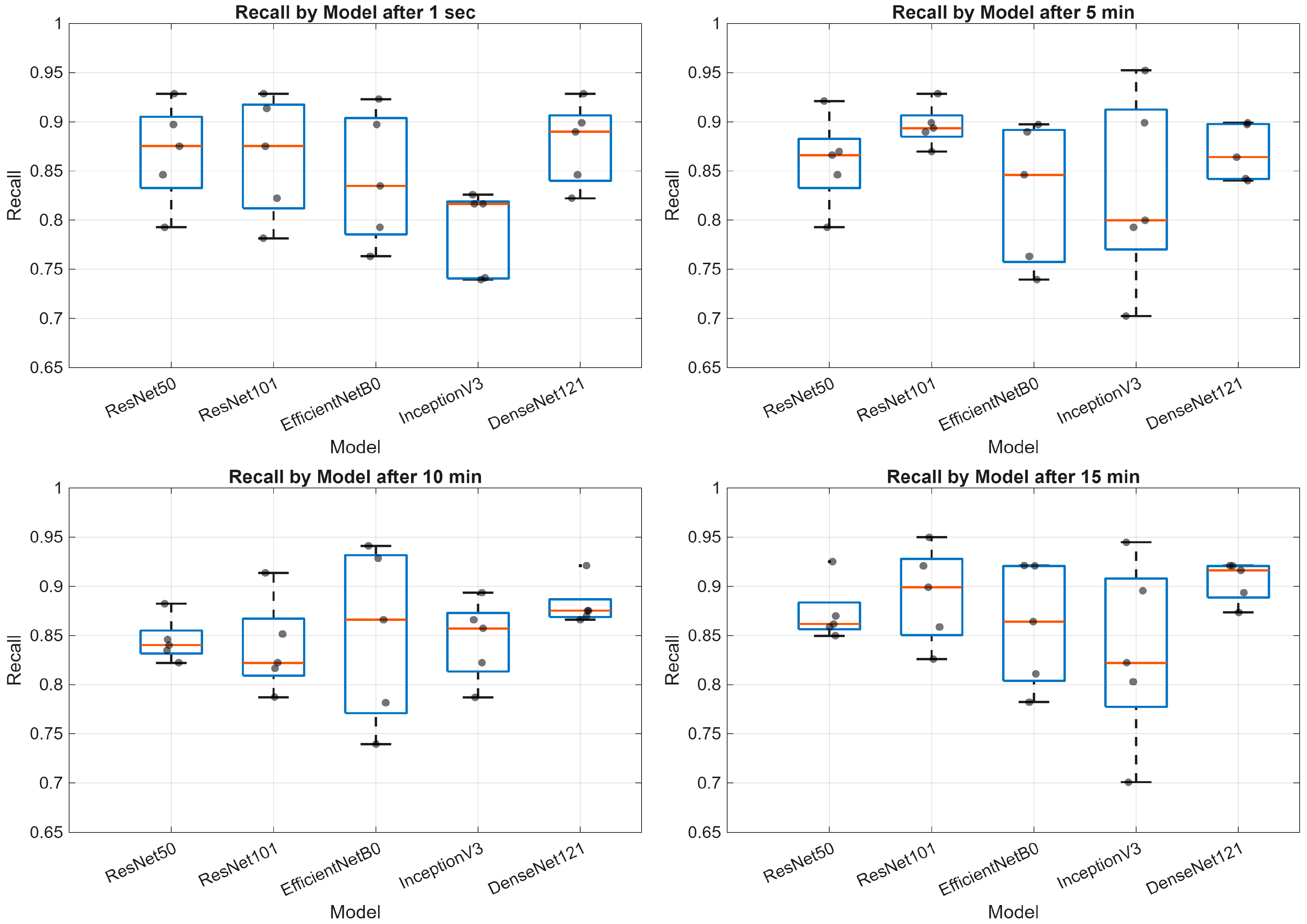
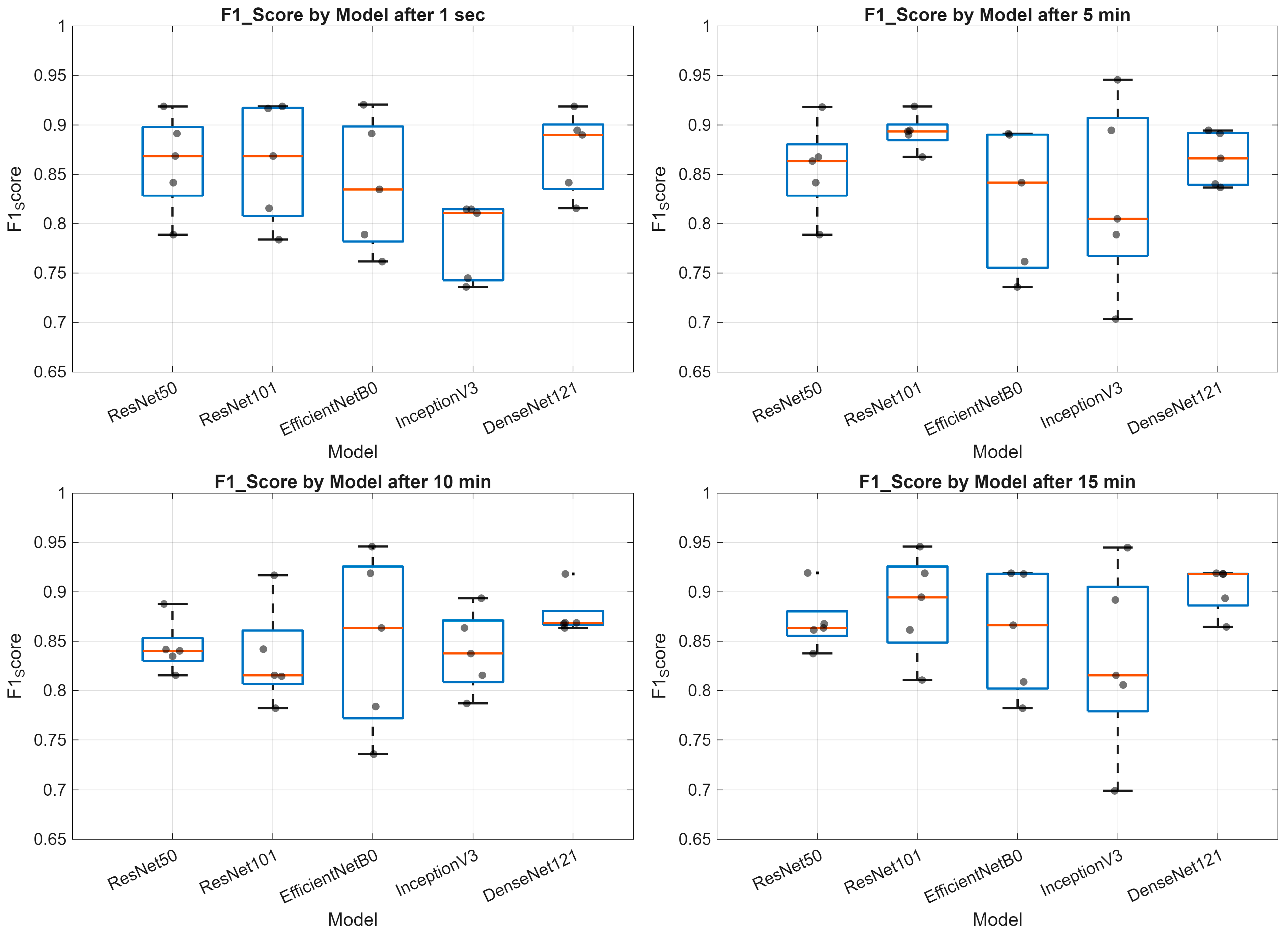
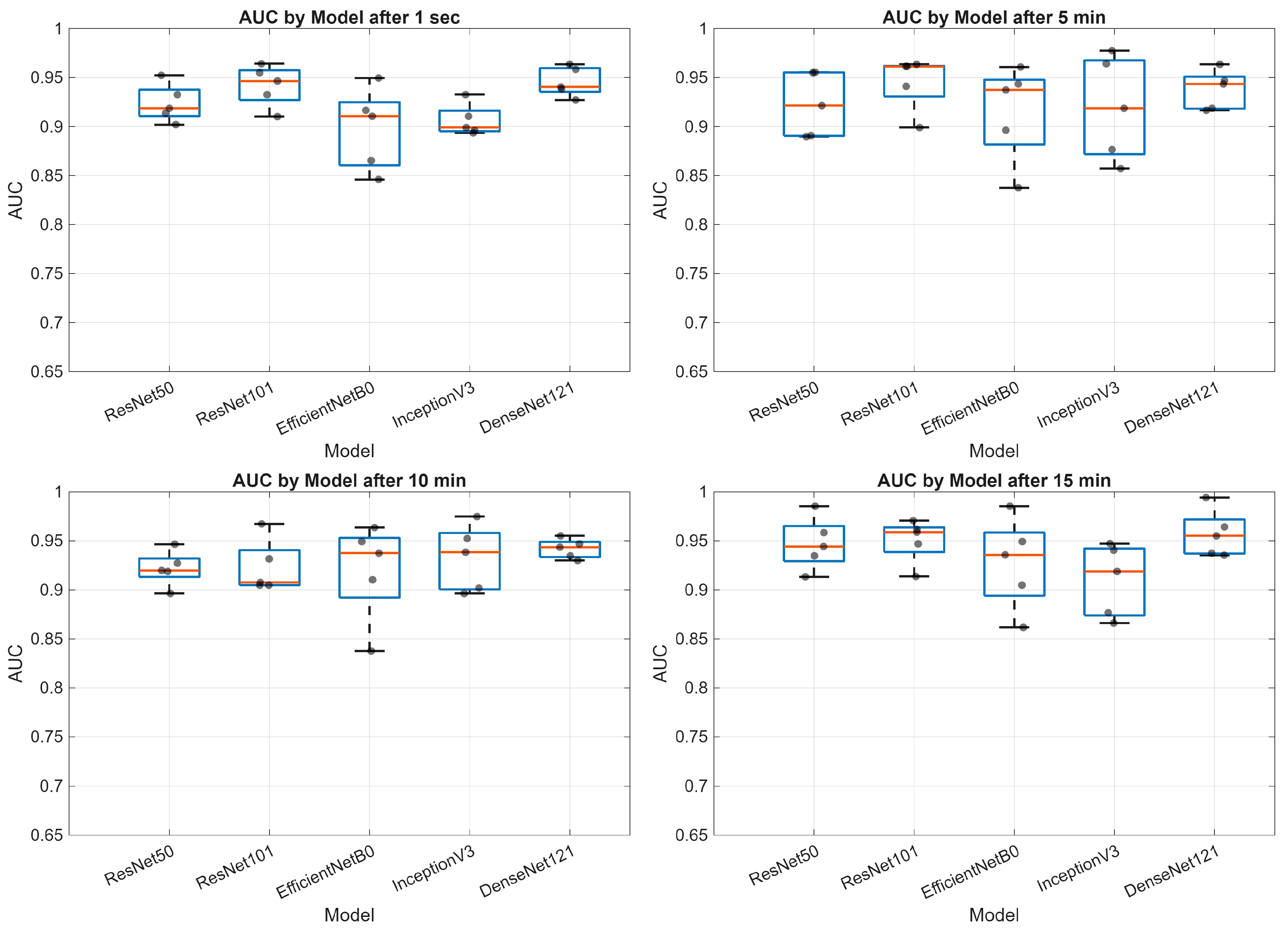
3. Results
4. Discussion
5. Conclusions
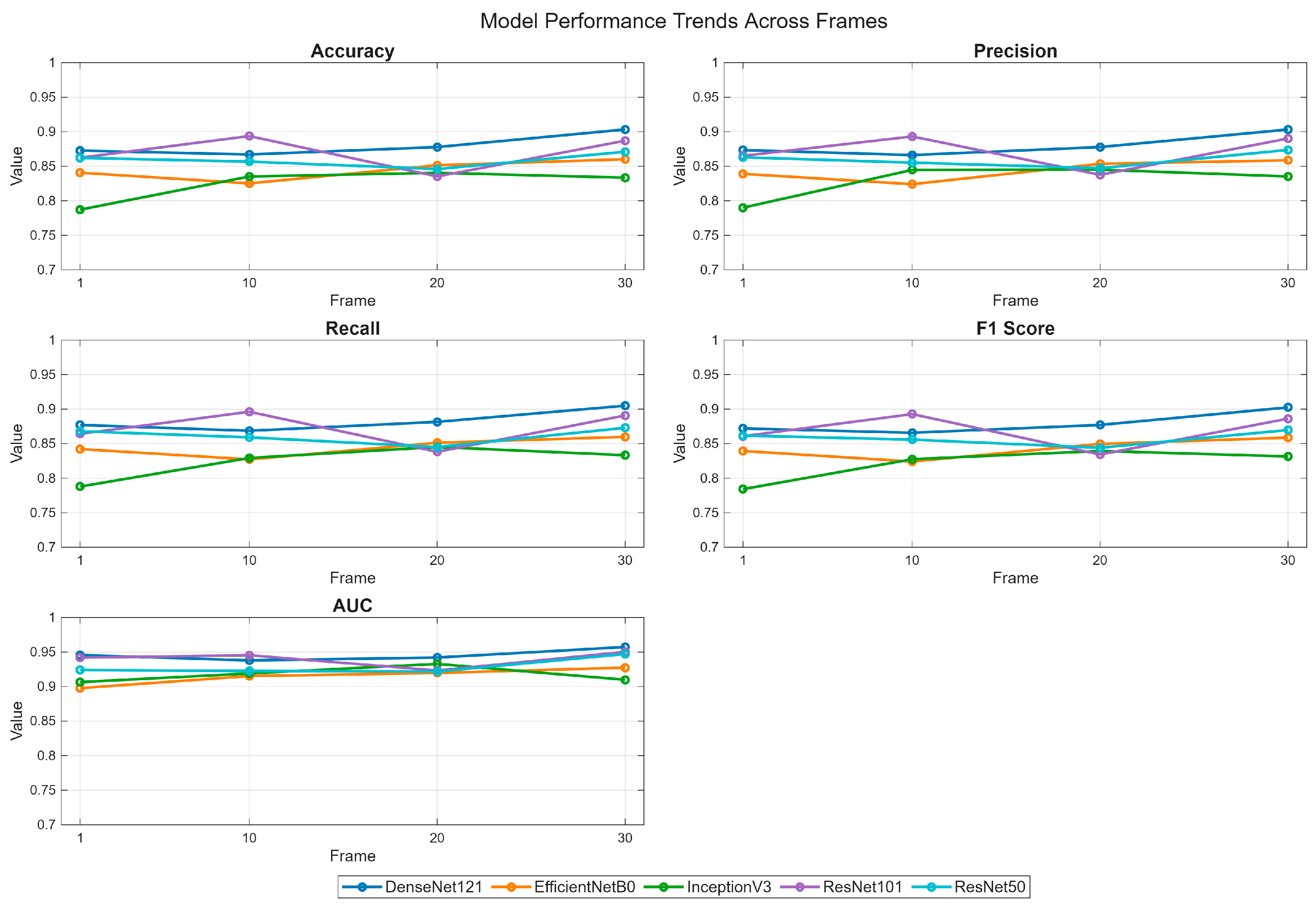
- Deep learning models demonstrate varying performance trajectories in the analysis of sequential skin reaction images. Each architecture exhibits a unique performance profile depending on the measurement time.
- The ResNet50 model proved to be the most stable overall, achieving high scores across all time points, making it a good candidate for clinical applications requiring consistent classification quality.
- DenseNet121 demonstrated progressive performance improvement, achieving the highest AUC values (up to 0.99) in the final phase of the study (15 min), suggesting its suitability for longer-term analyses.
