Could Hydroinfiltrators Made with Biochar Modify the Soil Microbiome? A Strategy of Soil Nature-Based Solution for Smart Agriculture
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site and Soil
2.2. Metagenomic Analysis of Soil at Different Depths near Olive Trees with and Without Biochar Hydroinfiltrators
2.2.1. DNA Extraction
2.2.2. Sequencing of 16S rRNA Gene Amplicons
2.2.3. Indexes of Bacterial Diversity
2.3. Microbiological Study Under In Vitro Conditions
2.3.1. Bacterial Strains
2.3.2. Culture Media and Conditions
2.3.3. Tolerance Tests and Determination of Enzymatic Activities
2.3.4. Experiments with Substrates of Soil and Biochar
3. Results and Discussion
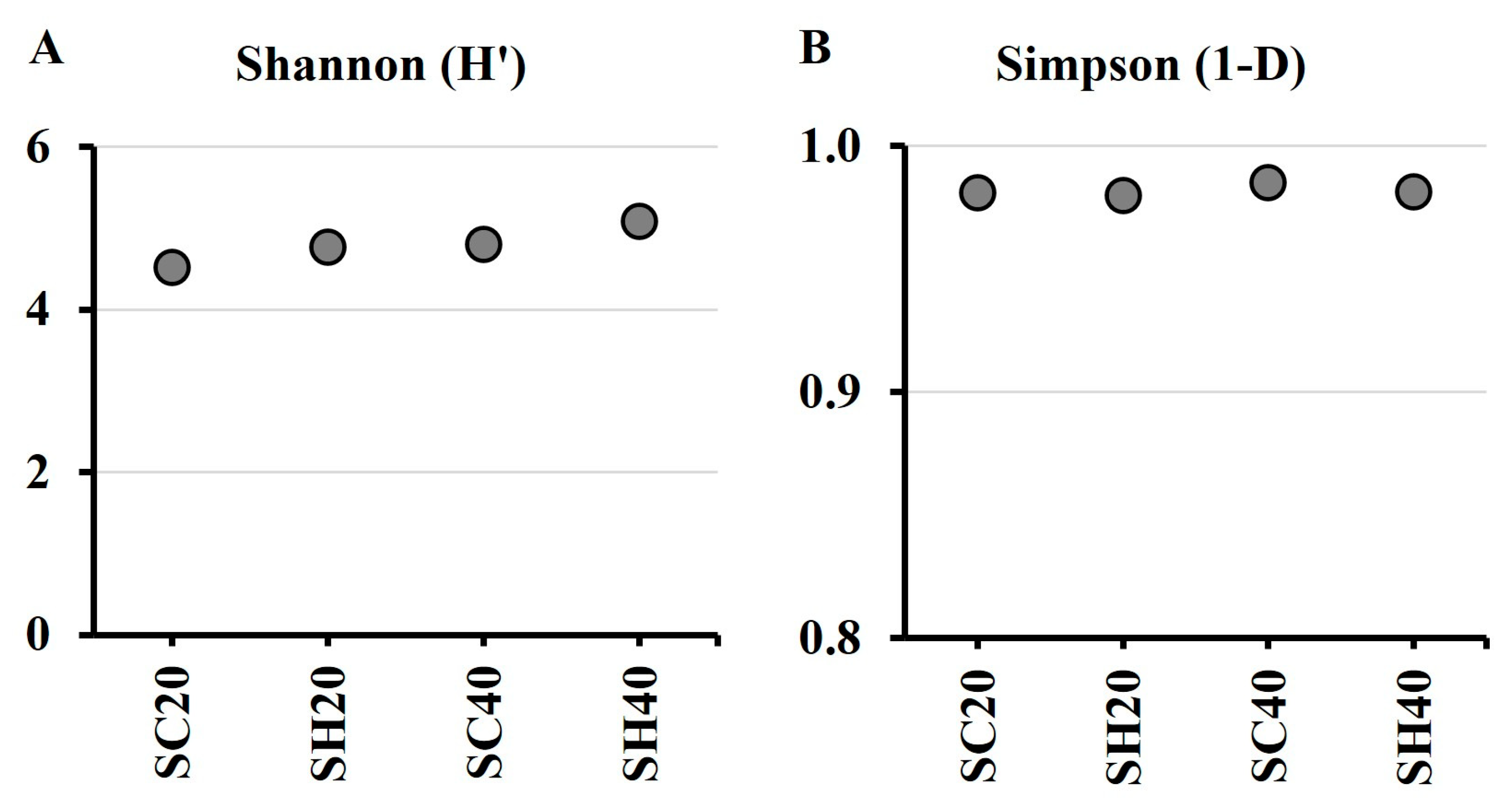
3.1. Metagenomic Analysis of Soil Samples with and Without Hydroinfiltrators
3.2. In Vitro Microbiological Study
3.2.1. Strain Selection Based on Tolerance Tests and Enzymatic Activities
3.2.2. Experiments with Substrates of Soil and Biochar
3.3. Influence of Biochar Hydroinfiltrators on Microbial Communities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Strain | Taxonomic Identification | Location | Origin |
|---|---|---|---|
| P6 | Pseudomonas segetis | Rhizosphere of Salicornia europea | Saladar El Margen (Granada, Spain) |
| B38 | Psychrobacter sp. | Endophyte (aerial part) of Salicornia hispanica | Saladar El Margen (Granada, Spain) |
| B23 | Kushneria endophytica | Endophyte (aerial part) of Arthrocaulon | Saladar El Margen (Granada, Spain) |
| N3 | Peribacillus castrilensis | Otter faeces | Castril (Granada) |
| 8C | Bacillus siamensis | Endophyte (aerial part) of Salicornia europea | Salobral de Ocaña (Toledo, Spain) |
| 11C | Bacillus cabrialesii | Endophyte (root) of Caroxylon vermiculatum | Salobral de Ocaña (Toledo, Spain) |
| 14C | Bacillus siamensis | Saline soil | Salobral de Ocaña (Toledo, Spain) |
| 15C | Pseudomonas neuropathica | Saline soil | Salobral de Ocaña (Toledo, Spain) |
| Strains | % NaCl | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.5 | 2 | 3.5 | 6 | 7.5 | 10 | 15 | 20 | |
| P6 | |||||||||
| B38 | |||||||||
| B23 | |||||||||
| N3 | |||||||||
| 8C | |||||||||
| 11C | |||||||||
| 14C | |||||||||
| 15C | |||||||||
| Strain | % Polyethylene Glycol (PEG) | |||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | |
| P6 | 0.702 | 0.664 | 0.530 | 0.653 | 0 | 0 |
| N3 | 1.151 | 1.257 | 1.293 | 1.065 | 0 | 0.035 |
| B23 | 1.343 | 1.455 | 1.418 | 1.080 | 0.105 | 0.050 |
| B38 | 1.686 | 1.809 | 1.492 | 1.254 | 0.114 | 0.093 |
| 8C | ND | 1.075 | 0.736 | 0.571 | 0.222 | 0.220 |
| 11C | 1.099 | 0.948 | 0.869 | 0.665 | 0.214 | 0.217 |
| 14C | 0.765 | 0.722 | 0.977 | 0.975 | 0.243 | 0.160 |
| 15C | 0.913 | 0.697 | 0.463 | 0.545 | 0.159 | 0.111 |
| Strain | Nase | ACC Desaminase | Alkaline Phosphatase | Acid Phosphatase | Fitase | Caseinase | Amilase |
|---|---|---|---|---|---|---|---|
| P6 | + | − | + | + | + | − | − |
