Nematicidal Activity of Alkyloxyalkanols Against Bursaphelenchus xylophilus
Abstract
1. Introduction
2. Materials and Methods
2.1. Pine Wood Nematodes
2.2. Chemicals
2.3. Instrumental Analysis
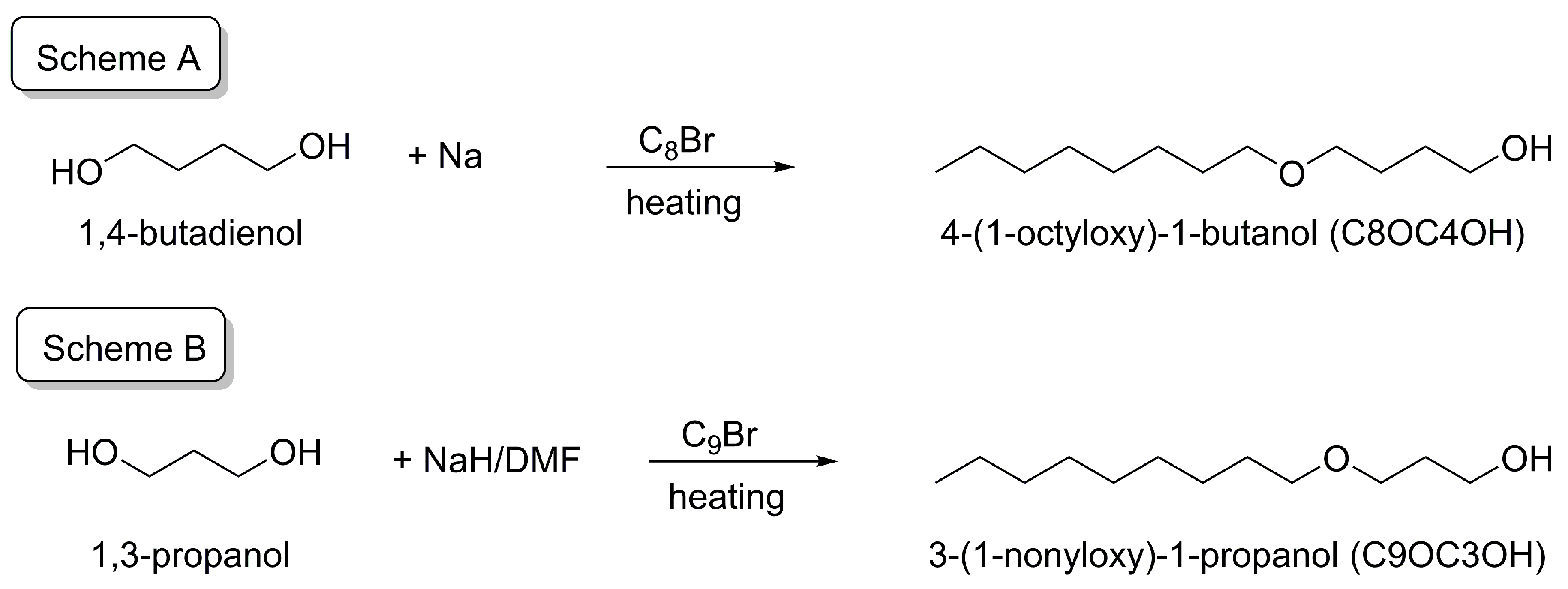
2.4. Synthesis of the Alkyloxyalkanols
2.4.1. 4-(1-Alkyloxyl)-1-butanol
2.4.2. 3-(1-Alkyloxy)-1-propanol, 5-(1-Alkyloxy)-1-pentanol, 6-(1-Alkyloxy)-1-hexanol, 7-(1-Alkyloxy)-1-heptanol, 8-(1-Alkyloxy)-1-octanol, and 9-(1-Alkyloxy)-1-nonanol
2.5. Nematicidal Activity
2.6. Statistical Analyses
3. Results
3.1. Nematicidal Activity of Alkyloxyalkanols
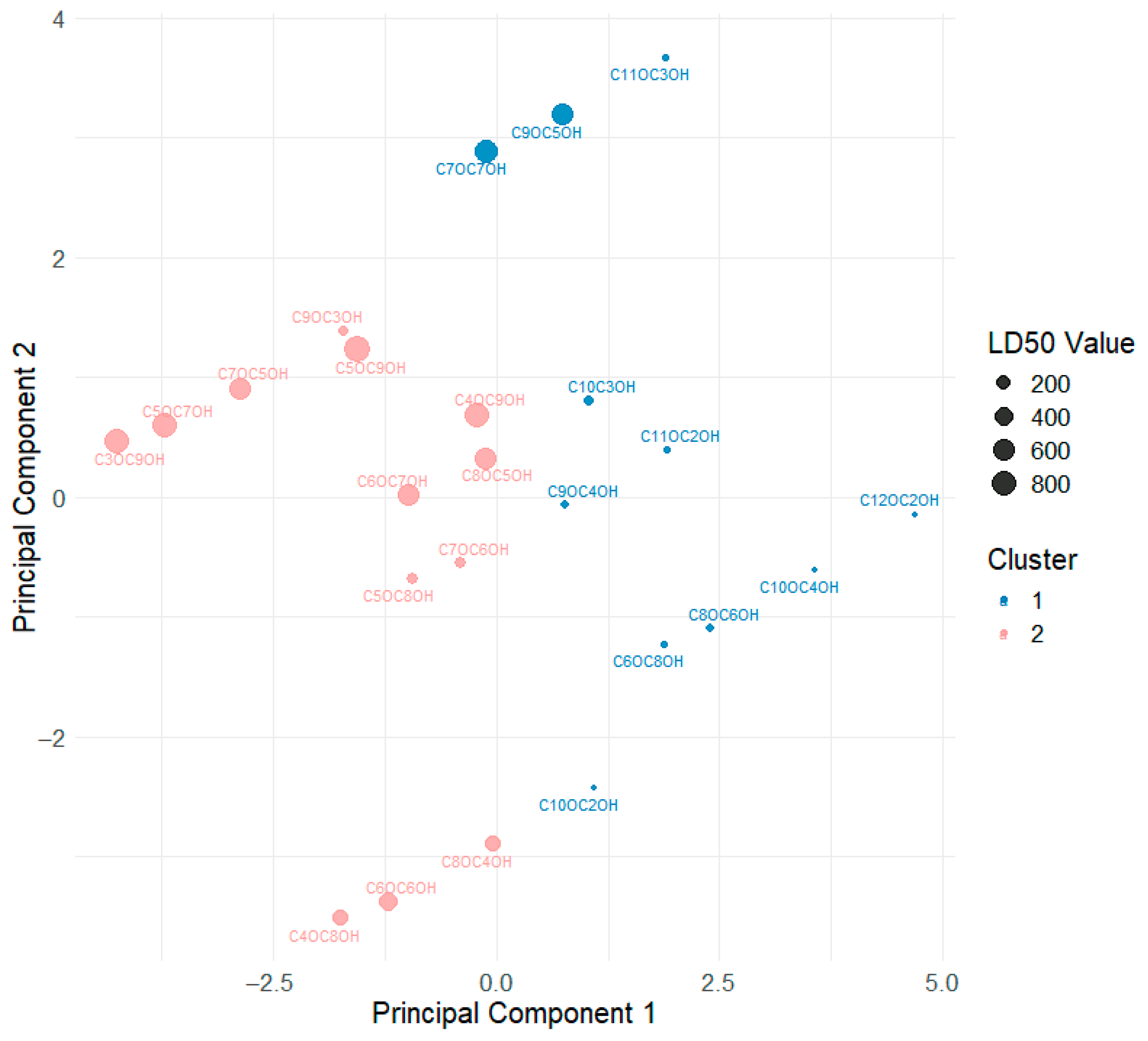
3.2. Multivariate Analysis of Nematicidal Activity and Alkyloxyalkanol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mota, M.M.; Vieira, P.R. Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Springer: Cham, The Netherlands, 2008; p. 405. [Google Scholar]
- Inácio, M.L.; Nóbrega, F.; Vieira, P.; Bonifácio, L.; Naves, P.; Sousa, E.; Mota, M. First detection of Bursaphelenchus xylophilus associated with Pinus nigra in Portugal and in Europe. For. Pathol. 2015, 45, 235–238. [Google Scholar] [CrossRef]
- Back, M.A.; Bonifácio, L.; Inácio, M.L.; Mota, M.; Boa, E. Pine wilt disease: A global threat to forestry. Plant Pathol. 2024, 73, 1026–1041. [Google Scholar] [CrossRef]
- Han, H.; Chung, Y.J.; Shin, S.C. First report of pine wilt disease on Pinus koraiensis in Korea. Plant Dis. 2008, 92, 1251. [Google Scholar] [CrossRef]
- Zamora, P.; Rodríguez, V.; Renedo, F.; Sanz, A.V.; Domínguez, J.C.; Pérez-Escolar, G.; Miranda, J.; Álvarez, B.; González-Casas, A.; Mayor, E.; et al. First report of Bursaphelenchus xylophilus causing pine wilt disease on Pinus radiata in Spain. Plant Dis. 2015, 99, 1449. [Google Scholar] [CrossRef]
- Korea Forest Service. Statistical Yearbook of Forestry; Korea Forest Service: Daejeon, Republic of Korea, 2017; p. 438. [Google Scholar]
- Soliman, T.; Mourits, M.C.M.; van der Werf, W.; Hengeveld, G.M.; Robinet, C.; Lansink, A.G.J.M.O. Framework for modelling economic impacts of invasive species, applied to pine wood nematode in Europe. PLoS ONE 2012, 7, e45505. [Google Scholar] [CrossRef] [PubMed]