- ResNet101 demonstrated high performance in the early phases (up to 5 min) but was less stable in the middle measurement period, which may be important for applications requiring rapid diagnostics.
- EfficientNetB0 demonstrated a systematic, albeit moderate, improvement in performance and may be valuable where computational efficiency is important (e.g., mobile devices and limited resources).
- InceptionV3 had the greatest improvement between minutes 1 and 10, but then reached a plateau, indicating its limited usefulness in late measurement phases.
- The stability of the AUC metric across all architectures and time points suggests that the models’ ability to differentiate classes (e.g., clinical vs. non-clinical groups) is less susceptible to temporal variation than other metrics, such as accuracy or sensitivity.
5.1. Clinical Significance Information
5.2. Limitations and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSFT | niacin skin flush test |
| SCH | schizophrenia |
| CS | chronic schizophrenia |
| SA | schizoaffective disorder |
| BD | bipolar affective disorder |
| C&RT | classification and regression tree |
| ROC | receiver operating characteristic curve |
References
- Nadalin, S.; Buretić-Tomljanović, A.; Rubeša, G.; Tomljanović, D.; Gudelj, L. Niacin Skin Flush Test: A Research Tool for Studying Schizophrenia. Psychiatr. Danub. 2010, 22, 14–27. [Google Scholar]
- Zhang, T.; Xu, L.; Wei, Y.; Tang, X.; Gan, R.; Zhang, D.; Yi, Z.; Liu, X.; Liu, H.; Wang, Z.; et al. Efficiency and Extent of Niacin-Induced Skin Flushing Patterns in Early Stages of Psychosis. J. Clin. Psychiatry 2025, 86, 24m15559. [Google Scholar] [CrossRef]
- Ju, M.; Long, B.; Wei, Y.; Tang, X.; Xu, L.; Gan, R.; Cui, H.; Tang, Y.; Yi, Z.; Liu, H.; et al. Cognitive Impairments in First-Episode Psychosis Patients with Attenuated Niacin Response. Schizophr. Res. Cogn. 2025, 40, 100346. [Google Scholar] [CrossRef]
- Thaker, G.K. Neurophysiological Endophenotypes Across Bipolar and Schizophrenia Psychosis. Schizophr. Bull. 2007, 34, 760–773. [Google Scholar] [CrossRef]
- Lyu, X.; Goperma, R.; Wang, D.; Wan, C.; Zhao, L. An Open Dataset and Machine Learning Algorithms for Niacin Skin-Flushing Response Based Screening of Psychiatric Disorders. BMC Psychiatry 2025, 25, 757. [Google Scholar] [CrossRef]
- Wyatt, R.J. Early Intervention for Schizophrenia: Can the Course of the Illness Be Altered? Biol. Psychiatry 1995, 38, 1–3. [Google Scholar] [CrossRef]
- McGorry, P.D.; Nelson, B.; Goldstone, S.; Yung, A.R. Clinical Staging: A Heuristic and Practical Strategy for New Research and Better Health and Social Outcomes for Psychotic and Related Mood Disorders. Can. J. Psychiatry 2010, 55, 486–497. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, X.; Wu, H.; Zeng, J.; Ye, J.; Gao, Y.; Hu, Y.; Xu, L.; Wei, Y.; Tang, X.; et al. Association of Attenuated Niacin Response with Inflammatory Imbalance and Prediction of Conversion to Psychosis from Clinical High-Risk Stage. J. Clin. Psychiatry 2023, 84, 47954. [Google Scholar] [CrossRef] [PubMed]
- Aucoin, M.; LaChance, L.; Cooley, K.; Kidd, S. Diet and Psychosis: A Scoping Review. Neuropsychobiology 2020, 79, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.G.; Gallego, J.A.; John, M.; Hanna, L.A.; Zhang, J.-P.; Birnbaum, M.L.; Greenberg, J.; Naraine, M.; Peters, B.D.; McNamara, R.K.; et al. A Potential Role for Adjunctive Omega-3 Polyunsaturated Fatty Acids for Depression and Anxiety Symptoms in Recent Onset Psychosis: Results from a 16 Week Randomized Placebo-Controlled Trial for Participants Concurrently Treated with Risperidone. Schizophr. Res. 2019, 204, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, J. Docosahexaenoic Acid (DHA): An Ancient Nutrient for the Modern Human Brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef]
- Sarris, J.; Ravindran, A.; Yatham, L.N.; Marx, W.; Rucklidge, J.J.; McIntyre, R.S.; Akhondzadeh, S.; Benedetti, F.; Caneo, C.; Cramer, H.; et al. Clinician Guidelines for the Treatment of Psychiatric Disorders with Nutraceuticals and Phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 2022, 23, 424–455. [Google Scholar] [CrossRef]