| B23 | + | − | + | − | + | + | + |
| B38 | − | − | − | + | + | − | + |
| N3 | + | − | − | − | + | − | − |
| 8C | + | + | − | − | + | + | + |
| 11C | + | − | + | − | + | + | + |
| 14C | + | + | − | − | + | + | + |
| 15C | + | + | − | + | + | + | + |
References
- Mishra, J.; Prakash, J.; Arora, N.K. Role of Beneficial Soil Microbes in Sustainable Agriculture and Environmental Management. Clim. Change Environ. Sustain. 2016, 4, 137. [Google Scholar] [CrossRef]
- Rojano-Cruz, R.; Martínez-Moreno, F.J.; Galindo-Zaldívar, J.; Lamas, F.; González-Castillo, L.; Delgado, G.; Párraga, J.; Ramírez-González, V.; Durán-Zuazo, V.H.; Cárceles-Rodríguez, B.; et al. Impacts of a Hydroinfiltrator Rainwater Harvesting System on Soil Moisture Regime and Groundwater Distribution for Olive Groves in Semi-Arid Mediterranean Regions. Geoderma 2023, 438, 116623. [Google Scholar] [CrossRef]
- Li, S.; Yang, L.; Jiang, T.; Ahmed, W.; Mei, F.; Zhang, J.; Zhang, T.; Yang, Y.; Peng, X.; Shan, Q.; et al. Unraveling the Role of Pyrolysis Temperature in Biochar-Mediated Modulation of Soil Microbial Communities and Tobacco Bacterial Wilt Disease. Appl. Soil Ecol. 2025, 206, 105845. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management; Routledge: London, UK, 2015. [Google Scholar]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of Microbial Communities to Biochar-Amended Soils: A Critical Review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Zhu, L.; Xiao, Q.; Shen, Y.; Li, S. Effects of Biochar and Maize Straw on the Short-Term Carbon and Nitrogen Dynamics in a Cultivated Silty Loam in China. Environ. Sci. Pollut. Res. Int. 2017, 24, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The Mechanisms of Biochar Interactions with Microorganisms in Soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.d.C.; Rocha-Granados, M.d.C.; Glick, B.R.; Santoyo, G. Microbiome Engineering to Improve Biocontrol and Plant Growth-Promoting Mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.M.; Elrys, A.S.; El-Mageed, T.A.; Semida, W.M.; Abdelkhalik, A.; Mosa, W.F.A.; Al Kafaas, S.S.; et al. Halotolerant Plant Growth-Promoting Rhizobacteria Improve Soil Fertility and Plant Salinity Tolerance for Sustainable Agriculture. Plant Stress 2024, 12, 100482. [Google Scholar] [CrossRef]
- Tu, Q.; Tang, S.; Huang, S. Mitigation of Salinity Stress via Improving Growth, Chlorophyll Contents and Antioxidants Defense in Sunflower with Bacillus pumilis and Biochar. Sci. Rep. 2025, 15, 9641. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group. WRB World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Rome, Italy, 2022. [Google Scholar]
- Gabriel, D.C.-F.; Juan Manuel, M.G.; Raúl, R.C.; José Ángel, R.H. Dispositivo Infiltrador; Universidad de Granada: Granada, Spain, 2019. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.C. Venny 2.1. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 20 January 2025).
