Radiological and Neuroradiological Features in Pediatric Mucopolysaccharidoses: A Retrospective Case Series from the Emilia-Romagna Regional Referral Center
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. MPS I
3.2. MPS II
3.3. MPS III
3.4. MPS IV
4. Discussion
4.1. MPS I
4.2. MPS II
4.3. MPS III
4.4. MPS IV
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPS | Mucopolysaccharidosis |
| ERT | Enzyme Replacement Therapy |
| GAG | Glycosaminoglycan |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| SDS | Standard Deviation Score |
| PVS | Perivascular Spaces |
| FLAIR | Fluid Attenuated Inversion Recovery |
| OSAS | Obstructive Sleep Apnea Syndrome |
References
- Filocamo, M.; Tomanin, R.; Bertola, F.; Morrone, A. Biochemical and molecular analysis in mucopolysaccharidoses: What a paediatrician must know. Ital. J. Pediatr. 2018, 44, 129. [Google Scholar] [CrossRef]
- Aldenhoven, M.; Sakkers, R.J.B.; Boelens, J.; De Koning, T.J.; Wulffraat, N.M. Musculoskeletal manifestations of lysosomal storage disorders. Ann. Rheum. Dis. 2009, 68, 1659–1665. [Google Scholar] [CrossRef]
- Galimberti, C.; Madeo, A.; Di Rocco, M.; Fiumara, A. Mucopolysaccharidoses: Early Diagnostic Signs in Infants and Children; BioMed Central Ltd.: London, UK, 2018. [Google Scholar] [CrossRef]
- Spina, V.; Barbuti, D.; Gaeta, A.; Palmucci, S.; Soscia, E.; Grimaldi, M.; Leone, A.; Manara, R.; Polonara, G. The role of imaging in the skeletal involvement of mucopolysaccharidoses. Ital. J. Pediatr. 2018, 44, 118. [Google Scholar] [CrossRef] [PubMed]
- Machnikowska-Sokołowska, M.; Myszczuk, A.; Wieszała, E.; Wieja-Błach, D.; Jamroz, E.; Paprocka, J. Mucopolysaccharidosis Type 1 among Children—Neuroradiological Perspective Based on Single Centre Experience and Literature Review. Metabolites 2023, 13, 209. [Google Scholar] [CrossRef]
