Differences in Lower Limb Muscle Activity and Gait According to Walking Speed Variation in Chronic Stroke
Abstract
1. Introduction
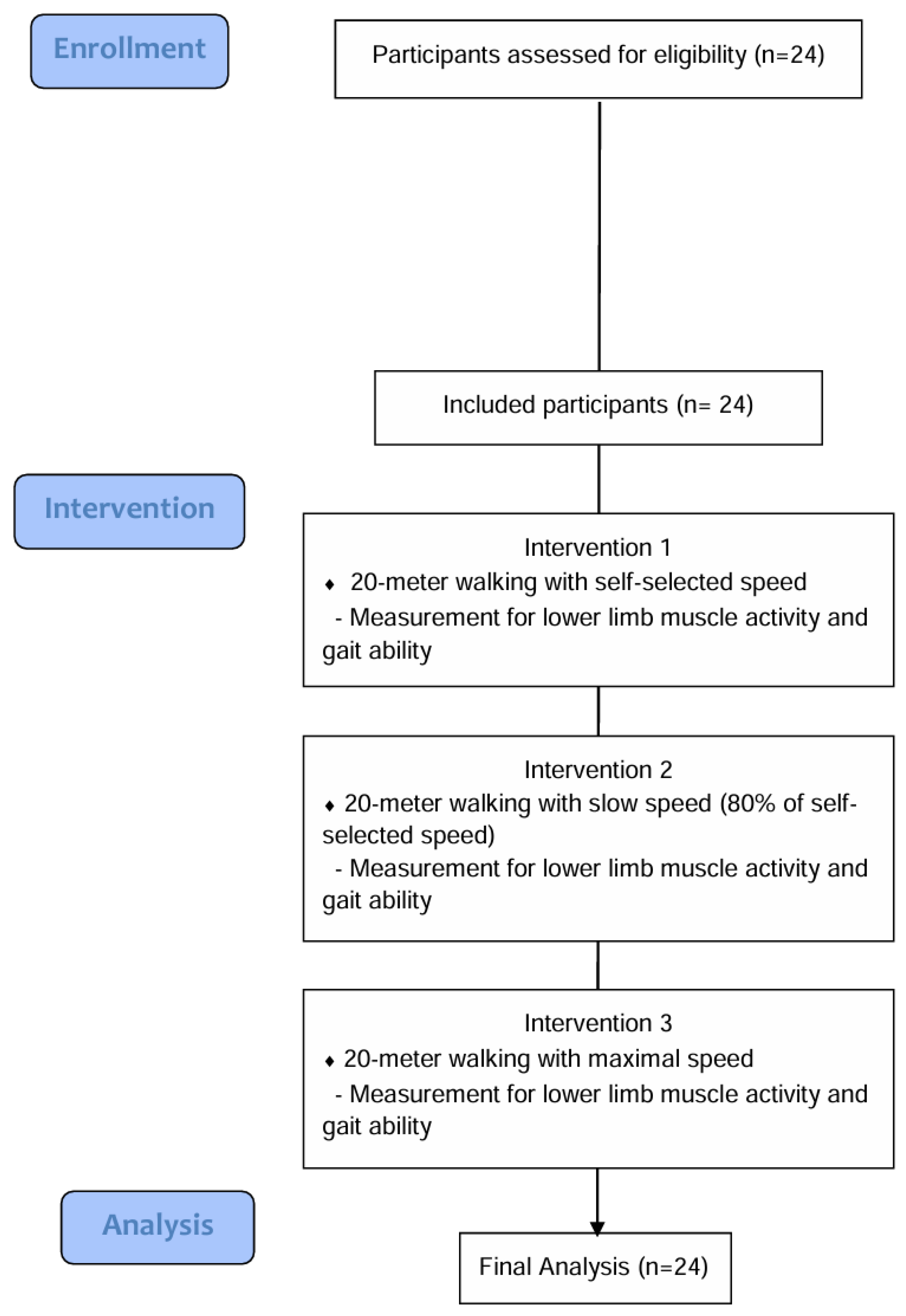
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Procedures
2.4. Muscle Activity and Gait Ability Measurements
2.5. Data Analysis
3. Results
3.1. General Characteristics of the Participants
3.2. Changes in Lower Limb Muscle Activity
3.3. Changes in Gait Ability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBS | Berg Balance Scale |
| BF | Biceps femoris |
| FAC | Functional Ambulation Category |
| GM | Gluteus medius |
| GN | Gastrocnemius |
| GAI | gait asymmetry index |
| GPS | Global Positioning System |
| MAS | Modified Ashworth Scale |
| MBI | Modified Barthel Index |
| MMSE-K | Mini-Mental State Examination—Korean version |
| MMT | Manual Muscle Testing |
| RF | Rectus femoris |
| RVC | Reference voluntary contraction |
| sEMG | Surface electromyography |
| TA | Tibialis anterior |
References
- Balaban, B.; Tok, F. Gait disturbances in patients with stroke. PM R J. Inj. Funct. Rehabil. 2014, 6, 635–642. [Google Scholar]
- Woolley, S.M. Characteristics of gait in hemiplegia. Top. Stroke Rehabil. 2001, 7, 1–18. [Google Scholar] [CrossRef]
- Vive, S.; Elam, C.; Bunketorp-Käll, L. Comfortable and maximum gait speed in individuals with chronic stroke and community-dwelling controls. J. Stroke Cerebrovasc. Dis. 2021, 30, 106023. [Google Scholar] [CrossRef]
- Schmid, A.; Duncan, P.W.; Studenski, S.; Lai, S.M.; Richards, L.; Perera, S.; Wu, S.S. Improvements in speed–based gait classifications are meaningful. Stroke 2007, 38, 2096–2100. [Google Scholar] [CrossRef]
- Kim, C.M.; Eng, J.J. Magnitude and pattern of 3D kinematic and kinetic gait profiles in persons with stroke, relationship to walking speed. Gait Posture 2004, 20, 140–146. [Google Scholar] [CrossRef]
- Tasseel-Ponche, S.; Delafontaine, A.; Godefroy, O.; Yelnik, A.P.; Doutrellot, P.L.; Duchossoy, C.; Hyra, M.; Sader, T.; Diouf, M. Walking speed at the acute and subacute stroke stage: A descriptive meta-analysis. Front. Neurol. 2022, 13, 989622. [Google Scholar] [CrossRef]
- Lee, D.G.; Ahn, S.H.; Lee, G.C. Discriminative power and predictive validity of the timed up and go test, figure-of-8 walk test, four square step test, and step test for classifying community ambulation levels in individuals with chronic stroke. J. Korean Phys. Ther. Sci. 2020, 27, 25–35. [Google Scholar] [CrossRef]
- Salbach, N.M.; O’Brien, K.; Brooks, D.; Irvin, E.; Martino, R.; Takhar, P.; Chan, S.; Howe, J.A. Speed and distance requirements for community ambulation: A systematic review. Arch. Phys. Med. Rehabil. 2014, 95, 117–128.e11. [Google Scholar] [CrossRef] [PubMed]