- Jones, H.J.; Borges, M.C.; Carnegie, R.; Mongan, D.; Rogers, P.J.; Lewis, S.J.; Thompson, A.D.; Zammit, S. Associations between Plasma Fatty Acid Concentrations and Schizophrenia: A Two-Sample Mendelian Randomisation Study. Lancet Psychiatry 2021, 8, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-Level Classification of Skin Cancer with Deep Neural Networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Tajbakhsh, N.; Shin, J.Y.; Gurudu, S.R.; Hurst, R.T.; Kendall, C.B.; Gotway, M.B.; Liang, J. Convolutional Neural Networks for Medical Image Analysis: Full Training or Fine Tuning? IEEE Trans. Med. Imaging 2016, 35, 1299–1312. [Google Scholar] [CrossRef]
- Shin, H.-C.; Roth, H.R.; Gao, M.; Lu, L.; Xu, Z.; Nogues, I.; Yao, J.; Mollura, D.; Summers, R.M. Deep Convolutional Neural Networks for Computer-Aided Detection: CNN Architectures, Dataset Characteristics and Transfer Learning. IEEE Trans. Med. Imaging 2016, 35, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Mahbod, A.; Schaefer, G.; Wang, C.; Dorffner, G.; Ecker, R.; Ellinger, I. Transfer Learning Using a Multi-Scale and Multi-Network Ensemble for Skin Lesion Classification. Comput. Methods Programs Biomed. 2020, 193, 105475. [Google Scholar] [CrossRef]
- Codella, N.; Cai, J.; Abedini, M.; Garnavi, R.; Halpern, A.; Smith, J.R. Deep Learning, Sparse Coding, and SVM for Melanoma Recognition in Dermoscopy Images. In Machine Learning in Medical Imaging; Zhou, L., Wang, L., Wang, Q., Shi, Y., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2015; Volume 9352, pp. 118–126. ISBN 978-3-319-24887-5. [Google Scholar]
- Romero-Lopez, A.; Giro-i-Nieto, X.; Burdick, J.; Marques, O. Skin Lesion Classification from Dermoscopic Images Using Deep Learning Techniques. In Proceedings of the Biomedical Engineering, Innsbruck, Austria, 20–21 February 2017; ACTA Press: Calgary, AB, Canada, 2017. [Google Scholar]
- Khan, M.A.; Akram, T.; Zhang, Y.-D.; Sharif, M. Attributes Based Skin Lesion Detection and Recognition: A Mask RCNN and Transfer Learning-Based Deep Learning Framework. Pattern Recognit. Lett. 2021, 143, 58–66. [Google Scholar] [CrossRef]
- Hasan, M.K.; Dahal, L.; Samarakoon, P.N.; Tushar, F.I.; Martí, R. DSNet: Automatic Dermoscopic Skin Lesion Segmentation. Comput. Biol. Med. 2020, 120, 103738. [Google Scholar] [CrossRef]
- El-Khatib, H.; Popescu, D.; Ichim, L. Deep Learning–Based Methods for Automatic Diagnosis of Skin Lesions. Sensors 2020, 20, 1753. [Google Scholar] [CrossRef]
- Tahir, M.; Naeem, A.; Malik, H.; Tanveer, J.; Naqvi, R.A.; Lee, S.-W. DSCC_Net: Multi-Classification Deep Learning Models for Diagnosing of Skin Cancer Using Dermoscopic Images. Cancers 2023, 15, 2179. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.S.; Roos, T. Transfer Learning with Ensembles of Deep Neural Networks for Skin Cancer Detection in Imbalanced Data Sets. Neural Process. Lett. 2023, 55, 4461–4479. [Google Scholar] [CrossRef]
- Venugopal, V.; Raj, N.I.; Nath, M.K.; Stephen, N. A Deep Neural Network Using Modified EfficientNet for Skin Cancer Detection in Dermoscopic Images. Decis. Anal. J. 2023, 8, 100278. [Google Scholar] [CrossRef]
- Bechelli, S.; Delhommelle, J. Machine Learning and Deep Learning Algorithms for Skin Cancer Classification from Dermoscopic Images. Bioengineering 2022, 9, 97. [Google Scholar] [CrossRef]
- Karakula-Juchnowicz, H.; Rog, J.; Wolszczak, P.; Jonak, K.; Stelmach, E.; Krukow, P. SKINREMS—A New Method for Assessment of the Niacin Skin Flush Test Response in Schizophrenia. JCM 2020, 9, 1848. [Google Scholar] [CrossRef]
- Sitarz, R.; Juchnowicz, D.; Karakuła, K.; Forma, A.; Baj, J.; Rog, J.; Karpiński, R.; Machrowska, A.; Karakuła-Juchnowicz, H. Niacin Skin Flush Backs—From the Roots of the Test to Nowadays Hope. J. Clin. Med. 2023, 12, 1879. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 26 June–1 July 2016; IEEE: New York, NY, USA, 2016; pp. 770–778. [Google Scholar]
