Influence of Thermally Treated Asbestos-Containing Materials on Cement Mortars Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
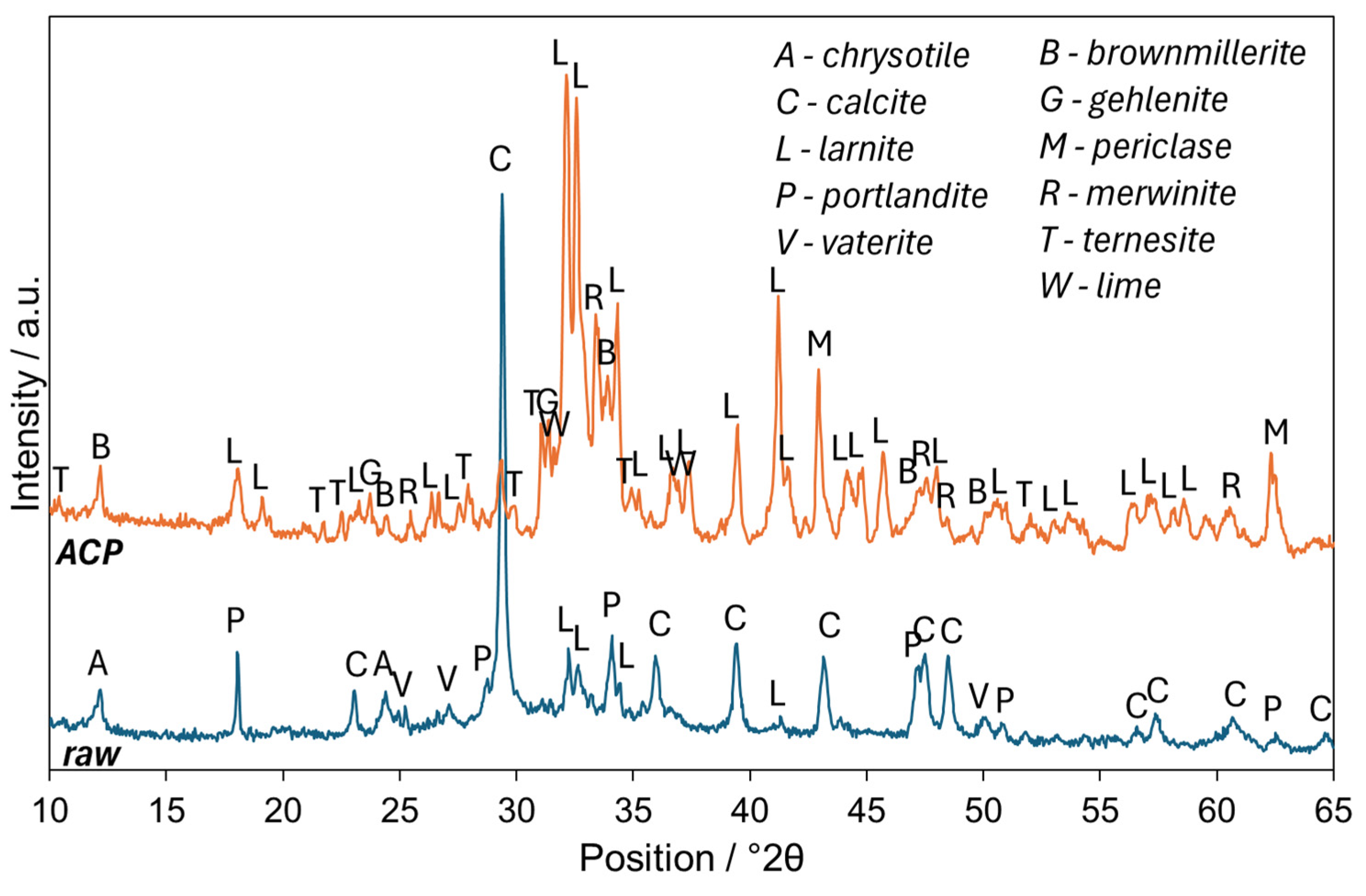
3.1. Characterization of ACP
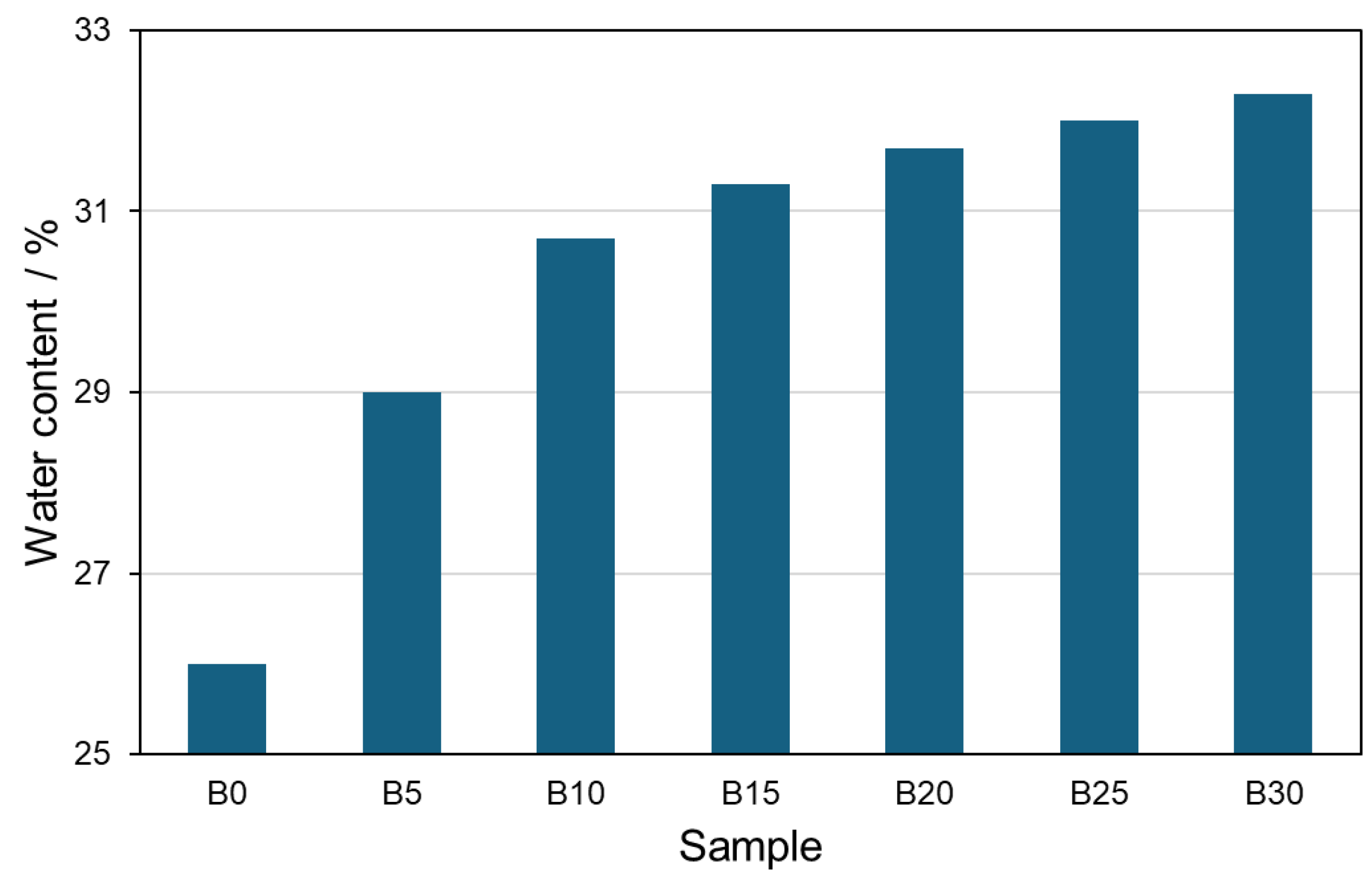
3.2. Characterization of Cement Pastes and Mortars
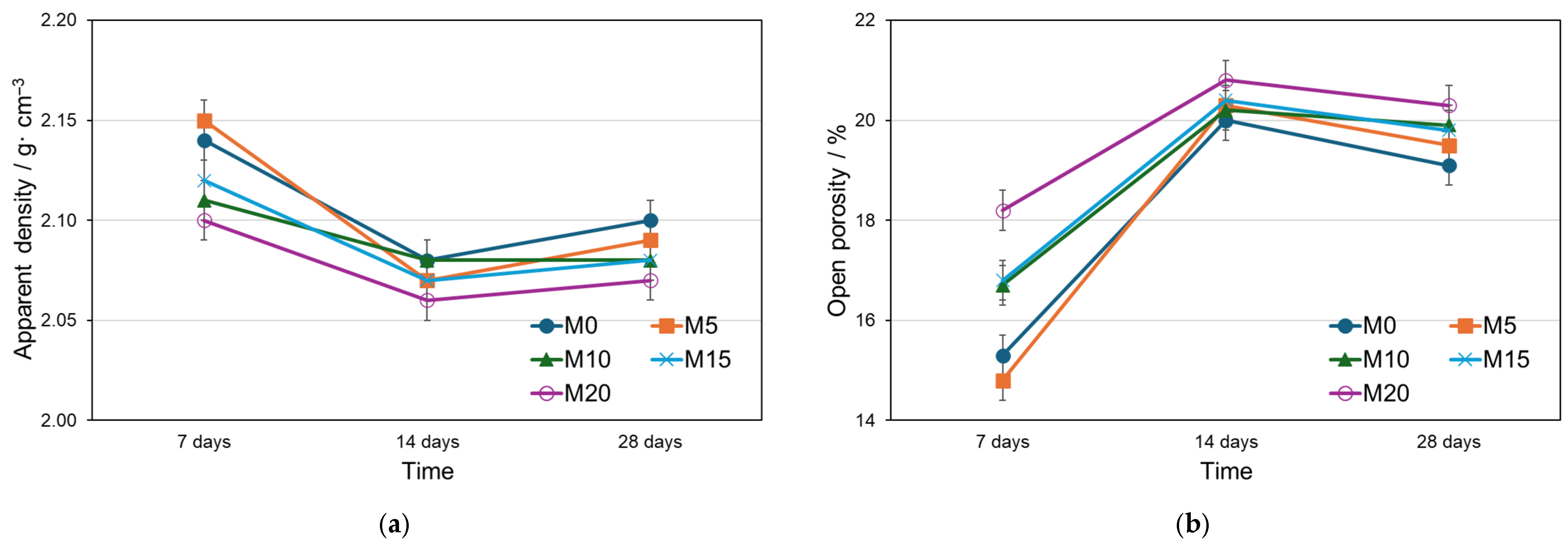
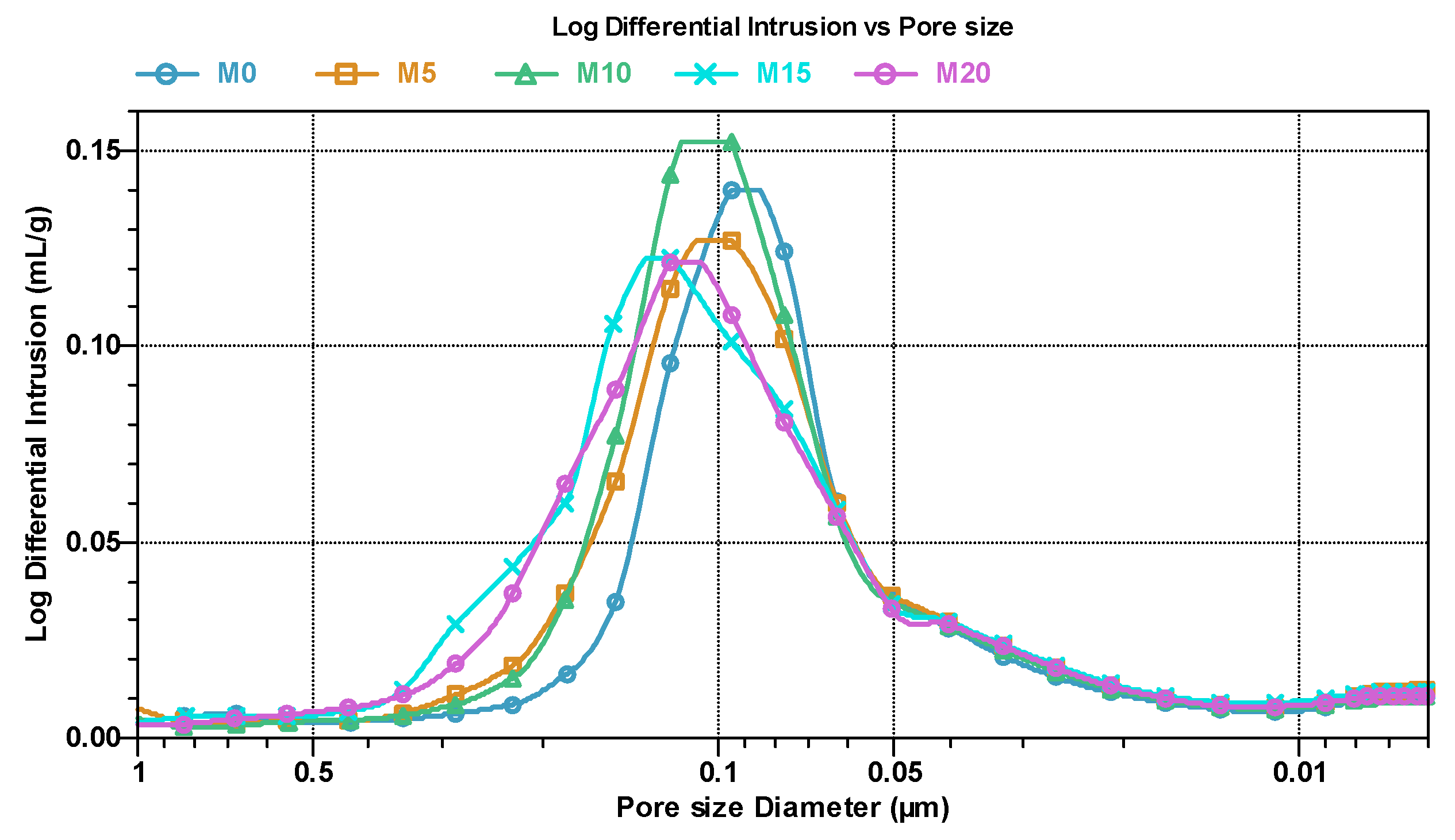
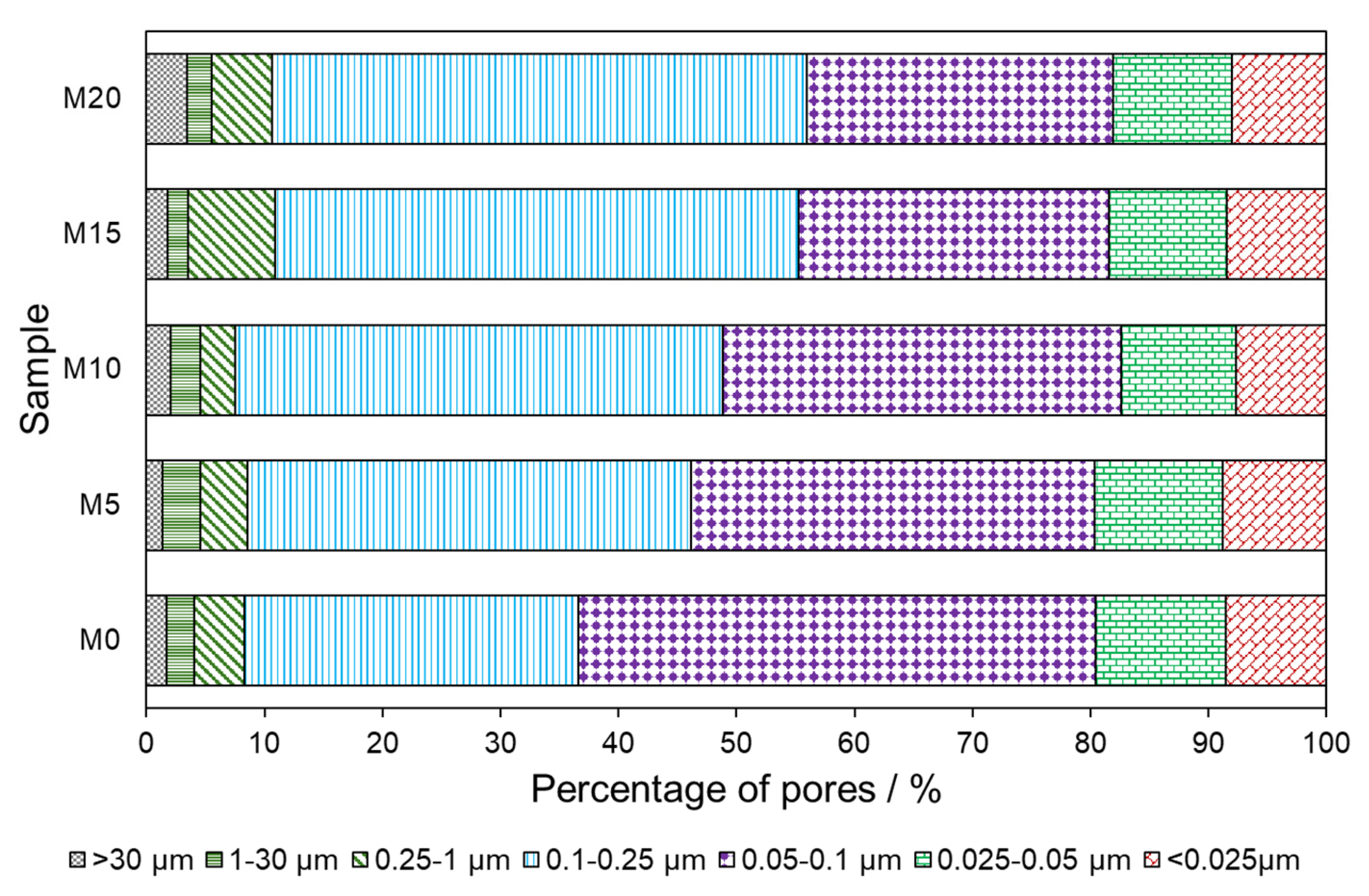