- Beyaert, C.; Vasa, R.; Frykberg, G.E. Gait post-stroke, pathophysiology and rehabilitation strategies. Neurophysiol. Clin. 2015, 45, 335–355. [Google Scholar] [CrossRef]
- Pohl, M.; Mehrholz, J.; Ritschel, C.; Rückriem, S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: A randomized controlled trial. Stroke 2002, 33, 553–558. [Google Scholar] [CrossRef]
- Lau, K.W.; Mak, M.K. Speed-dependent treadmill training is effective to improve gait and balance performance in patients with sub-acute stroke. J. Rehabil. Med. 2011, 43, 709–713. [Google Scholar]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Awad, L.N.; Reisman, D.S.; Wright, T.R.; Roos, M.A.; Binder-Macleod, S.A. Maximum walking speed is a key determinant of long distance walking function after stroke. Top. Stroke Rehabil. 2014, 21, 502–509. [Google Scholar] [CrossRef]
- Awad, L.N.; Palmer, J.A.; Pohlig, R.T.; Binder-Macleod, S.A.; Reisman, D.S. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabil. Neural Repair 2015, 29, 416–423. [Google Scholar] [CrossRef]
- Lamontagne, A.; Fung, J. Faster is better, implications for speed-intensive gait training after stroke. Stroke 2004, 35, 2543–2548. [Google Scholar] [CrossRef]
- Carvalho, C.; Sunnerhagen, K.S.; Willén, C. Walking speed and distance in different environments of subjects in the later stage post-stroke. Physiother. Theory Prac. 2010, 26, 519–527. [Google Scholar] [CrossRef]
- Burnfield, M. Gait analysis: Normal and pathological function. J. Sports Sci. Med. 2010, 9, 353. [Google Scholar]
- Marasović, T.; Cecić, M.; Zanchi, V. Analysis and interpretation of ground reaction forces in normal gait. WSEAS Trans. Syst. 2009, 8, 1105–1114. [Google Scholar]
- Wang, Y.; Mukaino, M.; Ohtsuka, K.; Otaka, Y.; Tanikawa, H.; Matsuda, F.; Tsuchiyama, K.; Yamada, J.; Saitoh, E. Gait characteristics of post-stroke hemiparetic patients with different walking speeds. Int. J. Rehabil. Res. 2020, 43, 69–75. [Google Scholar] [CrossRef]
- Liang, J.N.; Ho, K.Y.; Lee, Y.J.; Ackley, C.; Aki, K.; Arias, J.; Trinh, J. Slow walking in individuals with chronic post-stroke hemiparesis: Speed mediated effects of gait kinetics and ankle kinematics. Brain Sci. 2021, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Nymark, J.R.; Balmer, S.J.; Melis, E.H.; Lemaire, E.D.; Millar, S. Electromyographic and kinematic nondisabled gait differences at extremely slow overground and treadmill walking speeds. J. Rehabil. Res. Dev. 2005, 42, 523–534. [Google Scholar] [CrossRef]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; McIlroy, W.E. Gait asymmetry in community-ambulating stroke survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef]