- Schloss, P.D. Rarefaction Is Currently the Best Approach to Control for Uneven Sequencing Effort in Amplicon Sequence Analyses. mSphere 2024, 9, e0035423. [Google Scholar] [CrossRef] [PubMed]
- Hammer, D.A.T.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Khan, A.; Rao, T.S. Molecular Evolution of Xenobiotic Degrading Genes and Mobile DNA Elements in Soil Bacteria. In Microbial Diversity in the Genomic Era; Elsevier: Amsterdam, The Netherlands, 2018; pp. 657–678. ISBN 9780128148501. [Google Scholar]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-stimulated Plant Performance Is Strongly Linked to Microbial Diversity and Metabolic Potential in the Rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef]
- Sun, D.; Meng, J.; Chen, W. Effects of Abiotic Components Induced by Biochar on Microbial Communities. Acta Agric. Scand. B Soil Plant Sci. 2013, 63, 633–641. [Google Scholar] [CrossRef]
- Hu, L.; Cao, L.; Zhang, R. Bacterial and Fungal Taxon Changes in Soil Microbial Community Composition Induced by Short-Term Biochar Amendment in Red Oxidized Loam Soil. World J. Microbiol. Biotechnol. 2014, 30, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Eilers, K.G.; Debenport, S.; Anderson, S.; Fierer, N. Digging Deeper to Find Unique Microbial Communities: The Strong Effect of Depth on the Structure of Bacterial and Archaeal Communities in Soil. Soil Biol. Biochem. 2012, 50, 58–65. [Google Scholar] [CrossRef]
- Saxena, J.; Rana, G.; Pandey, M. Impact of Addition of Biochar Along with Bacillus sp. on Growth and Yield of French Beans. Sci. Hortic. 2013, 162, 351–356. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The Potential of Biochar as a Microbial Carrier for Agricultural and Environmental Applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Cui, L.; Zhang, M.; Wang, B. Characterization and Identification of a Chlorine-Resistant Bacterium, Sphingomonas TS001, from a Model Drinking Water Distribution System. Sci. Total Environ. 2013, 458–460, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Romanyà, J.; Li, N.; Xiang, Y.; Yang, J.; Han, X. Biochar Fertilization Effects on Soil Bacterial Community and Soil Phosphorus Forms Depends on the Application Rate. Sci. Total Environ. 2022, 843, 157022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Q.; Wang, D.; Shu, Y.-G.; Shi, H. Memory Effect on the Survival of Deinococcus radiodurans after Exposure in Near Space. Microbiol. Spectr. 2023, 11, e0347422. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Kong, Q.; Zheng, Y.; Zheng, H.; Liu, Y.; Cheng, Y.; Zhang, X.; Li, Z.; You, X.; Li, Y. Co-Application of Biochar and Pyroligneous Acid Improved Peanut Production and Nutritional Quality in a Coastal Soil. Environ. Technol. Innov. 2022, 28, 102886. [Google Scholar] [CrossRef]
- Guo, M.; Shang, X.; Ma, Y.; Zhang, K.; Zhang, L.; Zhou, Y.; Gong, Z.; Miao, R. Biochars Assisted Phytoremediation of Polycyclic Aromatic Hydrocarbons Contaminated Agricultural Soil: Dynamic Responses of Functional Genes and Microbial Community. Environ. Pollut. 2024, 345, 123476. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, X.; Awasthi, M.K.; Li, H.; Zhang, L.; Syed, A.; Bahkali, A.H.; Verma, M. Bacterial Community Diversity and Co-Occurrence Networks in Biochar as a Sustainable Soil Amendment Material for Apple Orchards. Ind. Crops Prod. 2023, 206, 117723. [Google Scholar] [CrossRef]
- Chen, J.; Aihemaiti, A.; Xia, Y.; Yan, F.; Zhang, Z. The Effect of Soil Amendment Derived from P-Enhanced Sludge Pyrochar on Ryegrass Growth and Soil Microbial Diversity. Sci. Total Environ. 2022, 813, 152526. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus Species in Soil as a Natural Resource for Plant Health and Nutrition. J. Appl. Microbiol. 2019, 128, 1583–1594. [Google Scholar] [CrossRef]
- Habibi, S.; Djedidi, S.; Prongjunthuek, K.; Mortuza, M.F.; Ohkama-Ohtsu, N.; Sekimoto, H.; Yokoyoma, T. Physiological and Genetic Characterization of Rice Nitrogen Fixer PGPR Isolated from Rhizosphere Soils of Different Crops. Plant Soil 2014, 379, 51–66. [Google Scholar] [CrossRef]