- Palmucci, S.; Attinà, G.; Lanza, M.L.; Belfiore, G.; Cappello, G.; Foti, P.V.; Milone, P.; Di Bella, D.; Barone, R.; Fiumara, A.; et al. Imaging findings of mucopolysaccharidoses: A pictorial review. Insights Imaging 2013, 4, 443–459. [Google Scholar] [CrossRef]
- De Ponti, G.; Donsante, S.; Frigeni, M.; Pievani, A.; Corsi, A.; Bernardo, M.E.; Riminucci, M.; Serafini, M. MPSI Manifestations and Treatment Outcome: Skeletal Focus. Int. J. Mol. Sci. 2022, 23, 11168. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Arn, P.; Giugliani, R.; Muenzer, J.; Okuyama, T.; Taylor, J.; Fallet, S. The natural history of MPS I: Global perspectives from the MPS I Registry. Genet. Med. 2014, 16, 759–765. [Google Scholar] [CrossRef]
- Blau, N.; Duran, M.; Gibson, K.M.; Vici, C.D. Physician’s Guide to the Diagnosis, Treatment, and Follow-Up of Inherited Metabolic Diseases; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Baronio, F.; Zucchini, S.; Zulian, F.; Salerno, M.; Parini, R.; Cattoni, A.; Deodato, F.; Gaeta, A.; Bizzarri, C.; Gasperini, S.; et al. Proposal of an Algorithm to Early Detect Attenuated Type I Mucopolysaccharidosis (MPS Ia) among Children with Growth Abnormalities. Medicina 2022, 58, 97. [Google Scholar] [CrossRef] [PubMed]
- Zafeiriou, D.I.; Batzios, S.P. Brain and Spinal MR Imaging Findings in Mucopolysaccharidoses: A Review. Am. J. Neuroradiol. 2013, 34, 5–13. [Google Scholar] [CrossRef]
- Martin, R.; Beck, M.; Eng, C.; Giugliani, R.; Harmatz, P.; Muñoz, V.; Muenzer, J. Recognition and Diagnosis of Mucopolysaccharidosis II (Hunter Syndrome). Pediatrics 2008, 121, e377–e386. [Google Scholar] [CrossRef]
- Link, B.; de Camargo Pinto, L.L.; Giugliani, R.; Wraith, J.E.; Guffon, N.; Eich, E.; Beck, M. Orthopedic manifestations in patients with mucopolysaccharidosis type II (Hunter syndrome) enrolled in the Hunter Outcome Survey. Orthop. Rev. 2010, 2, e16. [Google Scholar] [CrossRef][Green Version]