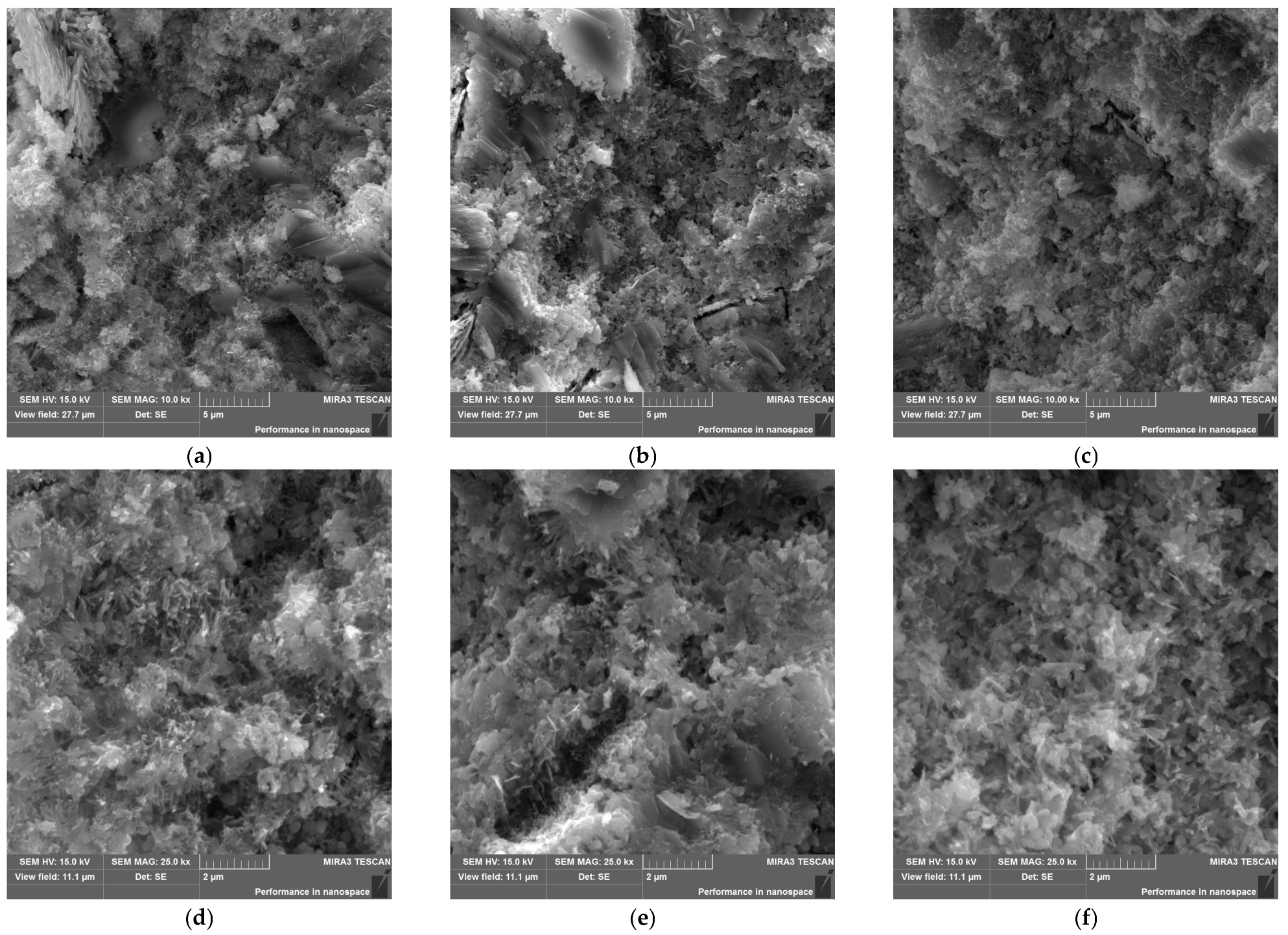
3.3. Porosimetry Analysis and Microstructure of Mortars
4. Conclusions
- water demand of cement, by increasing the w/c factor;
- setting of cement, by accelerating the hydration processes and shortening the initial setting time;
- compressive strength, by decreasing the strength development and decreasing the compressive strength after curing;
- flexural strength, by decreasing the flexural strength in comparison to the reference cement mortar;
- apparent density, by decreasing;
- open porosity, by increasing;
- the addition of up to 5% of cement gave the closest results to the reference sample.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartrip, P.W. History of asbestos related disease. Postgrad. Med. J. 2004, 80, 72–76. [Google Scholar] [CrossRef]
- Stayner, L.; Welch, L.S.; Lemen, R. The worldwide pandemic asbestos-related diseases. Annu. Rev. Public Health 2013, 34, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Musk, A.W.; de Klerk, N.; Reid, A.; Hui, J.; Franklin, P.; Brims, F. Asbestos-related diseases. Int. J. Tuberc. Lung Dis. 2020, 24, 562–567. [Google Scholar] [CrossRef] [PubMed]
- The International Ban Asbestos Secretariat IBAS. Available online: https://www.ibasecretariat.org/index.htm (accessed on 20 June 2025).