- Togashi, K.; Shigesada, N. Spread of the pinewood nematode vectored by the Japanese pine sawyer: Modeling and analytical approaches. Popul. Ecol. 2006, 48, 271–283. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Lu, M.; Wang, J.; CHen, H. Growth, physiological and biochemical responses of Pinus tabulaeformis to the infestation of Bursaphelenchus xylophilus. Plant Stress 2025, 16, 100848. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, L.; Zhou, J.; Yu, H.; Zhang, C.; Lv, Y.; Lin, Z.; Hu, S.; Zou, Z.; Sun, J. Enhancement of oxidative stress contributes to increased pathogenicity of the invasive pine wood nematode. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180323. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, S.; Stamps, W.T. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. For. Pathol. 2012, 42, 89–99. [Google Scholar] [CrossRef]
- National Institute of Forest Science. Manual for Control Method of Pine Wilt Disease; National Institute of Forest Science: Seoul, Republic of Korea, 2016; p. 21115. [Google Scholar]
- James, R.; Tisserat, N.; Todd, T. Prevention of pine wilt of Scots pine (Pinus sylvestris) with systemic abamectin injections. Arboric. Urban For. 2006, 32, 195–201. [Google Scholar] [CrossRef]
- Jansson, R.K.; Rabatin, S. Curative and residual efficacy of injection applications of avermectins for control of plant-parasitic nematodes on banana. J. Nematol. 1997, 29, 695–702. [Google Scholar] [PubMed]
- Saleem, M.; Hussain, D.; Ghouse, G.; Abbas, M.; Fisher, S.W. Monitoring of insecticide resistance in Spodoptera litura (Lepidoptera: Noctuidae) from four districts of Punjab, Pakistan to conventional and new chemistry insecticides. Crop Prot. 2015, 79, 177–184. [Google Scholar] [CrossRef]
- Xu, M.; Molento, M.; Blackhall, W.; Ribeiro, P.; Beech, R.; Prichard, R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol. Biochem. Parasitol. 1998, 91, 327–335. [Google Scholar] [CrossRef]