- Shin, M.H.; Lee, Y.M.; Park, J.M.; Kang, C.J.; Lee, B.D.; Moon, E.; Chung, Y.I. A combination of the Korean version of the mini-mental state examination and Korean dementia screening questionnaire is a good screening tool for dementia in the elderly. Psychiatry Investig. 2011, 8, 348–353. [Google Scholar] [CrossRef]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meiβner, D.; Pohl, M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef]
- Stegeman, D.; Hermens, H. Standards for surface electromyography, The European project Surface EMG for non-invasive assessment of muscles (SENIAM). Enschede Roessingh Res. Dev. 2007, 10, 8–12. [Google Scholar]
- Tabard-Fougère, A.; Mourot, L.; Genadry, E.; Armand, S. EMG Normalization Method Based on Grade 3 of Manual Muscle Testing: Within- and Between-Day Reliability of Normalization Tasks and Application to Gait Analysis. Gait Posture 2018, 60, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Roerdink, M.; Beek, P.J. Understanding Inconsistent Step-Length Asymmetries across Hemiplegic Stroke Patients: Impairments and Compensatory Gait. Neurorehabil. Neural Repair 2011, 25, 253–258. [Google Scholar] [CrossRef]
- Jang, J.S.; Sang, Y.L.; Myung, H.L.; Yong, W.C.; Hyun-Min, L.; Hyen-Ju, O. The relationship between gait speed, muscle activity, and plantar pressure in stroke patients. J. Korean Phys. Ther. 2009, 21, 47–52. [Google Scholar]
- Nadeau, S.; Gravel, D.; Arsenault, A.B.; Bourbonnais, D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin. Biomech. 1999, 14, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Kim, Y.; Chung, Y.; Hwang, S. Weight-shift training improves trunk control, proprioception, and balance in patients with chronic hemiparetic stroke. Tohoku J. Exp. Med. 2014, 232, 195–199. [Google Scholar] [CrossRef]
- Baik, S.M.; Cynn, H.S.; Kim, S.H. Understanding and exercise of gluteus medius weakness: A systematic review. Phys. Ther. Korea 2021, 28, 27–35. [Google Scholar] [CrossRef]
- Kim, J.H.; Chung, Y.; Kim, Y.; Hwang, S. Functional electrical stimulation applied to gluteus medius and tibialis anterior corresponding gait cycle for stroke. Gait Posture 2012, 36, 65–67. [Google Scholar] [CrossRef]
- Maguire, C.; Sieben, J.M.; Frank, M.; Romkes, J. Hip abductor control in walking following stroke the immediate effect of canes, taping and TheraTogs on gait. Clin. Rehabil. 2010, 24, 37–45. [Google Scholar] [CrossRef]
- Wang, S.; Bhatt, T. Gait kinematics and asymmetries affecting fall risk in people with chronic stroke: A retrospective study. Biomechanics 2022, 2, 453–465. [Google Scholar] [CrossRef]