- Parini, R.; Jones, S.A.; Harmatz, P.R.; Giugliani, R.; Mendelsohn, N.J. The natural history of growth in patients with Hunter syndrome: Data from the Hunter Outcome Survey (HOS). Mol. Genet. Metab. 2016, 117, 438–446. [Google Scholar] [CrossRef]
- Mao, S.J.; Chen, Q.Q.; Dai, Y.L.; Dong, G.P.; Zou, C.C. The diagnosis and management of mucopolysaccharidosis type II. Ital. J. Pediatr. 2024, 50, 207. [Google Scholar] [CrossRef]
- Wraith, J.E.; Scarpa, M.; Beck, M.; Bodamer, O.A.; De Meirleir, L.; Guffon, N.; Meldgaard Lund, A.; Malm, G.; Van der Ploeg, A.T.; Zeman, J. Mucopolysaccharidosis type II (Hunter syndrome): A clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur. J. Pediatr. 2008, 167, 267–277. [Google Scholar] [CrossRef]
- White, K.K.; Karol, L.A.; White, D.R.; Hale, S. Musculoskeletal Manifestations of Sanfilippo Syndrome (Mucopolysaccharidosis Type III). J. Pediatr. Orthop. 2011, 31, 594–598. [Google Scholar] [CrossRef]
- Harmatz, P.; Mengel, K.E.; Giugliani, R.; Valayannopoulos, V.; Lin, S.P.; Parini, R.; Guffon, N.; Burton, B.K.; Hendriksz, C.J.; Mitchell, J.J.; et al. The Morquio a Clinical Assessment Program: Baseline results illustrating progressive, multisystemic clinical impairments in Morquio A subjects. Mol. Genet. Metab. 2013, 109, 54–61. [Google Scholar] [CrossRef]
- Sung, J.; Kim, I.; Im, M.; Ahn, Y.J.; Kim, S.-M.; Jang, J.-H.; Park, H.-D.; Jeon, T.Y.; Ko, K.R.; Park, S.-J.; et al. Long-term outcomes of enzyme replacement therapy from a large cohort of Korean patients with mucopolysaccharidosis IVA (Morquio A syndrome). Mol. Genet. Metab. Rep. 2025, 42, 101189. [Google Scholar] [CrossRef] [PubMed]