- Ulewicz, M. Management of asbestos materials in terms of life and work safety. Prz. Bud. 2023, 9–10, 171–176. [Google Scholar]
- Paglietti, F.; Malinconico, S.; Conestabile della Staffa, B.; Bellagamba, S.; De Simone, P. Classification and management of asbestos-containing waste: European legislation and the Italian experience. Waste Manag. 2016, 50, 130–150. [Google Scholar] [CrossRef]
- Li, J.; Dong, Q.; Yu, K.; Liu, L. Asbestos and asbestos waste management in the Asian-Pacific region: Trends, challenges and solutions. J. Clean. Prod. 2014, 81, 218–226. [Google Scholar] [CrossRef]
- Brycht, N. Recycling of asbestos-cement waste—An opportunity or a threat? CzOTO 2022, 4, 10–18. [Google Scholar] [CrossRef]
- Council of Ministers of the Republic of Poland. Program for the Removal of Asbestos and Asbestos-Containing Products Used in Poland; Polish Government: Warsaw, Poland, 2002.
- Wallis, S.L.; Emmett, E.A.; Hardy, R.; Casper, B.B.; Blanchon, D.J.; Testa, J.R.; Menges, C.W.; Gonneau, C.; Jerolmack, D.J.; Seiphoori, A.; et al. Challenging global waste management—Bioremediation to detoxify asbestos. Front. Environ. Sci. 2020, 8, 20. [Google Scholar] [CrossRef]
- Avataneo, C.; Petriglieri, J.R.; Capella, S.; Tomatis, M.; Luiso, M.; Marangoni, G.; Lazzari, E.; Tinazzi, S.; Lasagna, M.; De Luca, D.A.; et al. Chrysotile asbestos migration in air from contaminated water: An experimental simulation. J. Hazard. Mater. 2022, 424, 127528. [Google Scholar] [CrossRef]
- Obmiński, A. Asbestos cement products and their impact on soil contamination in relation to various sources of anthropogenic and natural asbestos pollution. Sci. Total Environ. 2022, 848, 157275. [Google Scholar] [CrossRef]
- Pini, M.; Scarpellini, S.; Rosa, R.; Neri, P.; Gualtieri, A.F.; Ferrari, A.M. Management of asbestos containing materials: A detailed LCA comparison of different scenarios comprising first time asbestos characterization factor proposal. Environ. Sci. Technol. 2021, 55, 12672–12682. [Google Scholar] [CrossRef]
- Butkevics, J.; Atstaja, D. Diversion of asbestos-containing waste from landfilling: Opportunities and challenges. Sustainability 2025, 17, 4529. [Google Scholar] [CrossRef]
- European Commission. Communication COM(2022) 488 Final, 28 September 2022; European Commission: Brussels, Belgium, 2022; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2022%3A488%3AFIN (accessed on 25 July 2025).