- Tan, M.; Le, Q.V. EfficientNet: Rethinking Model Scaling for Convolutional Neural Networks. In Proceedings of the 36th International Conference on Machine Learning (PMLR), Long Beach, CA, USA, 9–15 June 2019. [Google Scholar] [CrossRef]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the Inception Architecture for Computer Vision. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), New York, NY, USA, 26 June–1 July 2016; IEEE: Las Vegas, NV, USA, 2016; pp. 2818–2826. [Google Scholar]
- Huang, G.; Liu, Z.; Van Der Maaten, L.; Weinberger, K.Q. Densely Connected Convolutional Networks. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; IEEE: New York, NY, USA, 2017; pp. 2261–2269. [Google Scholar]
- Machrowska, A.; Karpiński, R.; Maciejewski, M.; Jonak, J.; Krakowski, P.; Syta, A. Multi-Scale Analysis of Knee Joint Acoustic Signals for Cartilage Degeneration Assessment. Sensors 2025, 25, 706. [Google Scholar] [CrossRef]
- Matysiak, M.; Podkowiński, A.; Chorągiewicz, T.; Karpiński, R.; Dolecki, M.; Stęgierski, R.; Zimenkovskyy, A.; Shybinskyy, V.; Jonak, K.E.; Rejdak, R. AI-Assisted Fundus Image Analysis for Medical Diagnostics in Conflict Zones. Adv. Sci. Technol. Res. J. 2025, 20, 510–524. [Google Scholar]
- Gęca, J.; Głuchowski, D.; Podkowiński, A.; Chorągiewicz, T.; Wróbel-Dudzińska, D.; Karpiński, R.; Syta, A.; Jonak, K.; Wolińska, A.; Rejdak, R. Keratoconus Diagnosis Based on Dynamic Corneal Imaging Using 3D Convolutional Neural Networks. Adv. Sci. Technol. Res. J. 2025, 19, 257–272. [Google Scholar] [CrossRef]
- Syta, A.; Podkowiński, A.; Chorągiewicz, T.; Karpiński, R.; Gęca, J.; Wróbel-Dudzińska, D.; Jonak, K.E.; Głuchowski, D.; Maciejewski, M.; Rejdak, R.; et al. Machine Learning-Assisted Early Detection of Keratoconus: A Comparative Analysis of Corneal Topography and Biomechanical Data. Sci. Rep. 2025, 15, 24399. [Google Scholar] [CrossRef] [PubMed]
- Minaee, S.; Kafieh, R.; Sonka, M.; Yazdani, S.; Soufi, G.J. Deep-COVID: Predicting COVID-19 from Chest X-Ray Images Using Deep Transfer Learning. Med. Image Anal. 2020, 65, 101794. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.M.; Ghuffar, S.; Khurshid, K. Classification of Breast Cancer Histology Images Using Transfer Learning. In Proceedings of the 2019 16th International Bhurban Conference on Applied Sciences and Technology (IBCAST), Islamabad, Pakistan, 8–12 January 2019; IEEE: New York, NY, USA, 2019; pp. 328–332. [Google Scholar]
- Jiménez Gaona, Y.; Rodriguez-Alvarez, M.J.; Espino-Morato, H.; Castillo Malla, D.; Lakshminarayanan, V. DenseNet for Breast Tumor Classification in Mammographic Images. In Bioengineering and Biomedical Signal and Image Processing; Rojas, I., Castillo-Secilla, D., Herrera, L.J., Pomares, H., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 12940, pp. 166–176. ISBN 978-3-030-88162-7. [Google Scholar]
- Vesal, S.; Ravikumar, N.; Davari, A.; Ellmann, S.; Maier, A. Classification of Breast Cancer Histology Images Using Transfer Learning. In Image Analysis and Recognition; Lecture Notes in Computer Science Series; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Behar, N.; Shrivastava, M. ResNet50-Based Effective Model for Breast Cancer Classification Using Histopathology Images. Comput. Model. Eng. Sci. 2022, 130, 823–839. [Google Scholar] [CrossRef]
- Zhang, Q. A Novel ResNet101 Model Based on Dense Dilated Convolution for Image Classification. SN Appl. Sci. 2022, 4, 9. [Google Scholar] [CrossRef]
- Chhabra, M.; Kumar, R. A Smart Healthcare System Based on Classifier DenseNet 121 Model to Detect Multiple Diseases. In Mobile Radio Communications and 5G Networks; Marriwala, N., Tripathi, C.C., Jain, S., Kumar, D., Eds.; Lecture Notes in Networks and Systems; Springer Nature Singapore: Singapore, 2022; Volume 339, pp. 297–312. ISBN 978-981-16-7017-6. [Google Scholar]
- Aggarwal, S.; Sahoo, A.K.; Bansal, C.; Sarangi, P.K. Image Classification Using Deep Learning: A Comparative Study of VGG-16, InceptionV3 and EfficientNet B7 Models. In Proceedings of the 2023 3rd International Conference on Advance Computing and Innovative Technologies in Engineering (ICACITE), Greater Noida, India, 12–13 May 2023; IEEE: New York, NY, USA, 2023; pp. 1728–1732. [Google Scholar]