- Ding, X.; Peng, X.J.; Jin, B.S.; Xiao, M.; Chen, J.K.; Li, B.; Fang, C.M.; Nie, M. Spatial Distribution of Bacterial Communities Driven by Multiple Environmental Factors in a Beach Wetland of the Largest Freshwater Lake in China. Front. Microbiol. 2015, 6, 129. [Google Scholar] [CrossRef]
- Yousuf, J.; Thajudeen, J.; Alikunju, A.P.; Joseph, A.; Sukumaran, D.P.; Varghese, A.; Abdulla, M.H. Diversity and Activity of Culturable Nitrogen Fixing Heterotrophic Bacteria from Estuarine and Coastal Environments of Southeastern Arabian Sea (SEAS). Reg. Stud. Mar. Sci. 2020, 33, 100973. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate Solubilizing Microbes: Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Panjiar, N.; Kumar, S.; Saxena, A.K.; Suman, A. Assessment of Genetic Diversity and Plant Growth Promoting Attributes of Psychrotolerant Bacteria Allied with Wheat (Triticum aestivum) from the Northern Hills Zone of India. Ann. Microbiol. 2015, 65, 1885–1899. [Google Scholar] [CrossRef]
- Shakeel, M.; Rais, A.; Hassan, M.N.; Hafeez, F.Y. Root Associated Bacillus sp. Improves Growth, Yield and Zinc Translocation for Basmati Rice (Oryza sativa) Varieties. Front. Microbiol. 2015, 6, 1286. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rajawat, M.V.S.; Kaushik, R.; Prasanna, R.; Saxena, A.K. Beneficial Role of Endophytes in Biofortification of Zn in Wheat Genotypes Varying in Nutrient Use Efficiency Grown in Soils Sufficient and Deficient in Zn. Plant Soil 2017, 416, 107–116. [Google Scholar] [CrossRef]
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, I.C.; Ali, B. Auxin Production by Rhizobacteria Was Associated with Improved Yield of Wheat (Triticum aestivum L.) under Drought Stress. Arch. Agron. Soil Sci. 2018, 64, 574–587. [Google Scholar] [CrossRef]
- Danchin, A. Exploring Overlooked Growth-Promoting Mechanisms by Plant-Associated Bacteria. Sustain. Microbiol. 2024, 1, qvae011. [Google Scholar] [CrossRef]
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of Salt Stress in Wheat Seedlings by Halotolerant Bacteria Isolated from Saline Habitats. Springerplus 2013, 2, 6. [Google Scholar] [CrossRef]
- Goswami, D.; Dhandhukia, P.; Patel, P.; Thakker, J.N. Screening of PGPR from Saline Desert of Kutch: Growth Promotion in Arachis Hypogea by Bacillus licheniformis A2. Microbiol. Res. 2014, 169, 66–75. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Liu, J.; Zhang, Z.; Chen, M.; Yue, L.; Lu, W.; Ji, S.; Wang, D.; Zhu, H.; et al. Enzymatic Degradation, Antioxidant and Rheological Properties of a Sphingan WL Gum from Sphingomonas sp. WG. Int. J. Biol. Macromol. 2022, 210, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Mulissa, J.M.; Carolin, R.L.; Ruth, A.S.; Fassil, A. Phosphate Solubilization and Multiple Plant Growth Promoting Properties of Rhizobacteria Isolated from Chickpea (Cicer aeritinum L.) Producing Areas of Ethiopia. Afr. J. Biotechnol. 2016, 15, 1899–1912. [Google Scholar] [CrossRef]
- Lowman, S.; Kim-Dura, S.; Mei, C.; Nowak, J. Strategies for Enhancement of Switchgrass (Panicum virgatum L.) Performance under Limited Nitrogen Supply Based on Utilization of N-Fixing Bacterial Endophytes. Plant Soil 2016, 405, 47–63. [Google Scholar] [CrossRef]
- Yu, F.B.; Shan, S.D.; Luo, L.P.; Guan, L.B.; Qin, H. Isolation and Characterization of a Sphingomonas sp. Strain F-7 Degrading Fenvalerate and Its Use in Bioremediation of Contaminated Soil. J. Environ. Sci. Health Part B 2013, 48, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wong, M.H.; Wong, Y.S.; Tam, N.F.Y. Multi-Factors on Biodegradation Kinetics of Polycyclic Aromatic Hydrocarbons (PAHs) by Sphingomonas sp. a Bacterial Strain Isolated from Mangrove Sediment. Mar. Pollut. Bull. 2008, 57, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Halo, B.A.; Khan, A.L.; Waqas, M.; Al-Harrasi, A.; Hussain, J.; Ali, L.; Adnan, M.; Lee, I.J. Endophytic Bacteria (Sphingomonas sp. LK11) and Gibberellin Can Improve Solanum lycopersicum Growth and Oxidative Stress under Salinity. J. Plant Interact. 