- Akelytė, R.; Chiabrando, F.; Camboni, M.; Ledda, C.; Vencovsky, D.; Butera, S.; Dünger, O.; European Commission: Directorate-General for Environment. Study on Asbestos Waste Management Practices and Treatment Technologies; Publications Office of the European Union; European Commission: Brussels, Belgium, 2024. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Giacobbe, C.; Sardisco, L.; Saraceno, M.; Gualtieri, M.L.; Lusvardi, G.; Cavenati, C.; Zanatto, I. Recycling of the product of thermal inertization of cement–asbestos for various industrial applications. Waste Manag. 2011, 31, 91–100. [Google Scholar] [CrossRef]
- Spasiano, D.; Pirozzi, F. Treatments of asbestos containing wastes. J. Environ. Manag. 2017, 204, 82–91. [Google Scholar] [CrossRef]
- Paolini, V.; Tomassetti, L.; Segreto, M.; Borin, D.; Liotta, F.; Torre, M.; Petracchini, F. Asbestos treatment technologies. J. Mater. Cycles Waste Manag. 2019, 21, 205–226. [Google Scholar] [CrossRef]
- Iwaszko, J. Making asbestos-cement products safe using heat treatment. Case Stud. Constr. Mater. 2019, 10, e00221. [Google Scholar] [CrossRef]
- Gualtieri, A.F. Recycling asbestos-containing material (ACM) from construction and demolition waste (CDW). In Handbook of Recycled Concrete and Demolition Waste; Woodhead Publishing: Cambridge, UK, 2013; pp. 500–525. [Google Scholar]
- Curado, A.; Nunes, L.J.R.; Carvalho, A.; Abrantes, J.; Lima, E.; Tomé, M. Recovery of end-of-life building materials: Thermal decomposition and phase transformation of chrysotile in asbestos-containing fiber cement boards. Fibers 2025, 13, 62. [Google Scholar] [CrossRef]
- Viani, A.; Gualtieri, A.F.; Pollastri, S.; Rinaudo, C.; Croce, A.; Urso, G. Crystal chemistry of the high temperature product of transformation of cement-asbestos. J. Hazard. Mater. 2013, 248–249, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Bloise, A.; Catalano, M.; Barrese, E.; Gualtieri, A.F.; Gandolfi, N.B.; Capella, S.; Belluso, E. TG/DSC study of the thermal behavior of hazardous mineral fibres. J. Therm. Anal. Calorim. 2016, 123, 2225–2239. [Google Scholar] [CrossRef]
- Bloise, A.; Kusiorowski, R.; Lassinantti Gualtieri, M.; Gualtieri, A.F. Thermal behaviour of mineral fibres. In Mineral Fibers: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union and the Mineralogical Society of Great Britain & Ireland Minerals: Twickenham, UK, 2017. [Google Scholar]
- Bloise, A. Thermal behaviour of actinolite asbestos. J. Mater. Sci. 2019, 54, 11784–11795. [Google Scholar] [CrossRef]
- Vergani, F.; Galimberti, L.; Marian, N.M.; Giorgetti, G.; Viti, C.; Capitani, G. Thermal decomposition of cement–asbestos at 1100 °C: How much “safe” is “safe”? J. Mater. Cycles Waste Manag. 2022, 24, 297–310. [Google Scholar] [CrossRef]
- Bloise, A. On the thermal breakdown of tremolite: A new method for distinguishing between asbestos and non-asbestos tremolite samples. J. Mater. Sci. 2023, 58, 8779–8795. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Gerle, A.; Kujawa, M.; Śliwa, A.; Adamek, J. Charaterisation of asbestos-containing wastes by thermal analysis. J. Therm. Anal. Calorim. 2024, 149, 10681–10694. [Google Scholar] [CrossRef]
- Langer, A.M. Reduction of the biological potential of chrysotile asbestos arising from conditions of service on brake pads. Regul. Toxicol. Pharmacol. 2003, 38, 71–77. [Google Scholar] [CrossRef]
- Takata, A.; Yamauchi, H.; Yamashita, K.; Aminaka, M.; Hitomi, T.; Toya, T.; Kohyama, N. Mesothelioma carcinogenesis of chrysotile and forsterite compared and validated by intraperitoneal injection in rat. Ind. Health 2025, 63, 14–28. [Google Scholar] [CrossRef]
- Regulation of the Minister of the Environment of 27 September 2001 on the Waste Catalog (Journal of Laws No. 112, Item 1206). Available online: https://www.dziennikustaw.gov.pl/DU/2001/s/112/1206 (accessed on 20 June 2025).
- Sejm of the Republic of Poland, Waste Act (Journal of Laws of 4 January 2018, Item 21). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20180000021 (accessed on 20 June 2025).
- Carneiro, G.O.; Santos, T.A.; Simonelli, G.; Ribeiro, D.V.; Cilla, M.S.; Dias, C.M.R. Thermal treatment optimization of asbestos cement waste (ACW) potentializing its use as alternative binder. J. Clean. Prod. 2021, 320, 128801. [Google Scholar] [CrossRef]
- Capitani, G.; Dalpiaz, M.; Vergani, F.; Campanale, F.; Conconi, R.; Odorizzi, S. Recycling thermally deactivated asbestos cement in mortar: A possible route towards a rapid conclusion of the “asbestos problem”. J. Environ. Manag. 2024, 355, 120507. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Rao, B.; Asolekar, S.R. Geopolymer-based solidification and stabilization for environmentally sound disposal of asbestos-containing waste. J. Mater. Cycles Waste Manag. 2025, 27, 75–90. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Tartaglia, A. Thermal decomposition of asbestos and recycling in traditional ceramics. J. Eur. Ceram. Soc. 2000, 20, 1409–1418. [Google Scholar] [CrossRef]
- Leonelli, C.; Veronesi, P.; Boccaccini, D.N.; Rivasi, M.R.; Barbieri, L.; Andreola, F.; Lancellotti, I.; Rabitti, D.; Pellacani, G.C. Microwave thermal inertisation of asbestos containing waste and its recycling in traditional ceramics. J. Hazard. Mater. 2006, 135, 149–155. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Cavenati, C.; Zanatto, I.; Meloni, M.; Elmi, G.; Gualtieri, M.L. The transformation sequence of cement–asbestos slates up to 1200 °C and safe recycling of the reaction product in stoneware tile mixtures. J. Hazard. Mater. 2008, 152, 563–570. [Google Scholar] [CrossRef]
- Zaremba, T.; Krząkała, A.; Piotrowski, J.; Garczorz, D. Utilization of chrysotile asbestos for sintering ceramics production. Ceram. Mater. 2010, 62, 149–155. (In Polish) [Google Scholar]
- Capitani, G.; Vergani, F.; Conconi, R.; Mrvar, P.; Bombač, D.; Perše, L.S.; Oseli, A.; Bizjan, B. Recycling thermally detoxified asbestos-cement in stone-wool: An end-less-life material. Constr. Build. Mater. 2024, 440, 137351. [Google Scholar] [CrossRef]
- Borges, R.; Do Amaral, L.F.M.; Velloso, C.C.V.; Lodi, L.A.; Jacumazo, J.; Wypych, F.; Ribeiro, C. Recent advances in asbestos bioremediation: A short review and comparison with other methods. Sustain. Sci. Technol. 2025, 2, 032002. [Google Scholar] [CrossRef]
- Iwaszko, J.; Lubas, M.; Sitarz, M.; Zajemska, M.; Nowak, A. Production of vitrified material from hazardous asbestos-cement waste and CRT glass cullet. J. Clean. Prod. 2021, 317, 128345. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Lipowska, B.; Gerle, A. Synthesis of ye’elimite from anthropogenic waste. Minerals 2023, 13, 137. [Google Scholar] [CrossRef]
- Viani, A.; Gualtieri, A.F. Recycling the product of thermal transformation of cement-asbestos for the preparation of calcium sulfoaluminate clinker. J. Hazard. Mater. 2013, 260, 813–818. [Google Scholar] [CrossRef]
- PN-EN ISO 12677:2011 Standard; Chemical Analysis of Refractory Products by X-ray Fluorescence (XRF)—Fused Cast-Bead Method. ISO: Geneva, Switzerland, 2011.