- Gopal, R.M.; Pomroy, W.E.; West, D.M. Resistance of field isolates of Trichostrongylus colubriformis and Ostertagia circumcincta to ivermectin. Int. J. Parasitol. 1999, 29, 781–786. [Google Scholar] [CrossRef]
- McKenzie, C.L.; Byford, R.L. Continuous, alternating, and mixed insecticides affect development of resistance in the horn fly (Diptera: Muscidae). J. Econ. Entomol. 1993, 86, 1040–1048. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Disa, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 2010, 42, 8–16. [Google Scholar]
- Rajasekharan, S.K.; Lee, J.-H.; Ravichandran, V.; Lee, J. Assessments of iodoindoles and abamectin as inducers of methuosis in pinewood nematode, Bursaphelenchus xylophilus. Sci. Rep. 2017, 7, 6803. [Google Scholar] [CrossRef]
- Guo, Q.; Du, G.; Qi, H.; Zhang, Y.; Yue, T.; Wang, J.; Li, R. A nematicidal tannin from Punica granatum L. rind and its physiological effect on pine wood nematode (Bursaphelenchus xylophilus). Pestic. Biochem. Physiol. 2017, 135, 64–68. [Google Scholar] [CrossRef]
- Liu, G.; Lai, D.; Liu, Q.Z.; Zhou, L.; Liu, Z.L. Identification of nematicidal constituents of Notopterygium incisum rhizomes against Bursaphelenchus xylophilus and Meloidogyne incognita. Molecules 2016, 21, 1276. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-K.; Kim, J.-H.; Liu, M.J.; Jin, C.-Z.; Park, D.-J.; Kim, J.; Sung, B.-H.; Kim, C.J.; Son, K.-H. New discovery on the nematicidal activities of aureothin and alloaureothin isolated from endophytic bacteria, Streptomyces sp. AE170020. Sci. Rep. 2022, 12, 3947. [Google Scholar] [CrossRef]
- Nguyen, D.-M.-C.; Nguyen, V.-N.; Seo, D.-J.; Park, R.-D.; Jung, W.J. Nematicidal activity of compounds extracted from medicinal plants against the pine wood nematode Bursaphelenchus xylophilus. Nematology 2009, 11, 835–845. [Google Scholar] [CrossRef]
- Lee, H.-R.; Lee, S.-C.; Lee, D.H.; Choi, W.-S.; Jung, C.-S.; Jeon, J.-H.; Kim, J.-E.; Park, I.-K. Identification of the aggregation-sex pheromone produced by male Monochamus saltuarius, a major insect vector of the pine wood nematode. J. Chem. Ecol. 2017, 43, 67–678. [Google Scholar] [CrossRef]