- Patel, C.H.; Undaviya, D.; Dave, H.; Degadwala, S.; Vyas, D. EfficientNetB0 for Brain Stroke Classification on Computed Tomography Scan. In Proceedings of the 2023 2nd International Conference on Applied Artificial Intelligence and Computing (ICAAIC), Salem, India, 4–15 May 2023; IEEE: New York, NY, USA, 2023; pp. 713–718. [Google Scholar]
- Chicco, D.; Jurman, G. The Advantages of the Matthews Correlation Coefficient (MCC) over F1 Score and Accuracy in Binary Classification Evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef]
- Cao, C.; Chicco, D.; Hoffman, M.M. The MCC-F1 Curve: A Performance Evaluation Technique for Binary Classification. arXiv 2020, arXiv:2006.11278. [Google Scholar] [CrossRef]
- Diallo, R.; Edalo, C.; Awe, O.O. Machine Learning Evaluation of Imbalanced Health Data: A Comparative Analysis of Balanced Accuracy, MCC, and F1 Score. In Practical Statistical Learning and Data Science Methods; Awe, O.O., Vance, E.A., Eds.; STEAM-H: Science, Technology, Engineering, Agriculture, Mathematics & Health; Springer Nature Switzerland: Cham, Switzerland, 2025; pp. 283–312. ISBN 978-3-031-72214-1. [Google Scholar]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M. Comparison of Selected Classification Methods Based on Machine Learning as a Diagnostic Tool for Knee Joint Cartilage Damage Based on Generated Vibroacoustic Processes. Appl. Comput. Sci. 2023, 19, 136–150. [Google Scholar] [CrossRef]
- Machrowska, A.; Karpiński, R.; Maciejewski, M.; Jonak, J.; Krakowski, P.; Syta, A. Application of Recurrence Quantification Analysis in the Detection of Osteoarthritis of the Knee with the Use of Vibroarthrography. Adv. Sci. Technol. Res. J. 2024, 18, 19–31. [Google Scholar] [CrossRef]
- Lin, S.-H.; Liu, C.-M.; Chang, S.-S.; Hwu, H.-G.; Liu, S.K.; Hwang, T.J.; Hsieh, M.-H.; Guo, S.-C.; Chen, W.J. Familial Aggregation in Skin Flush Response to Niacin Patch Among Schizophrenic Patients and Their Nonpsychotic Relatives. Schizophr. Bull. 2007, 33, 174–182. [Google Scholar] [CrossRef]
- Ansarey, S.H. Inflammation and JNK’s Role in Niacin-GPR109A Diminished Flushed Effect in Microglial and Neuronal Cells with Relevance to Schizophrenia. Front. Psychiatry 2021, 12, 771144. [Google Scholar] [CrossRef]
- Feng, J.; Min, W.; Wang, D.; Yuan, J.; Chen, J.; Chen, L.; Chen, W.; Zhao, M.; Cheng, J.; Wan, C.; et al. Potential of Niacin Skin Flush Response in Adolescent Depression Identification and Severity Assessment: A Case-Control Study. BMC Psychiatry 2024, 24, 290. [Google Scholar] [CrossRef] [PubMed]
- Maroufi, M.; Tabatabaeian, M.; Tabatabaeian, M.; Mahaki, B.; Teimoori, G. Comparison of Niacin Skin Flush Response in Patients with Schizophrenia and Bipolar Disorder. Iran J. Psychiatry Behav. Sci. 2016, in press. [CrossRef]
- Nilsson, B.M.; Hultman, C.M.; Wiesel, F.-A. Niacin Skin-Flush Response and Electrodermal Activity in Patients with Schizophrenia and Healthy Controls. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 339–346. [Google Scholar] [CrossRef]
- Nilsson, B.M.; Holm, G.; Hultman, C.M.; Ekselius, L. Cognition and Autonomic Function in Schizophrenia: Inferior Cognitive Test Performance in Electrodermal and Niacin Skin Flush Non-Responders. Eur. Psychiatry 2015, 30, 8–13. [Google Scholar] [CrossRef]
- Yao, J.K.; Dougherty, G.G.; Gautier, C.H.; Haas, G.L.; Condray, R.; Kasckow, J.W.; Kisslinger, B.L.; Gurklis, J.A.; Messamore, E. Prevalence and Specificity of the Abnormal Niacin Response: A Potential Endophenotype Marker in Schizophrenia. Schizophr. Bull. 2016, 42, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, X.; Jiang, J.; Hu, X.; Qing, Y.; Wang, D.; Yang, T.; Yang, C.; Zhang, J.; Yang, P.; et al. Identification of the Niacin-Blunted Subgroup of Schizophrenia Patients from Mood Disorders and Healthy Individuals in Chinese Population. Schizophr. Bull. 2018, 44, 896–907. [Google Scholar] [CrossRef] [PubMed]


| Inclusion Criteria | |
|---|---|
| Group of patients | Control group |
|
|
| Exclusion criteria | |
|
|