- ASTM C204-18:2018; Standard Test Methods for Fineness of Hydraulic Cement by Air-Permeability Apparatus. ASTM International: West Conshohocken, PA, USA, 2018.
- PN-EN 196-3:2016 Standard; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. The Polish Committee for Standardization: Warsaw, Poland, 2016.
- PN-EN 196-1:2016 Standard; Methods of Testing Cement—Part 1: Determination of Strength. The Polish Committee for Standardization: Warsaw, Poland, 2016.
- Flanagan, D.M. Mineral Commodity Summaries 2025 (ver. 1.2): U.S.; Geological Survey: Reston, VA, USA, 2025; p. 38. [Google Scholar]
- Long, J.; Liu, W.; Zhang, N.; Zhang, H.; Xiao, Q.; Huang, S. New insight into the phase transition and kinetics of the dehydroxylation of bulk-to-nano chrysotile. Phys. Chem. Miner. 2024, 51, 28. [Google Scholar] [CrossRef]
- Kim, C.; Kim, Y.; Roh, Y. Thermal decomposition and phase transformation of chrysotile in asbestos-containing waste. Minerals 2025, 15, 344. [Google Scholar] [CrossRef]
- Viani, A.; Gualtieri, A.F.; Mácová, P.; Pollastri, S. Transformations through pseudomorphosis of asbestos minerals in thermally processed asbestos-containing materials investigated through SEM/EDS and micro-Raman spectroscopy: Implications for recycling of hazardous wastes. In Proceedings of the 18th International Microscopy Congress, Prague, Czech Republic, 7–12 September 2014. [Google Scholar]
- Kusiorowski, R.; Gerle, A.; Kujawa, M.; Bloise, A. Kinetic aspects of chrysotile asbestos thermal decomposition process. Minerals 2025, 15, 609. [Google Scholar] [CrossRef]
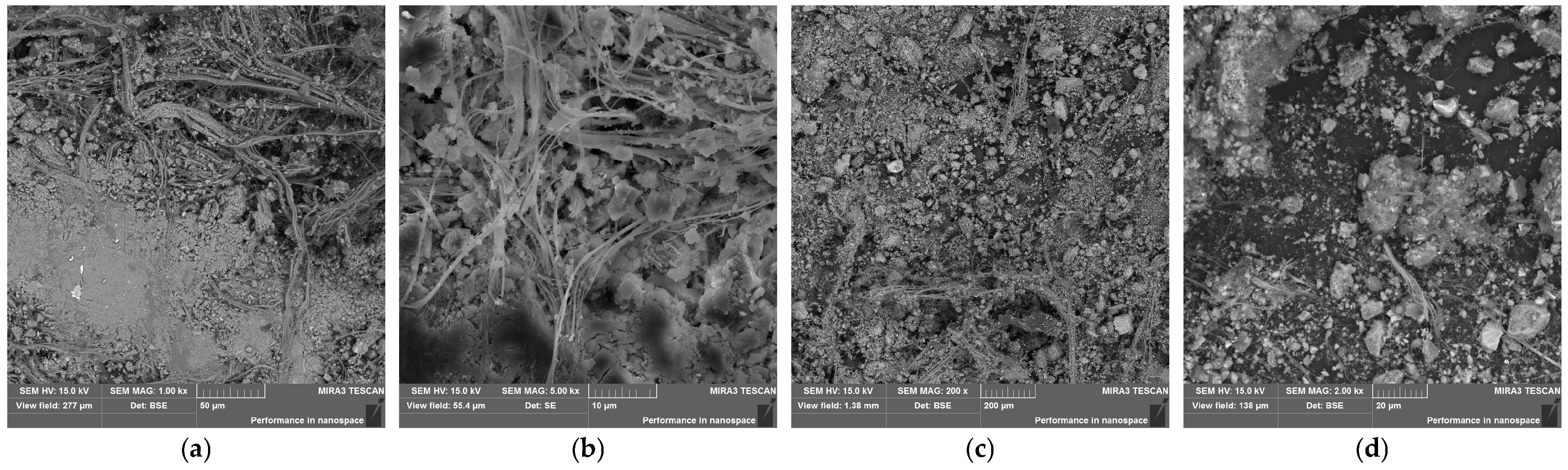

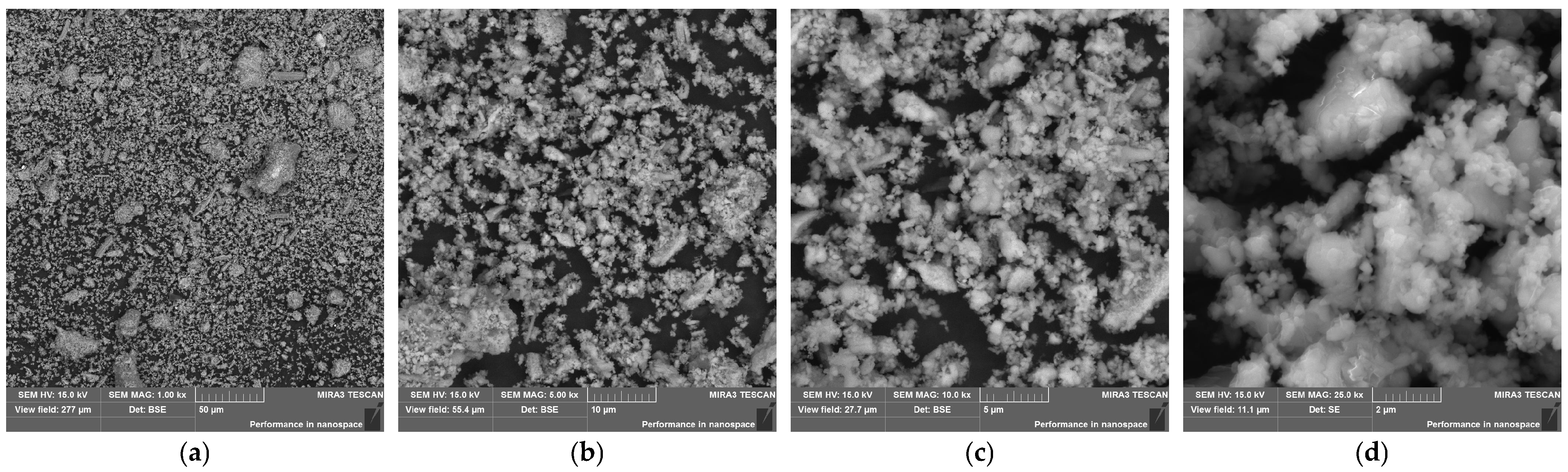

| Sample | Portland Cement | ACP |
|---|---|---|
| B0 | 100 | 0 |
| B5 | 95 | 5 |
| B10 | 90 | 10 |
| B15 | 85 | 15 |
| B20 | 80 | 20 |
| B25 | 75 | 25 |
| B30 | 70 | 30 |
| Sample | Portland Cement; g | ACP; g | Standard Sand; g | Water; g |
|---|---|---|---|---|
| M0 | 450 | 0 | 1350 | 225 |
| M5 | 427.5 | 22.5 | 1350 | 225 |