- Lee, H.-R.; Lee, S.-C.; Lee, D.H.; Jung, M.; Kwon, J.-H.; Huh, M.-J.; Kim, D.S.; Lee, J.-E.; Park, I.-K. Identification of aggregation-sex pheromone of the Korean Monochamus alternatus (Coleoptera: Cerambycidae) population, the main vector of pine wood nematode. J. Econ. Entomol. 2018, 111, 1768–1774. [Google Scholar] [CrossRef]
- Allison, J.D.; McKenney, J.L.; Millar, J.G.; McElfresh, J.S.; Mitchell, R.F.; Hanks, L.M. Response of the woodborers Monochamus carolinensis and Monochamus titillator (Coleoptera: Cerambycidae) to known cerambycid pheromones in the presence and absence of the host plant volatile α-pinene. Environ. Entomol. 2012, 41, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Pajares, J.A.; Alvarez, G.; Ibeas, F.; Gallego, D.; Hall, D.R.; Farman, D.I. Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, Monochamus galloprovincialis. J. Chem. Ecol. 2010, 36, 570–583. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.-M.; Park, C.G. Bursaphelenchus xylophilus is killed by homologues of 2-(1-undecyloxy)-1-ethanol. Sci. Rep. 2016, 6, 29300. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.J.; Park, J.O.; Yoon, K.A. Nematicidal activity of benzyloxyalkanols against pine wood nematode. Biomolecules 2021, 11, 384. [Google Scholar] [CrossRef]
- Han, H.-R.; Han, B.Y.; Chung, Y.J.; Shin, S.C. A simple PCR-RFLP for idenficiation of Bursaphelenchus spp. collected from Korea. Plant Pathol. J. 2008, 24, 150–163. [Google Scholar] [CrossRef]
- Loffredo, C.; Pires, P.A.R.; Imran, M.; El Seoud, O.A. ß-Carotene: A green, inexpensive, and convenient solvatochromic probe for the determination of solvent polarizability. Dyes Pigm. 2013, 96, 16–24. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Choi, I.-H.; Kim, J.; Shin, S.-C.; Park, I.-K. Nematicidal activity of monoterpenoids against the pine wood nematode (Bursaphelenchus xylophilus). Russ. J. Nematol. 2007, 15, 35–40. [Google Scholar]