- Lewek, M.D.; Bradley, C.E.; Wutzke, C.J.; Zinder, S.M. The relationship between spatiotemporal gait asymmetry and balance in individuals with chronic stroke. J. Appl. Biomech. 2014, 30, 31–36. [Google Scholar] [CrossRef]
- Chen, I.-H.; Yang, Y.R.; Chan, R.C.; Wang, R.Y. Turning-based treadmill training improves turning performance and gait symmetry after stroke. Neurorehabil. Neural. Repair 2014, 28, 45–55. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kim, S.H.; Shin, Y.I. Effects of power-assisted gait training with progressive speed increase and treadmill training on gait in stroke patients. J. Coach/Dev. 2007, 9, 113–125. [Google Scholar]
- Tao, W.; Chen, J.; Peng, J.; Xiao, W. Comparing the effectiveness of dual-task and single-task training on walking function in stroke recovery: A systematic review and meta-analysis. Medicine 2025, 104, e41776. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Chen, H.C.; Wong, M.K.; Tang, S.F.; Chen, R.S. Temporal Stride and Force Analysis of Cane-Assisted Gait in People with Hemiplegic Stroke. Arch. Phys. Med. Rehabil 2001, 82, 43–48. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Mean ± SD or Frequency (%) | ||
|---|---|---|---|
| Sex (male/female) | 13/11 (55.17/45.83) | ||
| Age (years) | 66.50 ± 11.81 | ||
| Height (cm) | 161.75 ± 8.32 | ||
| Time since stroke (days) | 275.66 ± 207.20 | ||
| Weight (kg) | 61.00 ± 7.73 | ||
| Etiology (hemorrhage/infarction) | 11/13 (45.83/55.17) | ||
| Hemiplegic side (left/right) | 10/14 (41.67/58.33) | ||
| Walking aid (mono cane/not use) | 13/11 (55.17/45.83) | ||
| FAC (2/3/4) | 7/7/10 (29.17/29.17/41.67) | ||
| MMT (1/2/3/4) | Hip | Flexors | 0/0/21/3 (0/0/87.5/12.5) |
| Extensors | 0/1/19/4 (0/4.17/79.17/16.67) | ||
| Knee | Flexors | 0/0/20/4 (0/0/83.33/16.67) | |
| Extensors | 0/1/19/4 (0/4.17/79.17/16.67) | ||
| Ankle | Dorsiflexors | 1/7/11/5 (4.17/29.17/45.83/20.83) | |
| Plantar flexors | 1/7/11/5 (4.17/29.17/45.83/20.83) | ||
| MAS (1/1+/2) | Ankle | Plantar flexors | 4/12/8 (16.67/50.00/33.33) |
| MMSE-K (points) | 25.70 ± 4.43 | ||
| BBS (points) | 42.16 ± 9.25 | ||
| MBI (points) | 75.41 ± 12.02 | ||
| Walking speed (m/s) | Slow | 0.51 ± 0.24 | |
| Self-selected | 0.67 ± 0.24 | ||
| Fast | 0.90 ± 0.36 | ||
| Parameters (%RVC) | Slow Speed Walking (A) | Self-Selected Speed Walking (B) | Maximum Speed Walking (C) | F(p) | Post hoc | |
|---|---|---|---|---|---|---|
| Stance phase | RF | 659.95 ± 725.60 | 797.15 ± 1169.16 | 1319.67 ± 1286.18 | 5.459 (0.007) | A < C |
| BF | 592.00 ± 505.34 | 668.03 ± 499.54 | 1013.80 ± 950.94 | 5.698 (0.006) | ||
| TA | 569.36 ± 579.40 | 854.86 ± 831.86 | 1082.51 ± 1626.82 | 1.716 (0.191) | ||