- Reichert, R.; Campos, L.G.; Vairo, F.; de Souza, C.F.M.; Pérez, J.A.; Duarte, J.Á.; Leiria, F.A.; Anés, M.; Vedolin, L.M. Neuroimaging Findings in Patients with Mucopolysaccharidosis: What You Really Need to Know. Radiographics 2016, 36, 1448–1462. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.C.; Harvey, K.; Beck, M.; Burin, M.G.; Chien, Y.H.; Church, H.J.; D’Almeida, V.; van Diggelen, O.P.; Fietz, M.; Giugliani, R.; et al. Diagnosing mucopolysaccharidosis IVA. J. Inherit. Metab. Dis. 2013, 36, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Solanki, G.A.; Martin, K.W.; Theroux, M.C.; Lampe, C.; White, K.K.; Shediac, R.; Lampe, C.G.; Beck, M.; Mackenzie, W.G.; Hendriksz, C.J.; et al. Spinal involvement in mucopolysaccharidosis IVA (Morquio-Brailsford or Morquio A syndrome): Presentation, diagnosis and management. J. Inherit. Metab. Dis. 2013, 36, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Amanatullah, D.F.; Strauss, E.J.; Di Cesare, P.E. Current management options for osteonecrosis of the femoral head: Part 1, diagnosis and nonoperative management. Am. J. Orthop. 2011, 40, E186–E192. [Google Scholar] [PubMed]
- Concolino, D.; Deodato, F.; Parini, R. Enzyme replacement therapy: Efficacy and limitations. Ital. J. Pediatr. 2018, 44, 120. [Google Scholar] [CrossRef] [PubMed]
- Opoka-Winiarska, V.; Jurecka, A.; Emeryk, A.; Tylki-Szymańska, A. Osteoimmunology in mucopolysaccharidoses type I, II, VI and VII. Immunological regulation of the osteoarticular system in the course of metabolic inflammation. Osteoarthr. Cartil. 2013, 21, 1813–1823. [Google Scholar] [CrossRef]
- Tajima, G.; Sakura, N.; Kosuga, M.; Okuyama, T.; Kobayashi, M. Effects of idursulfase enzyme replacement therapy for Mucopolysaccharidosis type II when started in early infancy: Comparison in two siblings. Mol. Genet. Metab. 2013, 108, 172–177. [Google Scholar] [CrossRef]
- Tylki-Szymanska, A.; Jurecka, A.; Zuber, Z.; Rozdzynska, A.; Marucha, J.; Czartoryska, B. Enzyme replacement therapy for mucopolysaccharidosis II from 3 months of age: A 3-year follow-up. Acta Paediatr. 2012, 101, e42–e47. [Google Scholar] [CrossRef]