| Patients N = 105 | Healthy Control N = 83 | Differences (p-Value) | |
|---|---|---|---|
| N (%) | |||
| Gender [females] | 52 (49.52) | 51 (61.45) | 0.134 |
| Cigarettes [smokers] | 31 (29.52) | 11 (13.25) | 0.178 |
| Somatic conditions [yes] | 22 (20.95) | 23 (27.71) | 0.388 |
| Psychoactive substances [users] | 26 (24.76) | 25 (30.12) | 0.521 |
| Median (Min–Max) | |||
| Age [years] | 26 (15–54) | 24 (19–32) | 0.342 |
| BMI [kg/m2] | 24.82 (17.64–37.89) | 22.79 (18.07–35.38) | 0.002 |
| Physical activity [min/week] | 0 (0–1370) | 47 (0–642) | 0.005 |
| Duration of illness [years] | 5 (1–29) | N/A | N/A |
| Hospitalizations [number] | 2 (0–17) | N/A | N/A |
| OLA equivalents [to 1 mg OLA] | 26 (0.71–129) | N/A | N/A |
| PANSS [total points] | 73 (34–144) | N/A | N/A |
| Accuracy | Precision | Recall | F1-Score | AUC | |
|---|---|---|---|---|---|
| ResNet50 | 0.87 ± 0.04 | 0.87 ± 0.04 | 0.87 ± 0.04 | 0.87 ± 0.04 | 0.94 ± 0.01 |
| ResNet101 | 0.84 ± 0.06 | 0.83 ± 0.06 | 0.84 ± 0.06 | 0.83 ± 0.06 | 0.89 ± 0.04 |
| EfficientNetB0 | 0.78 ± 0.03 | 0.79 ± 0.03 | 0.78 ± 0.04 | 0.78 ± 0.04 | 0.90 ± 0.01 |
| InceptionV3 | 0.86 ± 0.05 | 0.86 ± 0.05 | 0.86 ± 0.06 | 0.86 ± 0.06 | 0.94 ± 0.02 |
| DenseNet121 | 0.86 ± 0.04 | 0.86 ± 0.05 | 0.86 ± 0.05 | 0.86 ± 0.04 | 0.92 ± 0.01 |
| Accuracy | Precision | Recall | F1-Score | AUC | |
|---|---|---|---|---|---|
| ResNet50 | 0.86 ± 0.02 | 0.86 ± 0.02 | 0.86 ± 0.02 | 0.86 ± 0.02 | 0.93 ± 0.01 |
| ResNet101 | 0.82 ± 0.07 | 0.82 ± 0.07 | 0.82 ± 0.07 | 0.82 ± 0.07 | 0.91 ± 0.04 |
| EfficientNetB0 | 0.83 ± 0.08 | 0.84 ± 0.07 | 0.82 ± 0.09 | 0.82 ± 0.09 | 0.91 ± 0.05 |
| InceptionV3 | 0.89 ± 0.01 | 0.89 ± 0.01 | 0.89 ± 0.02 | 0.89 ± 0.01 | 0.94 ± 0.02 |
| DenseNet121 | 0.85 ± 0.04 | 0.85 ± 0.04 | 0.85 ± 0.04 | 0.85 ± 0.04 | 0.92 ± 0.03 |
| Accuracy | Precision | Recall | F1-Score | AUC | |
|---|---|---|---|---|---|
| ResNet50 | 0.87 ± 0.02 | 0.87 ± 0.02 | 0.88 ± 0.02 | 0.87 ± 0.02 | 0.94 ± 0.01 |
| ResNet101 | 0.85 ± 0.08 | 0.85 ± 0.09 | 0.85 ± 0.08 | 0.84 ± 0.08 | 0.91 ± 0.04 |
| EfficientNetB0 | 0.84 ± 0.04 | 0.84 ± 0.04 | 0.84 ± 0.04 | 0.83 ± 0.04 | 0.93 ± 0.03 |
| InceptionV3 | 0.83 ± 0.05 | 0.83 ± 0.05 | 0.83 ± 0.04 | 0.83 ± 0.05 | 0.92 ± 0.02 |
| DenseNet121 | 0.84 ± 0.02 | 0.84 ± 0.03 | 0.84 ± 0.02 | 0.84 ± 0.02 | 0.92 ± 0.01 |
| Accuracy | Precision | Recall | F1-Score | AUC | |
|---|---|---|---|---|---|
| ResNet50 | 0.90 ± 0.02 | 0.90 ± 0.02 | 0.90 ± 0.02 | 0.90 ± 0.02 | 0.95 ± 0.02 |
| ResNet101 | 0.86 ± 0.06 | 0.85 ± 0.06 | 0.85 ± 0.06 | 0.85 ± 0.06 | 0.92 ± 0.04 |
| EfficientNetB0 | 0.83 ± 0.09 | 0.83 ± 0.09 | 0.83 ± 0.09 | 0.83 ± 0.09 | 0.91 ± 0.03 |
| InceptionV3 | 0.88 ± 0.05 | 0.89 ± 0.04 | 0.89 ± 0.04 | 0.88 ± 0.05 | 0.95 ± 0.02 |
| DenseNet121 | 0.87 ± 0.02 | 0.87 ± 0.03 | 0.87 ± 0.03 | 0.86 ± 0.02 | 0.94 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitarz, R.; Syta, A.; Karpiński, R.; Machrowska, A.; Róg, J.; Karakuła, K.; Juchnowicz, D.; Karakuła-Juchnowicz, H. AI-Based Image Time-Series Analysis of the Niacin Skin Flush Test in Schizophrenia and Bipolar Disorder. Appl. Sci. 2025, 15, 12368. https://doi.org/10.3390/app152312368
Sitarz R, Syta A, Karpiński R, Machrowska A, Róg J, Karakuła K, Juchnowicz D, Karakuła-Juchnowicz H. AI-Based Image Time-Series Analysis of the Niacin Skin Flush Test in Schizophrenia and Bipolar Disorder. Applied Sciences. 2025; 15(23):12368. https://doi.org/10.3390/app152312368
Chicago/Turabian StyleSitarz, Ryszard, Arkadiusz Syta, Robert Karpiński, Anna Machrowska, Joanna Róg, Kaja Karakuła, Dariusz Juchnowicz, and Hanna Karakuła-Juchnowicz. 2025. "AI-Based Image Time-Series Analysis of the Niacin Skin Flush Test in Schizophrenia and Bipolar Disorder" Applied Sciences 15, no. 23: 12368. https://doi.org/10.3390/app152312368
APA StyleSitarz, R., Syta, A., Karpiński, R., Machrowska, A., Róg, J., Karakuła, K., Juchnowicz, D., & Karakuła-Juchnowicz, H. (2025). AI-Based Image Time-Series Analysis of the Niacin Skin Flush Test in Schizophrenia and Bipolar Disorder. Applied Sciences, 15(23), 12368. https://doi.org/10.3390/app152312368