2015, 10, 117–125. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From Diversity and Genomics to Functional Role in Environmental Remediation and Plant Growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Sbissi, I.; Chouikhi, F.; Ghodhbane-Gtari, F.; Gtari, M. Ecogenomic Insights into the Resilience of Keystone Blastococcus Species in Extreme Environments: A Comprehensive Analysis. BMC Genom. 2025, 26, 51. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent Advances on the Mechanisms of Kidney Stone Formation (Review). Int. J. Mol. Med. 2021, 48, 149. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of Plant Growth Promoting Rhizobacteria for Sustainable Development in Agriculture. Microbiol Res 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Xu, H.; Rice, S.A.; Chong, T.H.; Oh, H.S. Development of a Quorum Quenching-Column to Control Biofouling in Reverse Osmosis Water Treatment Processes. J. Ind. Eng. Chem. 2021, 94, 188–194. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zhou, X.; Guo, D.; Zhao, J.-H.; Yan, L.; Feng, G.-Z.; Gao, Q.; Yu, H.; Zhao, L.-P. Soil PH Is the Primary Factor Driving the Distribution and Function of Microorganisms in Farmland Soils in Northeastern China. Ann. Microbiol. 2019, 69, 1461–1473. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Z.; Zhao, H.; Hu, L.; Dahlgren, R.A.; Xu, J. Heavy Metal Effects on Multitrophic Level Microbial Communities and Insights for Ecological Restoration of an Abandoned Electroplating Factory Site. Environ. Pollut. 2023, 327, 121548. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yinghua, Z.; Xiu, Z.; Jing, S. Effects of Biochar on Bacterial Genetic Diversity in Soil Contaminated with Cadmium. Soil Use Manag. 2020, 37, 289–298. [Google Scholar] [CrossRef]
- Shang, X.; Wu, S.; Liu, Y.; Zhang, K.; Guo, M.; Zhou, Y.; Zhu, J.; Li, X.; Miao, R. Rice Husk and Its Derived Biochar Assist Phytoremediation of Heavy Metals and PAHs Co-Contaminated Soils but Differently Affect Bacterial Community. J. Hazard. Mater. 2024, 466, 133684. [Google Scholar] [CrossRef]
- Yang, F.; Wang, W.; Wu, Z.; Peng, J.; Xu, H.; Ge, M.; Lin, S.; Zeng, Y.; Sardans, J.; Wang, C.; et al. Fertilizer Reduction and Biochar Amendment Promote Soil Mineral-Associated Organic Carbon, Bacterial Activity, and Enzyme Activity in a Jasmine Garden in Southeast China. Sci. Total Environ. 2024, 954, 176300. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, X.; Suo, F.; You, X.; Yuan, Y.; Cheng, Y.; Zhang, C.; Li, Y. Effect of Biochar and Hydrochar from Cow Manure and Reed Straw on Lettuce Growth in an Acidified Soil. Chemosphere 2022, 298, 134191. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing Soil Microbial Diversity Is Associated with Decreasing Microbial Biomass under Nitrogen Addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Wang, B.; Teng, Y.; Xu, Y.; Chen, W.; Ren, W.; Li, Y.; Christie, P.; Luo, Y. Effect of Mixed Soil Microbiomes on Pyrene Removal and the Response of the Soil Microorganisms. Sci. Total Environ. 2018, 640–641, 9–17. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Barakat, M.; Verméglio, A.; Achouak, W.; Heulin, T. The Bacterial Genus Ramlibacter: Betaproteobacteria Capable of Surviving in Oligotrophic Environments Thanks to Several Shared Genetic Adaptation Traits. Environ. Microbiol. 2025, 27, e70059. [Google Scholar] [CrossRef]
- Radl, V.; Simões-Araújo, J.L.; Leite, J.; Passos, S.R.; Martins, L.M.V.; Xavier, G.R.; Rumjanek, N.G.; Baldani, J.I.; Zilli, J.E. Microvirga vignae sp. Nov., a Root Nodule Symbiotic Bacterium Isolated from Cowpea Grown in Semi-Arid Brazil. Int. J. Syst. Evol. Microbiol. 2014, 64, 725–730. [Google Scholar] [CrossRef]