- McGill, J.J.; Inwood, A.C.; Coman, D.J.; Lipke, M.L.; De Lore, D.; Swiedler, S.J.; Hopwood, J.J. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age—A sibling control study. Clin. Genet. 2010, 77, 492–498. [Google Scholar] [CrossRef]
- Gabrielli, O.; Clarke, L.A.; Bruni, S.; Coppa, G.V. Enzyme-Replacement Therapy in a 5-Month-Old Boy With Attenuated Presymptomatic MPS I: 5-Year Follow-up. Pediatrics 2010, 125, e183–e187. [Google Scholar] [CrossRef]
- Montanari, G.; Candela, E.; Baronio, F.; Ferrari, V.; Biasucci, G.; Lanari, M.; Ortolano, R. Early-Onset Inherited Metabolic Diseases: When Clinical Symptoms Precede Newborn Screening-Insights from Emilia-Romagna (Italy). Children 2025, 12, 464. [Google Scholar] [CrossRef] [PubMed]



| Subtype of MPS | Patient | Sex | Age at Diagnosis | Current Clinical Signs and Symptoms | Therapy |
|---|---|---|---|---|---|
| MPS I | 1 | F | 2.6 years | Coarse facial features; Heart valve disease; Hepatosplenomegaly; OSAS; Umbilical hernia; Mild neurodevelopmental delay; Short stature (age 6: −0.94 SDS, −3.40 SDS from target height) | Aldurazyme (started at 3 years) |
| MPS II | 2 | M | 14 months | Developmental delay; Overgrowth up to 6 years old; Hearing loss; Severe OSAS; Dysphagia; Mild mitral valve disease; Short stature (−3.43 SDS at 10, age of exitus) | Elaprase (started at 14 months) |
| MPS II | 3 | M | 4 years | Coarse features; Cardiomyopathy, heart valve disease; Hearing loss; Hepatosplenomegaly; Joint contractures; Short stature (final height −4.32 SDS); Carpal tunnel syndrome; Mild neurodevelopmental delay; OSAS | Elaprase (started at 15 years) |
| MPS IIIB | 4 | M | 1 year | Hepatosplenomegaly; Umbilical and inguinal hernia; Psychomotor regression; Agitation; Sleep disturbance; Dysphagia; Pes cavus; Recurrent otitis; Coarse facial features; Macroglossia; Short stature (−3.75 SDS at 14 years 6 months) | No therapy |