| GN | 347.63 ± 444.21 | 367.34 ± 347.68 | 469.60 ± 380.73 | 1.770 (0.182) | ||
| GM | 3114.99 ± 8154.58 | 1254.40 ± 1807.01 | 2114.04 ± 4878.40 | 1.636 (0.206) | ||
| Swing phase | RF | 407.49 ± 481.03 | 476.76 ± 528.75 | 849.66 ± 978.37 | 5.971 (0.005) | A < C |
| BF | 604.73 ± 563.99 | 743.73 ± 675.04 | 1118.23 ± 1069.70 | 5.143 (0.010) | A < C | |
| TA | 459.32 ± 580.81 | 605.41 ± 611.73 | 763.35 ± 1114.47 | 0.930 (0.402) | ||
| GN | 329.56 ± 286.61 | 392.68 ± 299.73 | 576.36 ± 299.73 | 6.487 (0.003) | A < C | |
| GM | 2836.31 ± 6223.01 | 1226.62 ± 1909.08 | 1792.19 ± 4168.99 | 2.406 (0.101) | ||
| Parameters | Slow Speed Walking (A) | Self-Selected Speed Walking (B) | Maximum Speed Walking (C) | F(p) | Post hoc | |
|---|---|---|---|---|---|---|
| Paratic side | STL (%height) | 46.22 ± 13.01 | 53.26 ± 12.14 | 60.00 ± 12.96 | 44.100 (<0.001) | A < B, B < C, A < C |
| STP (%cycle) | 57.25 ± 4.61 | 57.04 ± 5.26 | 57.17 ± 5.11 | 0.030 (0.970) | ||
| SWP (%cycle) | 42.75 ± 4.61 | 42.95 ± 5.26 | 42.82 ± 5.11 | 0.027 (0.973) | ||
| SLST (%cycle) | 37.14 ± 3.72 | 37.44 ± 4.83 | 38.05 ± 5.19 | 0.645 (0.529) | ||
| Non-paretic side | STL (%height) | 45.99 ± 13.07 | 53.23 ± 12.09 | 59.09 ± 13.32 | 41.318 (<0.001) | A < B, B < C, A < C |
| STP (%cycle) | 62.09 ± 5.57 | 62.66 ± 4.86 | 61.87 ± 5.19 | 0.302 (0.741) | ||
| SWP (%cycle) | 36.97 ± 3.77 | 37.33 ± 4.86 | 38.12 ± 5.19 | 1.011 (0.372) | ||
| SLST (%cycle) | 42.81 ± 4.63 | 43.00 ± 5.22 | 42.73 ± 5.11 | 0.053 (0.949) | ||
| GAI | STL | −0.25 ± 1.31 | −0.02 ± 0.51 | −0.82 ± 4.51 | 0.534 (0.590) | |
| STP | 3.98 ± 7.33 | 4.72 ± 7.47 | 3.94 ± 8.23 | 0.173 (0.841) | ||
| SWP | −7.15 ± 9.15 | −6.93 ± 11.08 | −5.82 ± 11.93 | 0.305 (0.738) | ||
| SLST | 6.99 ± 9.05 | 6.85 ± 11.02 | 5.80 ± 11.96 | 0.258 (0.774) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.G.; Cho, K.H. Differences in Lower Limb Muscle Activity and Gait According to Walking Speed Variation in Chronic Stroke. Appl. Sci. 2025, 15, 8479. https://doi.org/10.3390/app15158479
Shin YG, Cho KH. Differences in Lower Limb Muscle Activity and Gait According to Walking Speed Variation in Chronic Stroke. Applied Sciences. 2025; 15(15):8479. https://doi.org/10.3390/app15158479
Chicago/Turabian StyleShin, Yong Gyun, and Ki Hun Cho. 2025. "Differences in Lower Limb Muscle Activity and Gait According to Walking Speed Variation in Chronic Stroke" Applied Sciences 15, no. 15: 8479. https://doi.org/10.3390/app15158479
APA StyleShin, Y. G., & Cho, K. H. (2025). Differences in Lower Limb Muscle Activity and Gait According to Walking Speed Variation in Chronic Stroke. Applied Sciences, 15(15), 8479. https://doi.org/10.3390/app15158479