- Ardley, J.K.; Parker, M.A.; De Meyer, S.E.; Trengove, R.D.; O’Hara, G.W.; Reeve, W.G.; Yates, R.J.; Dilworth, M.J.; Willems, A.; Howieson, J.G. Microvirga lupini sp. Nov., Microvirga lotononidis sp. Nov. and Microvirga zambiensis sp. Nov. Are Alphaproteobacterial Root-Nodule Bacteria That Specifically Nodulate and Fix Nitrogen with Geographically and Taxonomically Separate Legume Hosts. Int. J. Syst. Evol. Microbiol. 2012, 62, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, J.; Qiao, C.; Yang, J.; Li, J.; Zheng, X.; Wang, C.; Cao, P.; Li, Y.; Chen, Q. Rhizosphere Bacterial Community Is Mainly Determined by Soil Environmental Factors, but the Active Bacterial Diversity Is Mainly Shaped by Plant Selection. BMC Microbiol. 2024, 24, 450. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Du, M.; Ruan, Y.; Wang, Y.; Guo, J.; Yang, Q.; Shao, R.; Wang, H. Metagenomic Insights into Microbial Variation and Carbon Cycling Function in Crop Rotation Systems. Sci. Total Environ. 2024, 947, 174529. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Guo, X.; Wang, D.; Chu, H. Effects of Long-Term Application of Chemical and Organic Fertilizers on the Abundance of Microbial Communities Involved in the Nitrogen Cycle. Appl. Soil. Ecol. 2015, 95, 171–178. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Li, D.; Wang, H.; Chen, F.; Fu, X.; Fang, X.; Sun, X.; Yu, G. Impacts of Nitrogen and Phosphorus Additions on the Abundance and Community Structure of Ammonia Oxidizers and Denitrifying Bacteria in Chinese Fir Plantations. Soil Biol. Biochem. 2016, 103, 284–293. [Google Scholar] [CrossRef]
- Frêne, C.; Núñez-Ávila, M.; Castro, B.; Armesto, J.J. Seasonal Partitioning of Rainfall in Second-Growth Evergreen Temperate Rainforests in Chiloé Island, Southern Chile. Front. For. Glob. Change 2022, 4, 781663. [Google Scholar] [CrossRef]
- Motta Escobar, S.; Salazar Cabezas, L.D.; Sánchez Leal, L.C. Perspectiva Del Uso de Pseudomonas spp. Como Biocontrol de Fitopatógenos En Cultivos de Hortalizas En Colombia: Una Revisión Sistemática. Rev. Mutis 2022, 12. [Google Scholar] [CrossRef]
- Parra-Cota, F.I.; Bruno, I.; García-Montelongo, M.; González-Villarreal, S.; Villarreal-Delgado, M.F.; Córdova-Albores, L.C.; Escalante-Beltrán, A.; de los Santos-Villalobos, S. The Genus Bacillus as Biological Control Agent against Pests and Pathogens for Sustainable Agriculture. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2024, 42. [Google Scholar] [CrossRef]
- de los Santos-Villalobos, S.; Valenzuela-Ruiz, V.; Montoya-Martínez, A.C.; Parra-Cota, F.I.; Santoyo, G.; Larsen, J. Bacillus cabrialesii subsp. Cabrialesii subsp. Nov. and Bacillus cabrialesii subsp. Tritici subsp. Nov., Plant Growth-Promoting Bacteria and Biological Control Agents Isolated from Wheat (Triticum turgidum subsp. Durum) in the Yaqui Valley, Mexico. Int. J. Syst. Evol. Microbiol. 2023, 73, 005779. [Google Scholar] [CrossRef]
- Gray, D.A.; Dugar, G.; Gamba, P.; Strahl, H.; Jonker, M.J.; Hamoen, L.W. Extreme Slow Growth as Alternative Strategy to Survive Deep Starvation in Bacteria. Nat. Commun. 2019, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Seshadri, B.; Sarkar, B.; Wang, H.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; Yang, X.; Bolan, N. Biochar Modulates Heavy Metal Toxicity and Improves Microbial Carbon Use Efficiency in Soil. Sci. Total. Environ. 2018, 621, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Ayman, M.; Fahmy, M.A.; Elnahal, A.S.M.; Alfassam, H.E.; Rudayni, H.A.; Allam, A.A.; Farahat, E.M. Enhancing Wheat Tolerance to Salinity Using Nanomaterials, Proline, and Biochar-Inoculated with Bacillus subtilis. Heliyon 2024, 10, e37160. [Google Scholar] [CrossRef]
- Koyro, H.-W.; Huchzermeyer, B. From Soil Amendments to Controlling Autophagy: Supporting Plant Metabolism under Conditions of Water Shortage and Salinity. Plants 2022, 11, 1654. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-Char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Schmalenberger, A.; Fox, A. Bacterial Mobilization of Nutrients From Biochar-Amended Soils. Adv. Appl. Microbiol. 2016, 94, 109–159. [Google Scholar] [CrossRef]