| MPS IV | 5 | F | 1.5 years | Short stature (−2.37 SDS at 2 years and 6 months); Normal psychomotor development; Facial asymmetry with left-sided hypoplasia; Short neck; Pectus carinatum; Limited elbow extension; | Vimizim (started at 23 months) |
| MPS IV | 6 | M | 8.9 years | Joint stiffness; Flat feet; Mandibular laterodeviation; Hypermetropia and astigmatism; Short trunk; Pectus carinatum; Umbilical hernia; Short stature (final height: −3.45 SDS) | Vimizim (started at 8.9 years) |
| MPS IV | 7 | F | 11 years | Developmental delay (onset 4–5 years); Coarse facial features; Corneal clouding; Joint hyperlaxity; Severe short stature (−7.81 SDS at 11) with a short trunk; Pectus carinatum | No therapy |
| MPS IV | 8 | M | 17 years | Developmental delay (onset ~5 years); Coarse facial features; Corneal clouding; OSAS; Severe short stature (−10.61 SDS at 17) with a short trunk; Pectus carinatum; Short limbs; Joint hyperlaxity (except for contractures of inferior limbs); Macrobrachycephaly | No therapy |
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| MPS Type | I | II | II | III | IV | IV | IV | IV |
| Short stature | + | + | + | + | + | + | + | + |
| Overgrowth in the first years of life | + | |||||||
| Claw hands | + | |||||||
| Odontoid hypoplasia | + | + | + | + | ||||
| Craniocervical instability | + | + | ||||||
| Pectus carinatum | + | + | + | + | ||||
| Genu valgum | + | + | + | + | ||||
| Scoliosis | + | + | + | + | + | |||
| Lumbar hyperlordosis | + | + | ||||||
| Hip dysplasia | + | + | + | + | + | + | + | + |
| Dysmorphic long bones | + | + | + | + | + | + | ||
| Radio–ulnar deformity | + | + | + | |||||
| Oar-shaped ribs | + | + | + | + | + | + | ||
| Short, wide clavicles | + | |||||||
| Platyspondyly with beaking of vertebral bodies | + | + | + | + | + | + | + | + |
| Macro and dolicocephaly | + | |||||||
| Thickened skull | + | + | ||||||