| M10 | 405 | 45 | 1350 | 225 |
| M15 | 382.5 | 67.5 | 1350 | 225 |
| M20 | 360 | 90 | 1350 | 225 |
| SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | Cr2O3 | SO3 | L.O.I. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25.86 | 0.22 | 5.08 | 3.75 | 0.07 | 8.02 | 54.17 | 0.16 | 0.18 | 0.12 | 0.04 | 2.32 | 0.10 |
| Phase | Raw | ACP |
|---|---|---|
| calcite | 18.0 ± 0.5 | - |
| vaterite | 0.7 ± 0.2 | - |
| portlandite | 3.1 ± 0.2 | - |
| chrysotile | 3.7 ± 0.2 | - |
| larnite | 2.6 ± 0.2 | 33.7 ± 0.4 |
| brownmillerite | - | 0.8 ± 0.1 |
| gehlenite | - | 0.9 ± 0.1 |
| ternesite | - | 2.1 ± 0.2 |
| periclase | - | 2.8 ± 0.2 |
| merwinite | - | 7.5 ± 0.3 |
| lime | - | 0.8 ± 0.2 |
| amorphous | 71.9 ± 0.7 | 51.4 ± 0.6 |
| Sample | Initial Setting Time | Final Setting Time | Setting Time |
|---|---|---|---|
| B0 | 125 | 210 | 85 |
| B5 | 130 | 215 | 85 |
| B10 | 120 | 245 | 125 |
| B15 | 90 | 240 | 150 |
| B20 | 90 | 225 | 135 |
| B25 | 25 | 130 | 105 |
| B30 | 10 | 25 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusiorowski, R.; Gerle, A.; Kujawa, M.; Śliwa, A. Influence of Thermally Treated Asbestos-Containing Materials on Cement Mortars Properties. Appl. Sci. 2025, 15, 9225. https://doi.org/10.3390/app15169225
Kusiorowski R, Gerle A, Kujawa M, Śliwa A. Influence of Thermally Treated Asbestos-Containing Materials on Cement Mortars Properties. Applied Sciences. 2025; 15(16):9225. https://doi.org/10.3390/app15169225
Chicago/Turabian StyleKusiorowski, Robert, Anna Gerle, Magdalena Kujawa, and Andrzej Śliwa. 2025. "Influence of Thermally Treated Asbestos-Containing Materials on Cement Mortars Properties" Applied Sciences 15, no. 16: 9225. https://doi.org/10.3390/app15169225
APA StyleKusiorowski, R., Gerle, A., Kujawa, M., & Śliwa, A. (2025). Influence of Thermally Treated Asbestos-Containing Materials on Cement Mortars Properties. Applied Sciences, 15(16), 9225. https://doi.org/10.3390/app15169225