- Chintala, R.; Schumacher, T.E.; Kumar, S.; Malo, D.D.; Rice, J.A.; Bleakley, B.; Chilom, G.; Clay, D.E.; Julson, J.L.; Papiernik, S.K.; et al. Molecular Characterization of Biochars and Their Influence on Microbiological Properties of Soil. J. Hazard Mater. 2014, 279, 244–256. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Arunrat, N.; Uttarotai, T.; Kongsurakan, P.; Sereenonchai, S.; Hatano, R. Bacterial Community Structure in Soils With Fire-Deposited Charcoal Under Rotational Shifting Cultivation of Upland Rice in Northern Thailand. Ecol. Evol. 2025, 15, e70851. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Hua, M.; Reckling, M.; Wirth, S.; Bellingrath-Kimura, S.D. Potential Effects of Biochar-Based Microbial Inoculants in Agriculture. Environ. Sustain. 2018, 1, 19–24. [Google Scholar] [CrossRef]
- Yu, M.; Meng, J.; Yu, L.; Su, W.; Afzal, M.; Li, Y.; Brookes, P.C.; Redmile-Gordon, M.; Luo, Y.; Xu, J. Changes in Nitrogen Related Functional Genes along Soil PH, C and Nutrient Gradients in the Charosphere. Sci. Total Environ. 2019, 650, 626–632. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘Charosphere’—Does Biochar in Agricultural Soil Provide a Significant Habitat for Microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Karppinen, E.M.; Mamet, S.D.; Stewart, K.J.; Siciliano, S.D. The Charosphere Promotes Mineralization of 13 C-Phenanthrene by Psychrotrophic Microorganisms in Greenland Soils. J. Environ. Qual. 2019, 48, 559–567. [Google Scholar] [CrossRef]
- Luo, Y.; Durenkamp, M.; De Nobili, M.; Lin, Q.; Devonshire, B.J.; Brookes, P.C. Microbial Biomass Growth, Following Incorporation of Biochars Produced at 350 °C or 700 °C, in a Silty-Clay Loam Soil of High and Low PH. Soil Biol. Biochem. 2013, 57, 513–523. [Google Scholar] [CrossRef]
- Pei, J.; Zhuang, S.; Cui, J.; Li, J.; Li, B.; Wu, J.; Fang, C. Biochar Decreased the Temperature Sensitivity of Soil Carbon Decomposition in a Paddy Field. Agric. Ecosyst. Environ. 2017, 249, 156–164. [Google Scholar] [CrossRef]
- Pingree, M.R.A.; DeLuca, T.H. Function of Wildfire-Deposited Pyrogenic Carbon in Terrestrial Ecosystems. Front. Environ. Sci. 2017, 5, 53. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides Producing Bacteria: A Review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial Extracellular Polysaccharides Involved in Biofilm Formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef] [PubMed]
- Halverson, L.J. BACTERIA | Soil. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; pp. 115–122. [Google Scholar]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Wood, B.J.B. Lactic Acid Bacteria: Biodiversity and Taxonomy; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A New Functional Genus with Potential Probiotic Properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Dekio, I.; Asahina, A.; Shah, H.N. Unravelling the Eco-Specificity and Pathophysiological Properties of Cutibacterium Species in the Light of Recent Taxonomic Changes. Anaerobe 2021, 71, 102411. [Google Scholar] [CrossRef] [PubMed]
- Hawver, L.A.; Giulietti, J.M.; Baleja, J.D.; Ng, W.-L. Quorum Sensing Coordinates Cooperative Expression of Pyruvate Metabolism Genes To Maintain a Sustainable Environment for Population Stability. mBio 2016, 7, e01863-16. [Google Scholar] [CrossRef] [PubMed]
- Masiello, C.A.; Chen, Y.; Gao, X.; Liu, S.; Cheng, H.-Y.; Bennett, M.R.; Rudgers, J.A.; Wagner, D.S.; Zygourakis, K.; Silberg, J.J. Biochar and Microbial Signaling: Production Conditions Determine Effects on Microbial Communication. Environ. Sci. Technol. 2013, 47, 11496–11503. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, B.; Xu, G.; Shao, H. Effects of Wheat Straw Biochar on Carbon Mineralization and Guidance for Large-Scale Soil Quality Improvement in the Coastal Wetland. Ecol. Eng. 2014, 62, 43–47. [Google Scholar] [CrossRef]