| J-shaped turcica | + | + | + | + | ||||
| Kyphosis | + | + | + | + | + | + |
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| MPS Type | I | II | II | III | IV | IV | IV | IV |
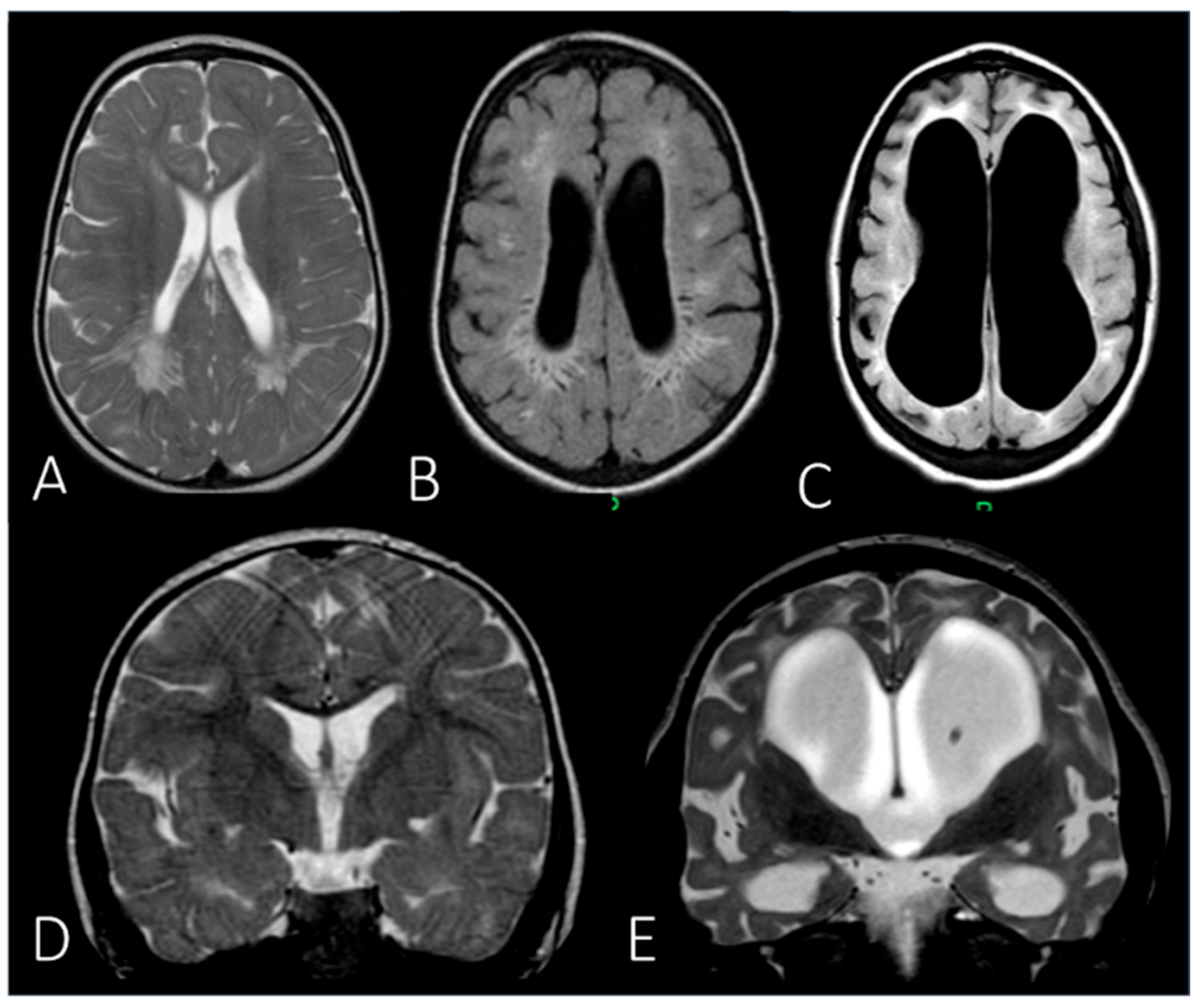
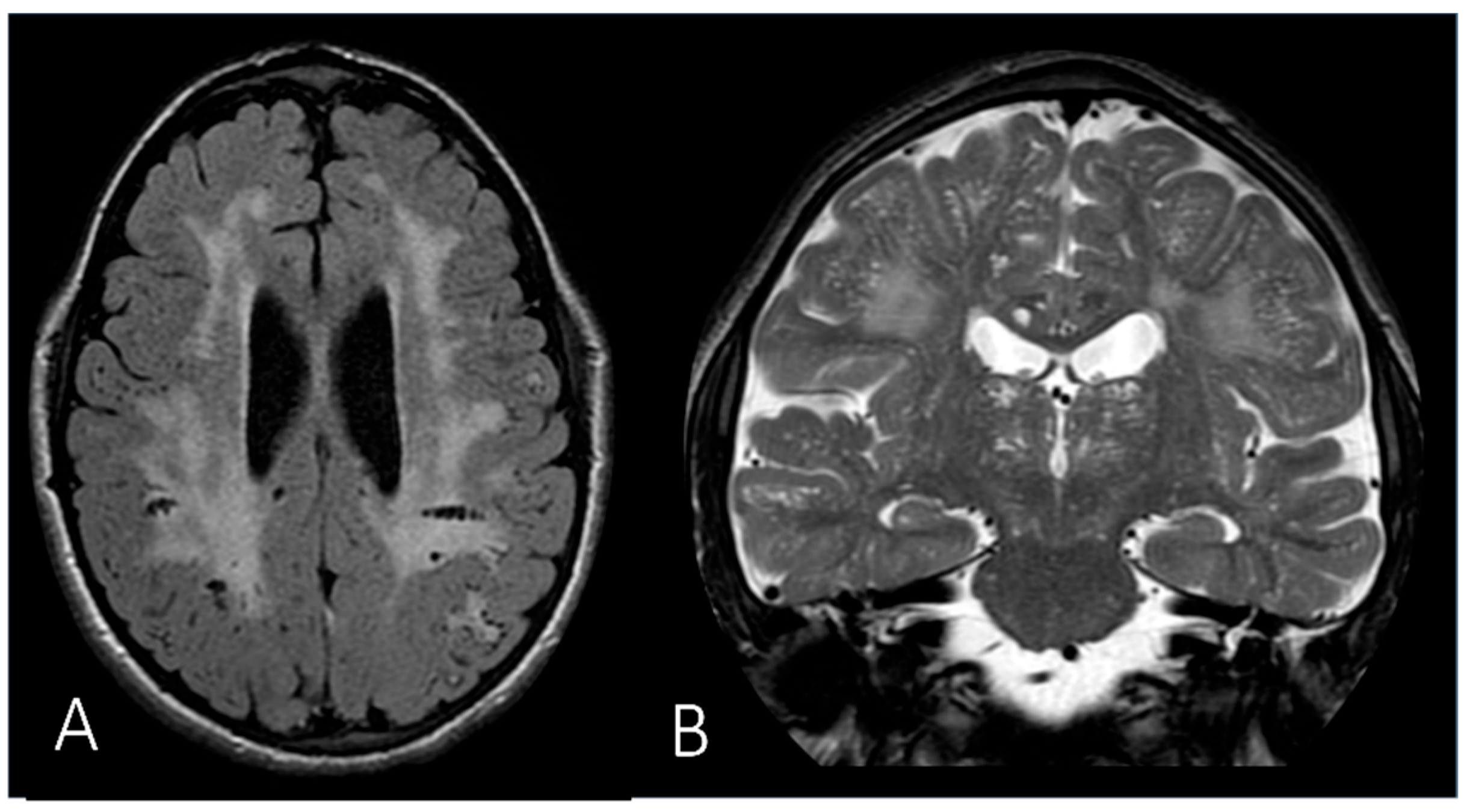
| White matter abnormality | + (mild) | + | + | N.A. | + | |||
| Brain atrophy | + | + | N.A. | |||||
| Hydrocephalus | + (mild) | + | N.A. | |||||
| Spinal canal/ foramen magnum stenosis | N.A. | + | + | + | + | |||
| Enlarged perivascular spaces | + | + | + | N.A. | ||||
| Odontoid hypoplasia | N.A. | + | + | + | + | |||
| Compressive myelopathy | N.A. | + | + | + |
| Patient | Timepoint | Imaging Modality | Location of Lesions | Key Findings |
|---|---|---|---|---|
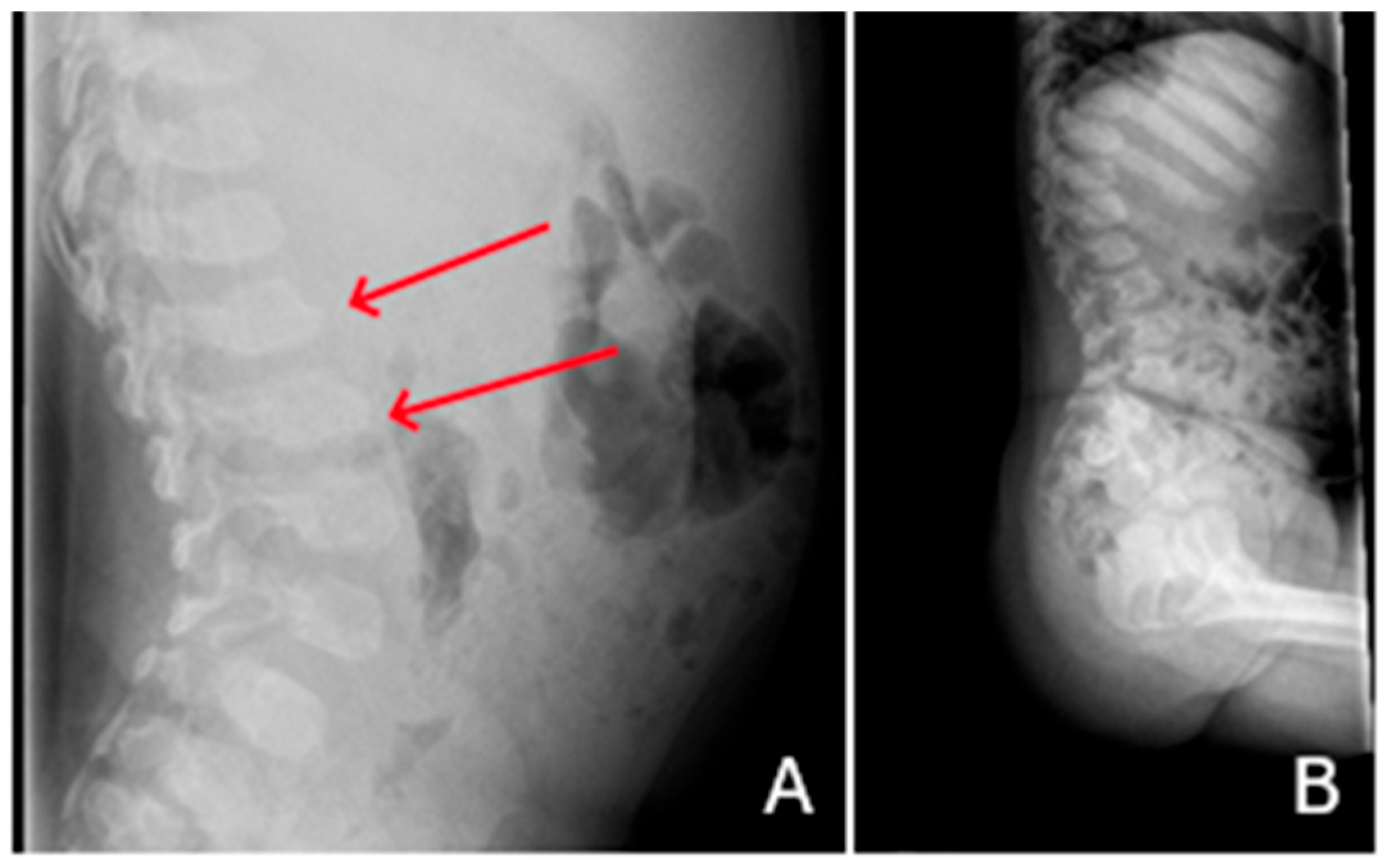
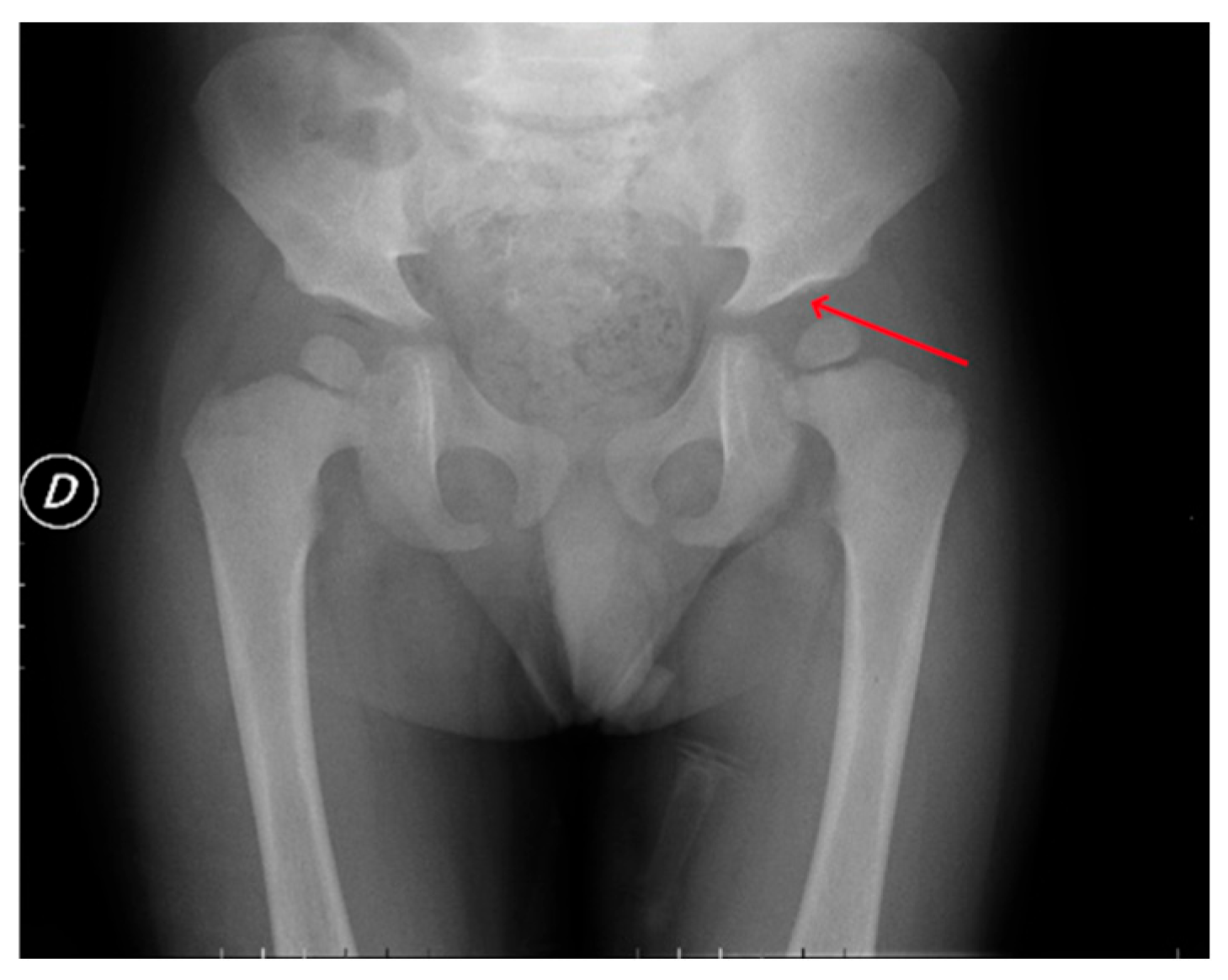
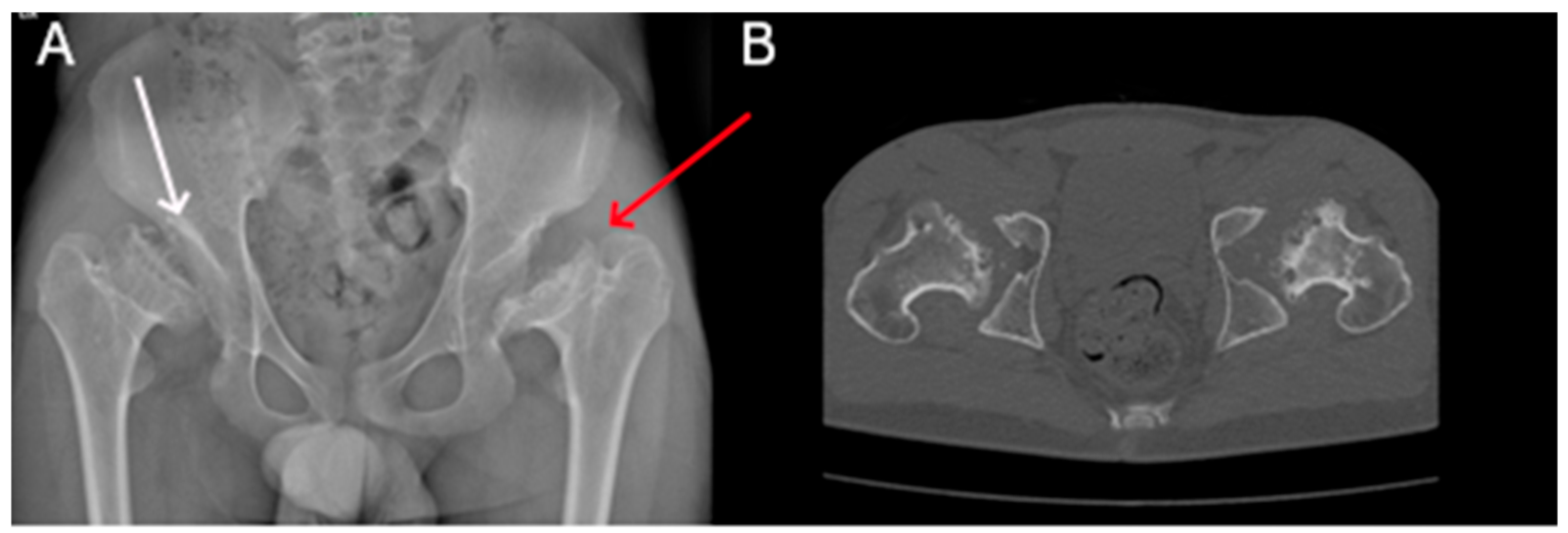
| P1 (MPSI) | Baseline | RX | Pelvis | Mild iliac wing hypoplasia; shifty acetabular roofs. |
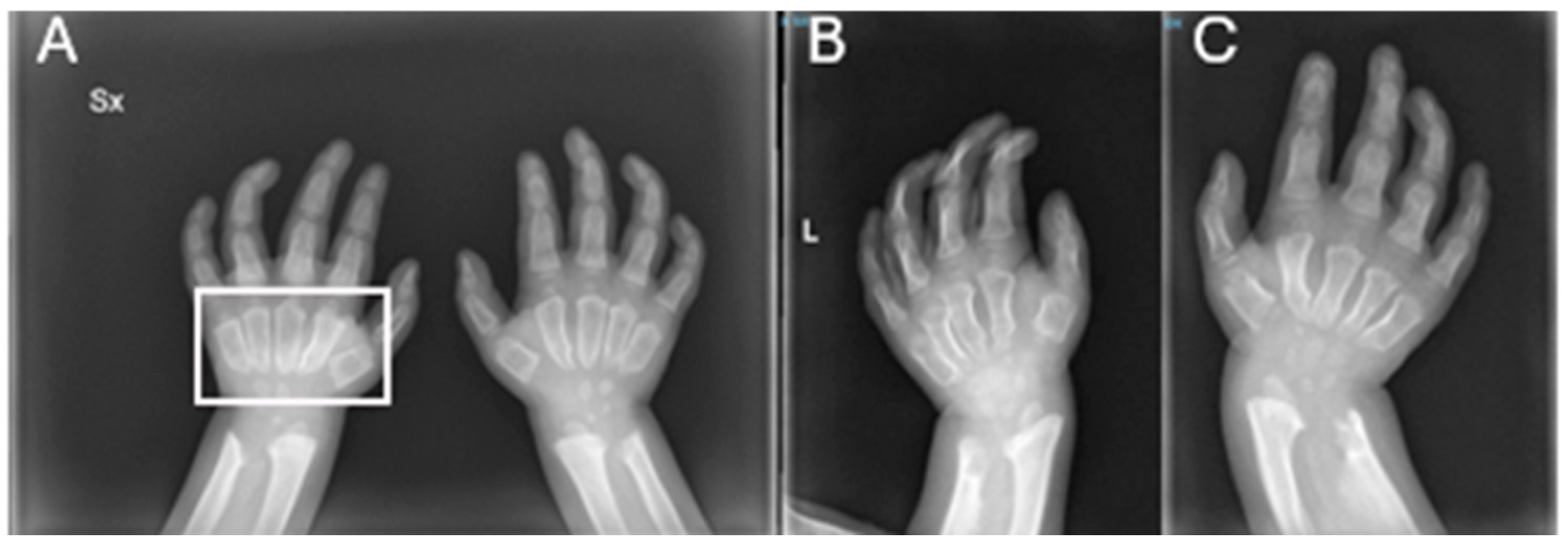
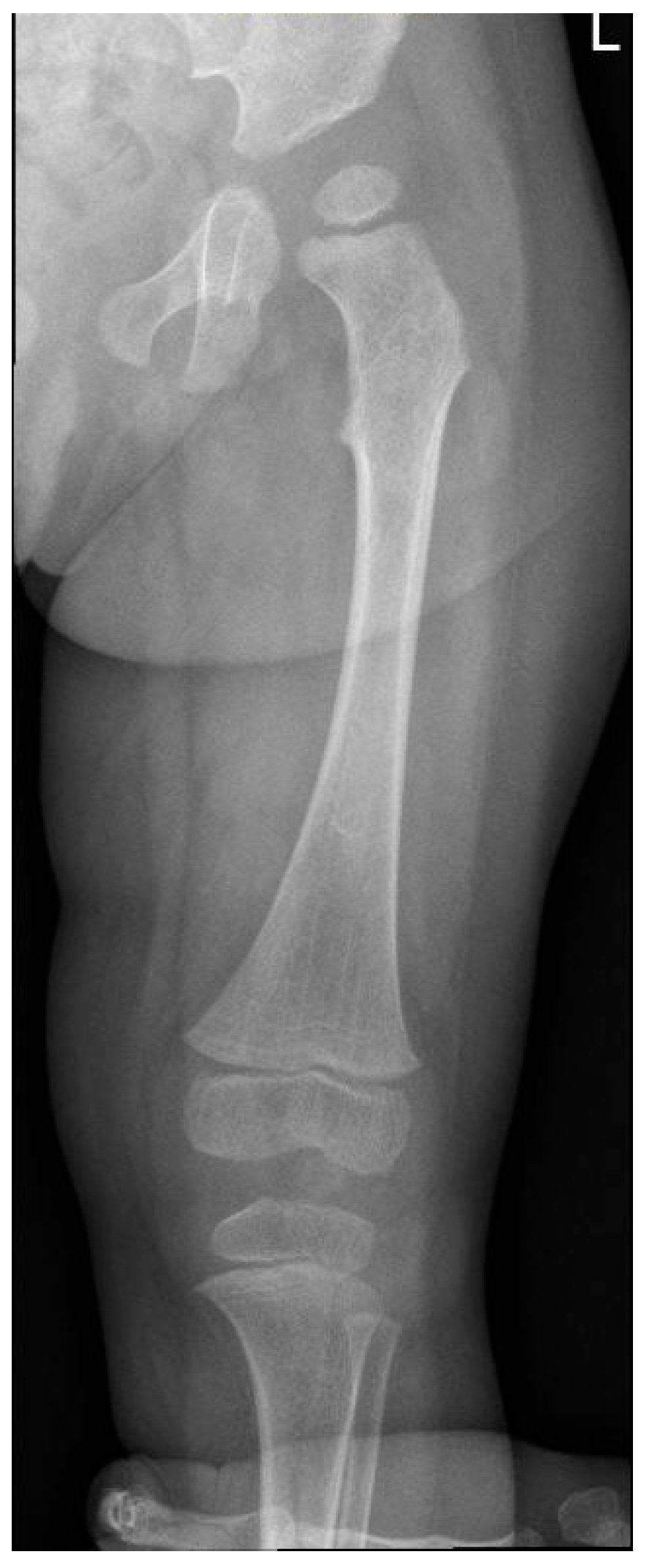
| RX | Hands | Short, thickened metacarpal bones; tapered proximal ends. | ||
| After 3 years of ERT | RX | Pelvis | Thickening of the acetabular roofs. | |
| RX | Hands | Progression of the metacarpal deformities; malformative aspects of radius and ulna. | ||
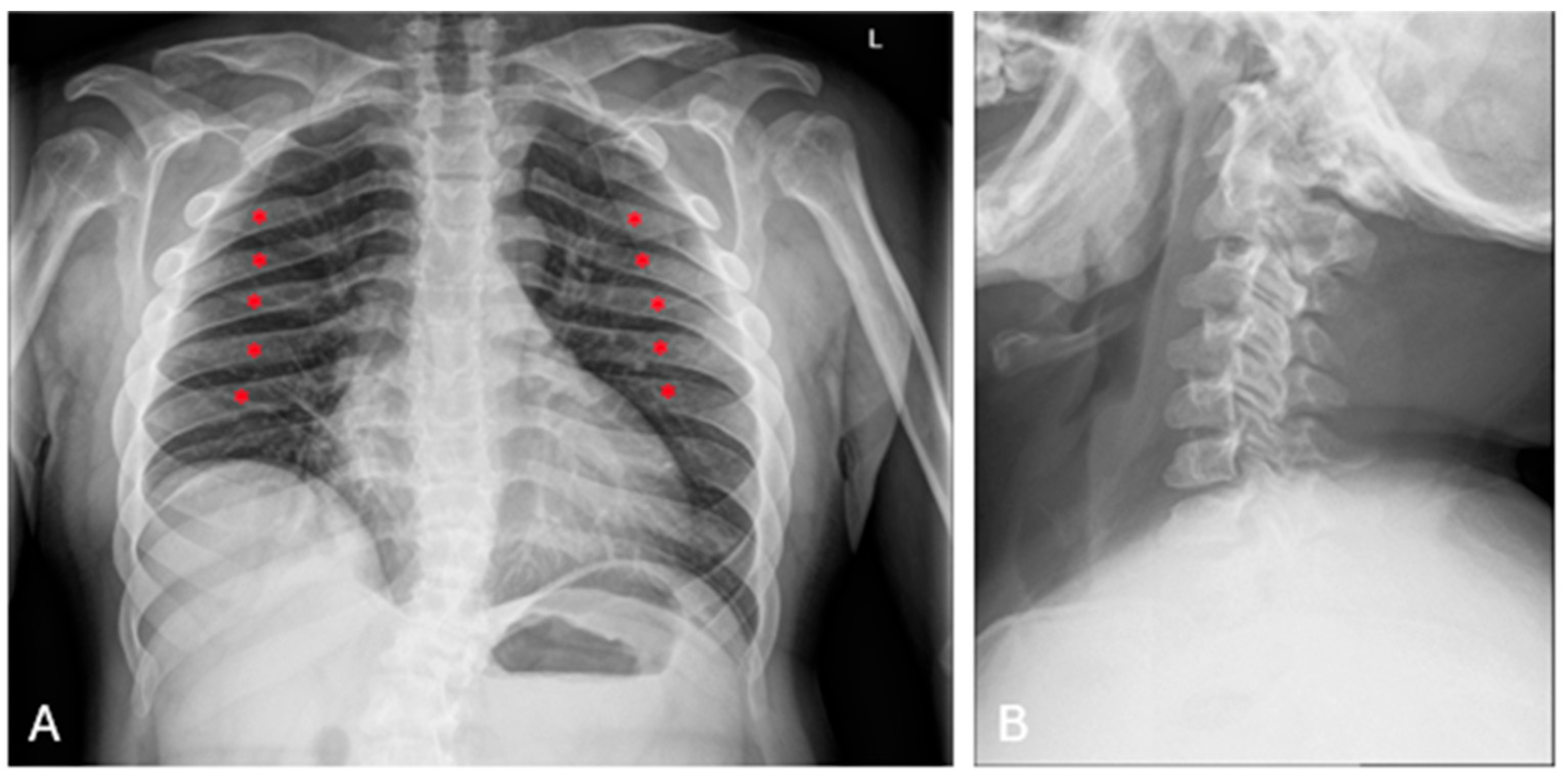
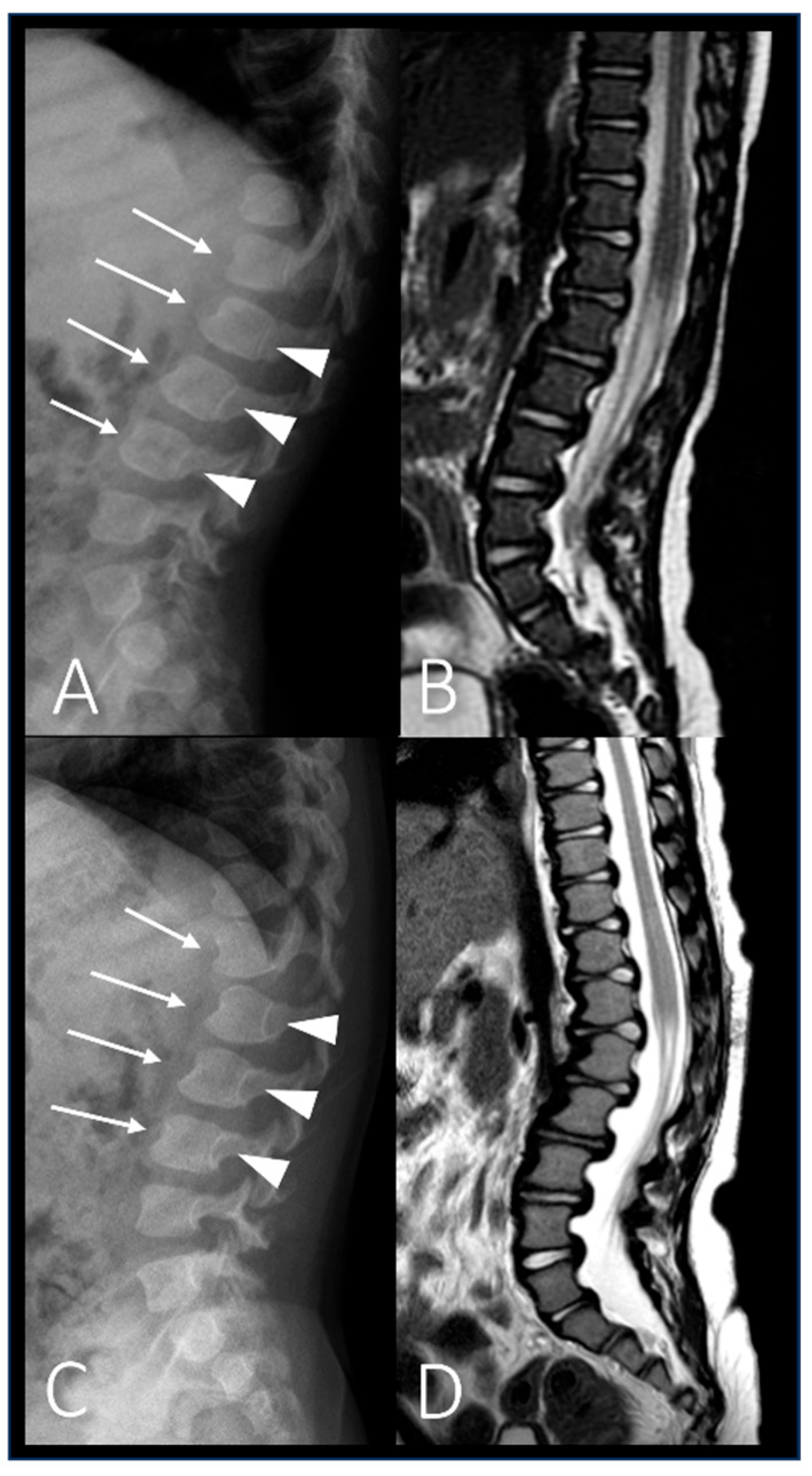
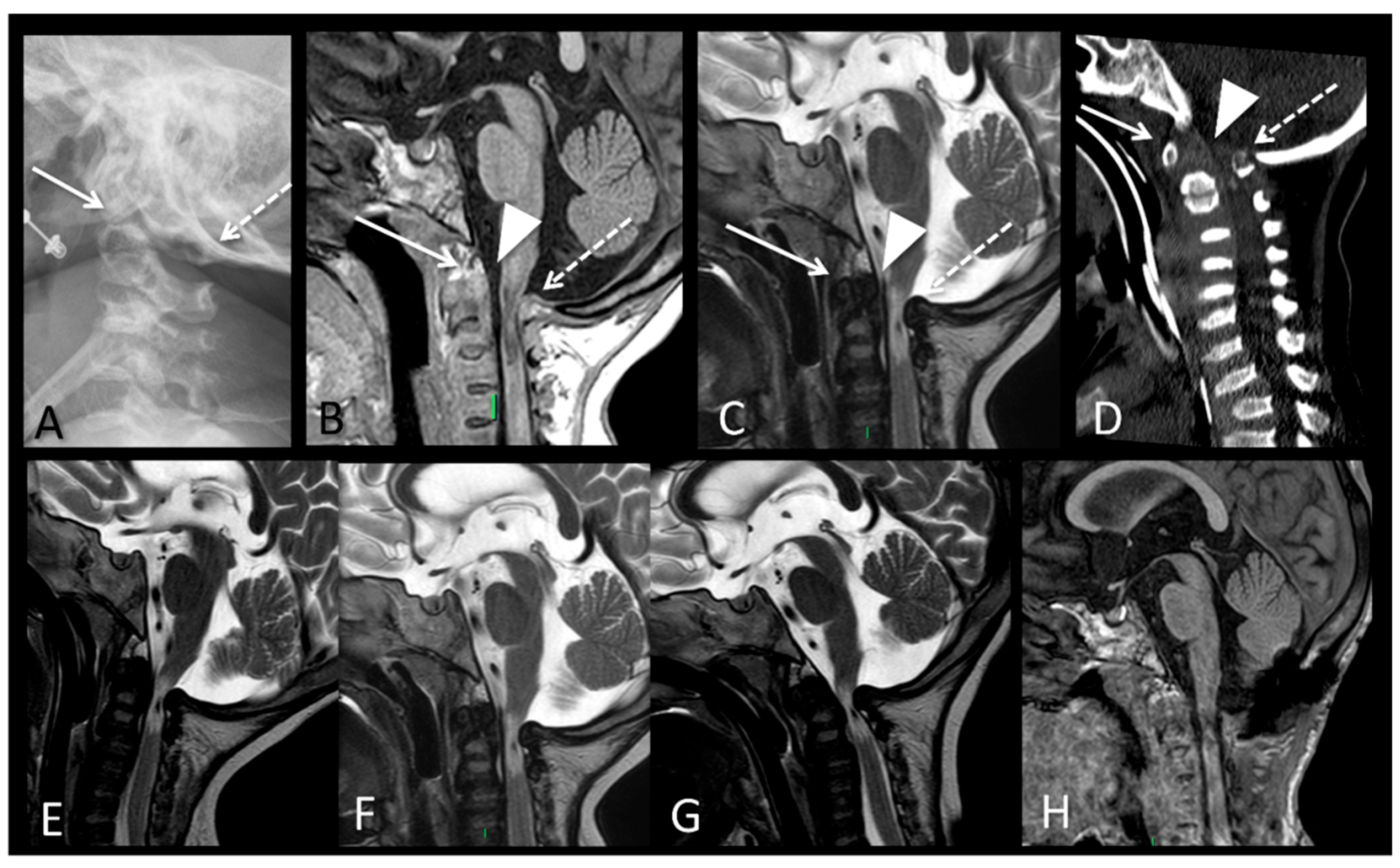
| P2 (MPSII) | Baseline | RX and MRI | Spine | Vertebral body deformities; posterior bulging of the intervertebral discs. |
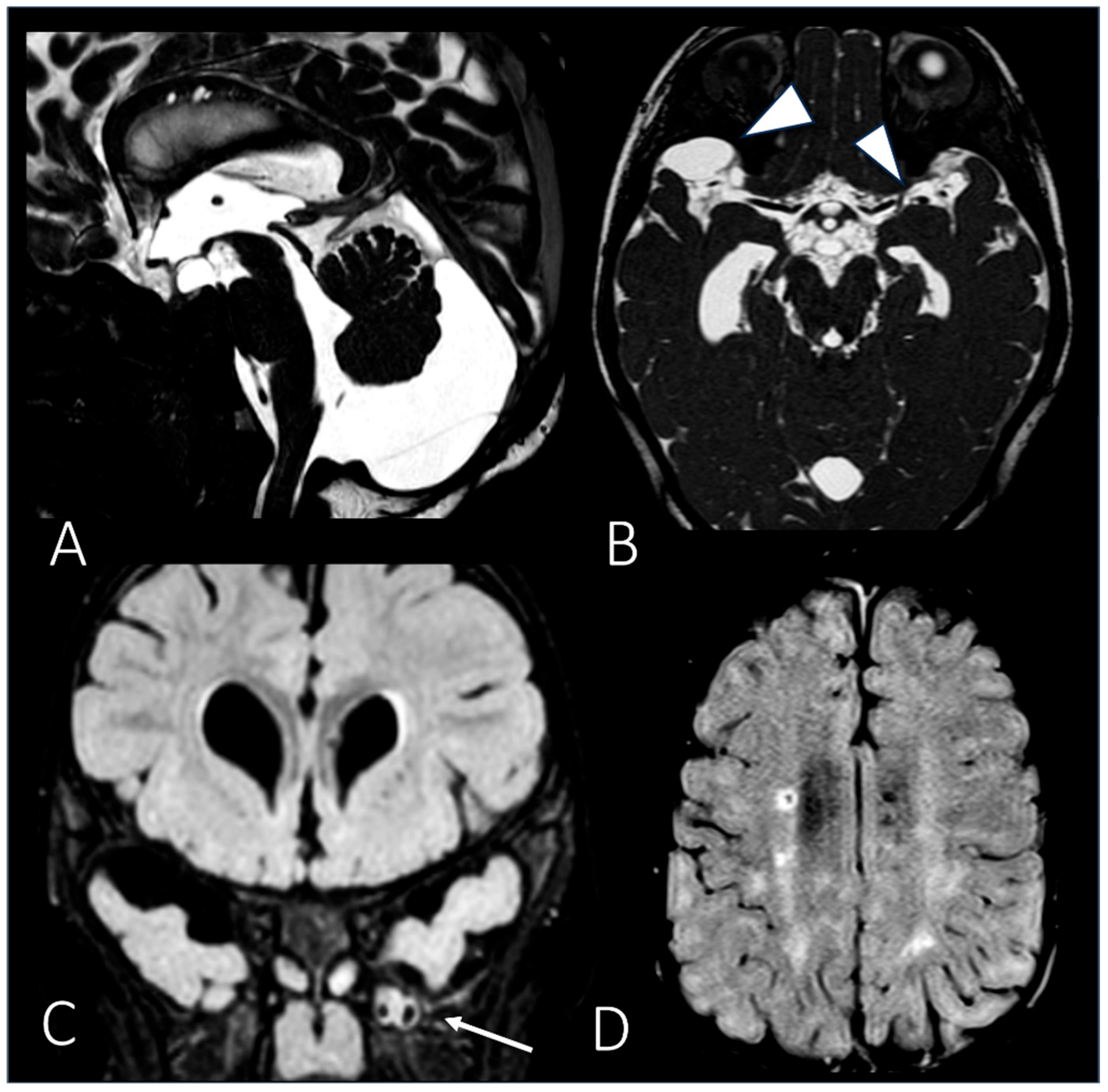
| MRI | Brain | Periventricular white matter abnormalities associated with enlarged perivascular spaces. | ||
| After 6 years of ERT | RX and MRI | Spine | Thoracolumbar kyphosis; anterior beaking and posterior scalloping of vertebral bodies. | |
| MRI | Brain | Extension of the white matter signal alterations; severe dilation of the ventricular system; cerebral atrophy. | ||