- Yu, S.; Mao, T.; Shang, Y.; Sun, J.; Liu, P.; Jin, L.H. Nematicidal activity of 3-indoleacetonitrile against Bursaphelenchus xylophilus: Inhibition of development and reproduction through induction of excessive reactive oxygen species. Pestic. Biochem. Physiol. 2025, 213, 106509. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.-Z.; Sun, D.-A.; Jin, H.; Guo, H.-R.; Qin, B. Design and synthesis of novel coumarin analogs and their nematicidal activity against five phytonematodes. Chin. Chem. Lett. 2016, 27, 375–379. [Google Scholar] [CrossRef]
- Seo, S.-M.; Kim, J.; Kim, E.; Park, H.-M.; Kim, Y.-J.; Park, I.-K. Structure-activity relationship of aliphatic compounds for nematicidal activity against pine wood nematode (Bursaphelenchus xylophilus). J. Agric. Food Chem. 2010, 58, 1823–1827. [Google Scholar] [CrossRef]
- Fiume, M.M.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of alkyl PEG/PPG ethers as used in cosmetics. Int. J. Toxicol. 2016, 35 (Suppl. 1), 60S–89S. [Google Scholar] [CrossRef]
- Kang, J.S.; Moon, Y.-S.; Lee, S.H.; Park, I.-K. Inhibition of acetylcholinesterase and glutathione S-transferase of the pinewood nematode (Bursaphelenchus xylophilus) by aliphatic compounds. Pestic. Biochem. Physiol. 2013, 105, 184–188. [Google Scholar] [CrossRef]
- El-Helw, E.A.E.; Abou-Elmagd, W.S.I.; Hosni, E.M.; Kamal, M.; Hashem, A.I.; Ramadan, S.K. Synthesis of benzo[h]quinoline derivatives and evaluation of their insecticidal activity against Culex pipiens L. larvae. Eur. J. Med. Chem. 2025, 290, 117565. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Bernasek, S. Undetstanding odd-even effects in organic self-assembled monolayers. Chem. Rev. 2007, 107, 1408–1453. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, I.; Berg, M.C.; Petridis, L.; Smith, J.C.; Gautam, S. Dynamic odd-even effect in n-alkane systems: A molecular dynamics study. Phys. Chem. Chem. Phys. 2022, 24, 28403–28410. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Pajares, J.A.; Ibeas, F.; Diez, J.J.; Gallego, D. Attractive responses by Monochamus galloprovincialis (Col., Cerambycidae) to host and bark beetle semiochemicals. J. Appl. Entomol. 2004, 128, 633–638. [Google Scholar] [CrossRef]

| IUPAC Name | Abbreviation | Purity |