- Thies, C.; Haenke, S.; Scherber, C.; Bengtsson, J.; Bommarco, R.; Clement, L.W.; Ceryngier, P.; Dennis, C.; Emmerson, M.; Gagic, V.; et al. The Relationship between Agricultural Intensification and Biological Control: Experimental Tests across Europe. Ecol. Appl. 2011, 21, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qi, G.; Ma, G.; Zhao, X. Biochar Amendment Controlled Bacterial Wilt through Changing Soil Chemical Properties and Microbial Community. Microbiol. Res. 2020, 231, 126373. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Haider, S.; Song, J.; Zhang, D.; Chang, S.; Bai, J.; Hao, J.; Yang, G.; Ren, G.; Han, X.; et al. Regulation of Soil Microbial Nitrogen Limitation by Soybean Rhizosphere Diazotrophs under Long-Term No-till Mulching. Appl. Soil Ecol. 2025, 206, 105873. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.; Sun, L.; Qi, G.; Chen, S.; Zhao, X. Microbial Community Composition Is Related to Soil Biological and Chemical Properties and Bacterial Wilt Outbreak. Sci. Rep. 2017, 7, 343. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Li, X.; Ping, Q.; Yuan, X.; Agathokleous, E.; Feng, Z. Interactive Effects of Ozone Exposure and Nitrogen Addition on the Rhizosphere Bacterial Community of Poplar Saplings. Sci. Total Environ. 2021, 754, 142134. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.C.; de Moraes, D.; da Nóbrega, S.W.; Barboza, M.G. Ammonia Adsorption in a Fixed Bed of Activated Carbon. Bioresour. Technol. 2007, 98, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological Nitrogen Fixation by Common Beans (Phaseolus vulgaris L.) Increases with Bio-Char Additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar Induced Soil Microbial Community Change: Implications for Biogeochemical Cycling of Carbon, Nitrogen and Phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, T.; Van Zwieten, L.; Zhu, Q.; Yan, T.; Xue, J.; Wu, Y. Soil Microbial Community Structure Shifts Induced by Biochar and Biochar-Based Fertilizer Amendment to Karst Calcareous Soil. Soil Sci. Soc. Am. J. 2019, 83, 398–408. [Google Scholar] [CrossRef]
- Li, Q.; Ji, H.; Zhang, C.; Cui, Y.; Peng, C.; Chang, S.X.; Cao, T.; Shi, M.; Li, Y.; Wang, X.; et al. Biochar Amendment Alleviates Soil Microbial Nitrogen and Phosphorus Limitation and Increases Soil Heterotrophic Respiration under Long-Term Nitrogen Input in a Subtropical Forest. Sci. Total Environ. 2024, 951, 175867. [Google Scholar] [CrossRef] [PubMed]

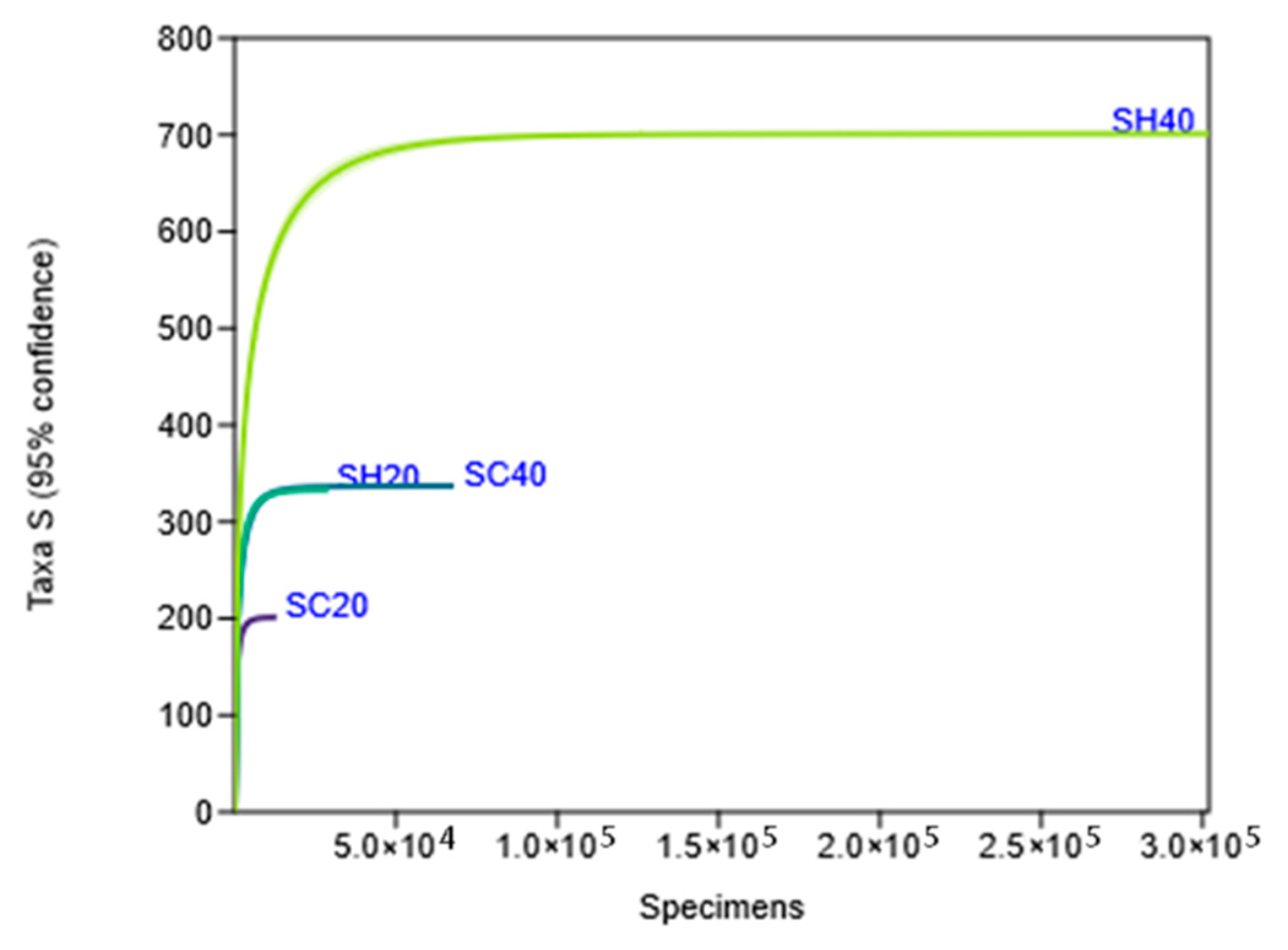
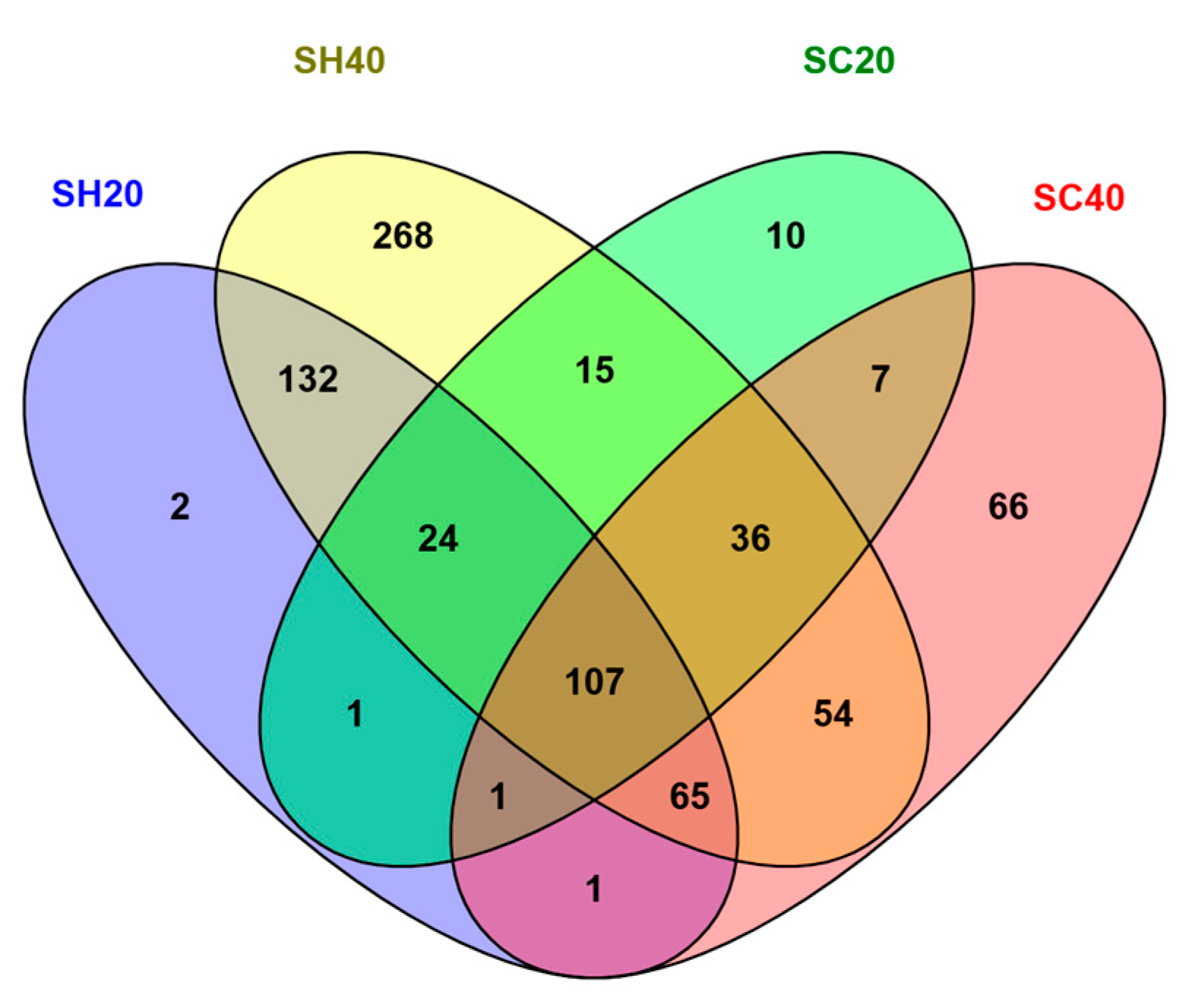



| Sample | Substrate | Moisture (%) |
|---|---|---|
| S | Soil 100% | 25.5 |
| SB10 | Soil/biochar 90/10% | 57.4 |
| SB20 | Soil/biochar 80/20% | 53.8 |
| B | Biochar 100% | 60.9 |
| (a) Jaccard Index (IJ) | ||||
| SC20 | SH20 | SC40 | SH40 | |
| SC20 | 1.000 | 0.332 | 0.390 | 0.253 |
| SH20 | 1.000 | 0.351 | 0.465 | |
| SC40 | 1.000 | 0.338 | ||
| SH40 | 1.000 | |||
| (b) Whittaker Index (IW) | ||||
| SC20 | SH20 | SC40 | SH40 | |
| SC20 | 0.000 | 0.502 | 0.439 | 0.596 |
| SH20 | 0.000 | 0.481 | 0.366 | |
| SC40 | 0.000 | 0.495 | ||
| SH40 | 0.000 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, A.; del Moral, A.; Delgado, G.; Párraga, J.; Rufián, J.Á.; Rojano, R.; Martín-García, J.M. Could Hydroinfiltrators Made with Biochar Modify the Soil Microbiome? A Strategy of Soil Nature-Based Solution for Smart Agriculture. Appl. Sci. 2025, 15, 8503. https://doi.org/10.3390/app15158503
Navarro A, del Moral A, Delgado G, Párraga J, Rufián JÁ, Rojano R, Martín-García JM. Could Hydroinfiltrators Made with Biochar Modify the Soil Microbiome? A Strategy of Soil Nature-Based Solution for Smart Agriculture. Applied Sciences. 2025; 15(15):8503. https://doi.org/10.3390/app15158503
Chicago/Turabian StyleNavarro, Azahara, Ana del Moral, Gabriel Delgado, Jesús Párraga, José Ángel Rufián, Raúl Rojano, and Juan Manuel Martín-García. 2025. "Could Hydroinfiltrators Made with Biochar Modify the Soil Microbiome? A Strategy of Soil Nature-Based Solution for Smart Agriculture" Applied Sciences 15, no. 15: 8503. https://doi.org/10.3390/app15158503
APA StyleNavarro, A., del Moral, A., Delgado, G., Párraga, J., Rufián, J. Á., Rojano, R., & Martín-García, J. M. (2025). Could Hydroinfiltrators Made with Biochar Modify the Soil Microbiome? A Strategy of Soil Nature-Based Solution for Smart Agriculture. Applied Sciences, 15(15), 8503. https://doi.org/10.3390/app15158503