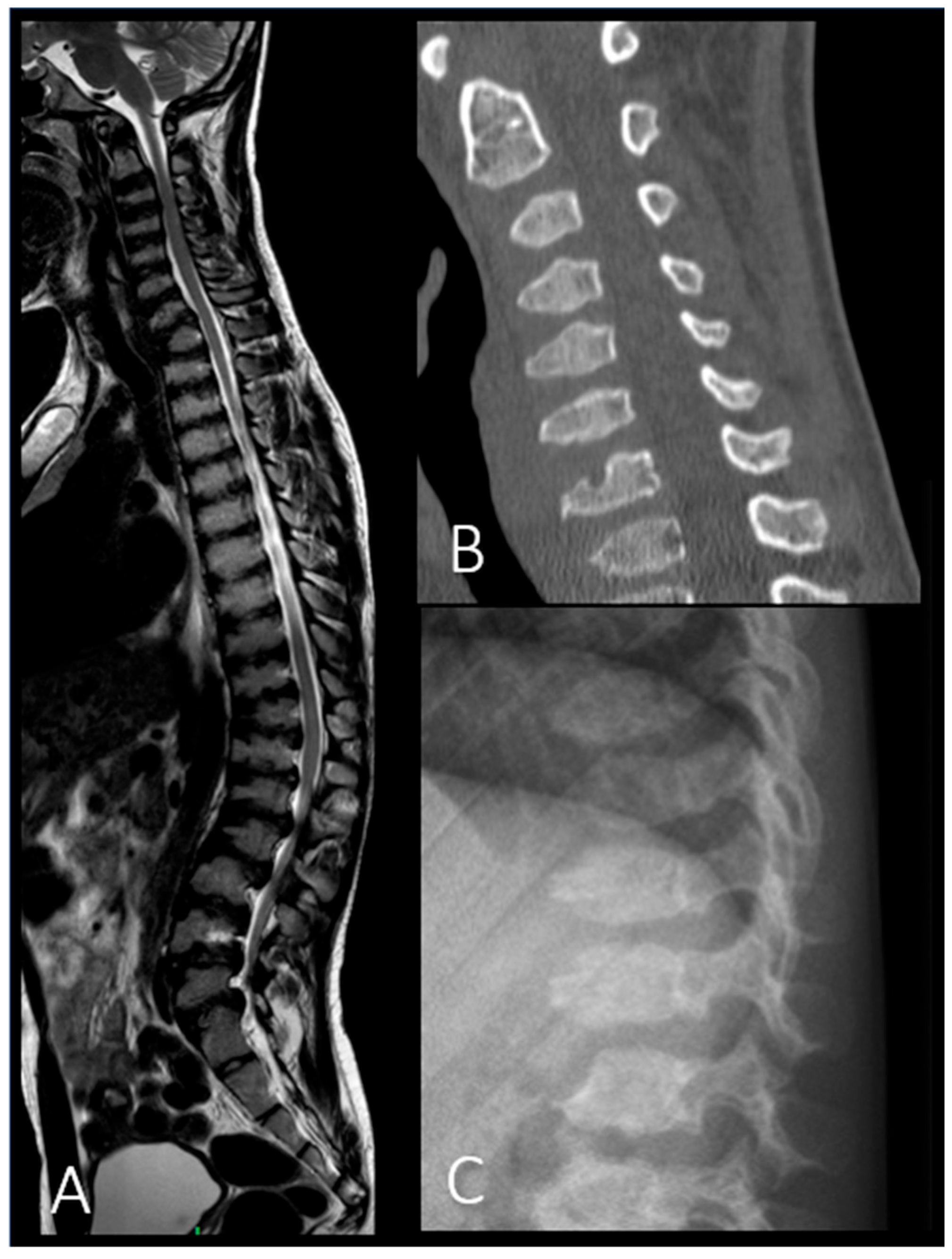
| P6 (MPSIV) | Baseline | CT | Spine | Platyspondyly in both cervical and thoracolumbar regions; anterior vertebral body beaking. |
| After 8 years of ERT | MRI | Spine | Deformities of the vertebral bodies and posterior bulging of the intervertebral discs; spinal canal impression. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.; Bortolamedi, E.; Baldazzi, M.; Toni, F.; Ortolano, R.; Candela, E.; Biasucci, G.; Lanari, M.; Baronio, F. Radiological and Neuroradiological Features in Pediatric Mucopolysaccharidoses: A Retrospective Case Series from the Emilia-Romagna Regional Referral Center. Appl. Sci. 2025, 15, 9093. https://doi.org/10.3390/app15169093
Silva G, Bortolamedi E, Baldazzi M, Toni F, Ortolano R, Candela E, Biasucci G, Lanari M, Baronio F. Radiological and Neuroradiological Features in Pediatric Mucopolysaccharidoses: A Retrospective Case Series from the Emilia-Romagna Regional Referral Center. Applied Sciences. 2025; 15(16):9093. https://doi.org/10.3390/app15169093
Chicago/Turabian StyleSilva, Giovanni, Elisa Bortolamedi, Michelangelo Baldazzi, Francesco Toni, Rita Ortolano, Egidio Candela, Giacomo Biasucci, Marcello Lanari, and Federico Baronio. 2025. "Radiological and Neuroradiological Features in Pediatric Mucopolysaccharidoses: A Retrospective Case Series from the Emilia-Romagna Regional Referral Center" Applied Sciences 15, no. 16: 9093. https://doi.org/10.3390/app15169093
APA StyleSilva, G., Bortolamedi, E., Baldazzi, M., Toni, F., Ortolano, R., Candela, E., Biasucci, G., Lanari, M., & Baronio, F. (2025). Radiological and Neuroradiological Features in Pediatric Mucopolysaccharidoses: A Retrospective Case Series from the Emilia-Romagna Regional Referral Center. Applied Sciences, 15(16), 9093. https://doi.org/10.3390/app15169093