|---|---|---|
| 2-(1-Decyloxy)-1-ethanol | C10OC2OH | 98.6 |
| 2-(1-Undecyloxy)-1-ethanol | C11OC2OH | 98.3 |
| 2-(1-Dodecyloxy)-1-ethanol | C12OC2OH | 97.2 |
| 3-(1-Nonyloxy)-1-propanol | C9OC3OH | 96.2 |
| 3-(1-Decyloxy)-1-propanol | C10C3OH | 98.9 |
| 3-(1-Undecyloxy)-1-propanol | C11OC3OH | 96.1 |
| 4-(1-Octyloxy)-1-butanol | C8OC4OH | 97.3 |
| 4-(1-Nonyloxy)-1-butanol | C9OC4OH | 98.3 |
| 4-(1-Decyloxy)-1-butanol | C10OC4OH | 97.2 |
| 5-(1-Heptyloxy)-1-pentanol | C7OC5OH | 98.2 |
| 5-(1-Octyloxy)-1-pentanol | C8OC5OH | 96.5 |
| 5-(1-Nonyloxy)-1-pentanol | C9OC5OH | 98.0 |
| 6-(1-Hexyloxy)-1-hexanol | C6OC6OH | 95.5 |
| 6-(1-Heptyloxy)-1-hexanol | C7OC6OH | 99.0 |
| 6-(1-Octyloxy)-1-hexanol | C8OC6OH | 98.9 |
| 7-(1-Pentyloxy)-1-heptanol | C5OC7OH | 96.5 |
| 7-(1-Hexyloxy)-1-heptanol | C6OC7OH | 95.8 |
| 7-(1-Heptyloxy)-1-heptanol | C7OC7OH | 98.4 |
| 8-(1-Butyloxy)-1-octanol | C4OC8OH | 99.5 |
| 8-(1-Pentyloxy)-1-octanol | C5OC8OH | 99.3 |
| 8-(1-Hexyloxy)-1-octanol | C6OC8OH | 99.5 |
| 9-(1-Propyloxy)-1-nonanol | C3OC9OH | 97.8 |
| 9-(1-Butyloxy)-1-nonanol | C4OC9OH | 98.3 |
| 9-(1-Pentyloxy)-1-nonanol | C5OC9OH | 95.7 |
| Compound | LD50 (95% CI; ppm) | LD90 (95% CI; ppm) | Slope ± SE | Goodness of Fit | |
|---|---|---|---|---|---|
| χ2 | df | ||||
| C10OC2OH | 42.8 (39.6–45.4) | 69.8 (65.7–75.9) | 2.62 ± 0.28 | 9.4 | 14 |
| C9OC3OH | 96.8 (86.4–110.2) | 227.6 (184.9–310.2) | 3.45 ± 0.37 | 445.4 | 46 |
| C8OC4OH | 276.5 (215.9–370.8) | 2534.0 (1548.0–5033.0) | 1.33 ± 0.12 | 548.5 | 51 |
| C7OC5OH | 560.5 (363.5–861.8) | 1603.0 (993.4–6602.0) | 2.81 ± 0.76 | 1804.1 | 28 |
| C6OC6OH | 390.2 (288.5–552.0) | 1932.0 (1170.0–4629.0) | 1.84 ± 0.28 | 1842.6 | 50 |
| C5OC7OH | 800.9 (746.2–851.0) | 1057.5 (990.3–1151.5) | 10.62 ± 1.15 | 249.5 | 22 |
| C4OC8OH | 239.2 (190.7–301.4) | 1272.0 (891.5–2099.0) | 1.77 ± 0.18 | 760.4 | 46 |
| C3OC9OH | 802.3 (706.4–879.1) | 1042.2 (949.6–1196.9) | 11.28 ± 1.99 | 591.4 | 22 |
| C11OC2OH | 46.1 (40.9–51.3) | 120.7 (104.4–145.7) | 3.07 ± 0.26 | 1343.6 | 99 |
| C10C3OH | 73.4 (67.5–79.9) | 150.2 (128.6–189.9) | 4.11 ± 0.46 | 328.5 | 46 |
| C9OC4OH | 65.5 (53.5–80.5) | 236.6 (176.6–357.3) | 2.30 ± 0.24 | 933.5 | 51 |
| C8OC5OH | 576.8 (353.2–920.8) | 1440.0 (907.5–7557) | 3.22 ± 1.00 | 2405.9 | 28 |
| C7OC6OH | 131.2 (87.3–194.8) | 1176.0 (648.1–3240.0) | 1.35 ± 0.20 | 3478.8 | 74 |
| C6OC7OH | 565.8 (n.a.) | 626.6 (n.a.) | 28.90 ± 32.89 | 61.3 | 22 |
| C5OC8OH | 107.9 (85.9–132.5) | 487.3 (368.1–715.0) | 1.96 ± 0.19 | 691.3 | 46 |
| C4OC9OH | 734.7 (689.4–779.7) | 936.1 (879.1–1008.6) | 12.18 ± 1.05 | 212.7 | 22 |
| C12OC2OH | 42.6 (36.1–49.0) | 231.6 (201.2–273.8) | 0.76 ± 0.22 | 233.3 | 14 |
| C11OC3OH | 49.2 (43.4–54.4) | 145.8 (127.1–175.9) | 2.72 ± 0.25 | 164.5 | 46 |
| C10OC4OH | 43.1 (32.4–56.4) | 188.4 (130.3–328.4) | 2.00 ± 0.25 | 1357.2 | 51 |
| C9OC5OH | 514.4 (347.0–725.9) | 1420.0 (939.7–4014.0) | 2.91 ± 0.71 | 1480.2 | 28 |
| C8OC6OH | 55.1 (46.2–64.7) | 184.0 (147.4–248.4) | 2.45 ± 0.24 | 1291.2 | 74 |
| C7OC7OH | 627.2 (601.3–664.6) | 766.2 (714.0–849.1) | 14.70 ± 1.40 | 68.4 | 22 |
| C6OC8OH | 48.0 (35.2–64.0) | 152.6 (106.8–275.1) | 2.55 ± 0.40 | 228.5 | 58 |
| C5OC9OH | 871.7 (847.2–896.3) | 1222.0 (1174–1283) | 8.73 ± 0.46 | 46.8 | 22 |
| Abamectin | 2.2 (1.87–2.55) | 13.1 (11.4–15.9) | 1.66 ± 0.09 | 1008.1 | 154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.O.; Lee, S.; Kim, M.-J.; Kim, J. Nematicidal Activity of Alkyloxyalkanols Against Bursaphelenchus xylophilus. Appl. Sci. 2025, 15, 9862. https://doi.org/10.3390/app15189862
Park JO, Lee S, Kim M-J, Kim J. Nematicidal Activity of Alkyloxyalkanols Against Bursaphelenchus xylophilus. Applied Sciences. 2025; 15(18):9862. https://doi.org/10.3390/app15189862
Chicago/Turabian StylePark, Joon Oh, Sujin Lee, Min-Jung Kim, and Junheon Kim. 2025. "Nematicidal Activity of Alkyloxyalkanols Against Bursaphelenchus xylophilus" Applied Sciences 15, no. 18: 9862. https://doi.org/10.3390/app15189862
APA StylePark, J. O., Lee, S., Kim, M.-J., & Kim, J. (2025). Nematicidal Activity of Alkyloxyalkanols Against Bursaphelenchus xylophilus. Applied Sciences, 15(18), 9862. https://doi.org/10.3390/app15189862







