From Evidence to Practice: A Systematic Review and Meta-Analysis on the Effects of Supervised Exercise on Fatigue in Breast and Prostate Cancer Survivors
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Analysis
3. Results
3.1. Systematic Review
3.1.1. Study Selection
3.1.2. Study and Intervention Characteristics
3.1.3. Characteristics of Included Studies
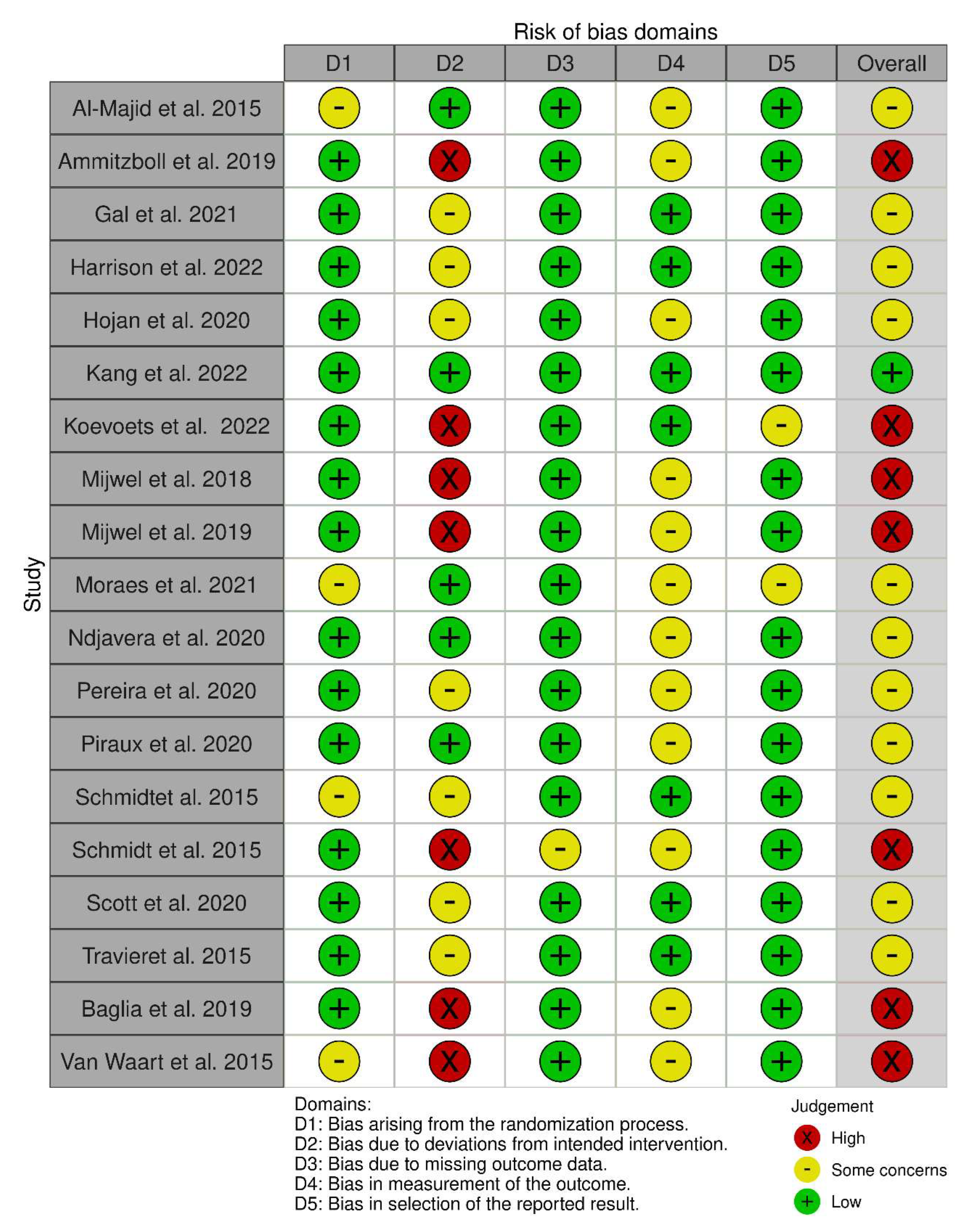
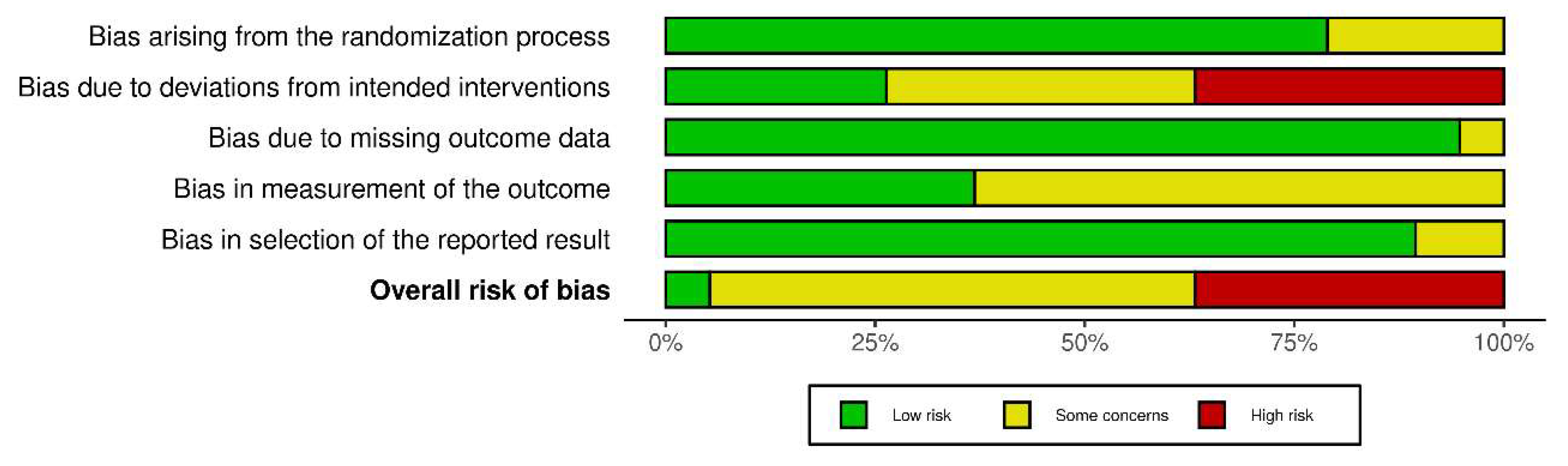
3.1.4. Quality Assessment and Risk of Bias
3.2. Data Synthesis
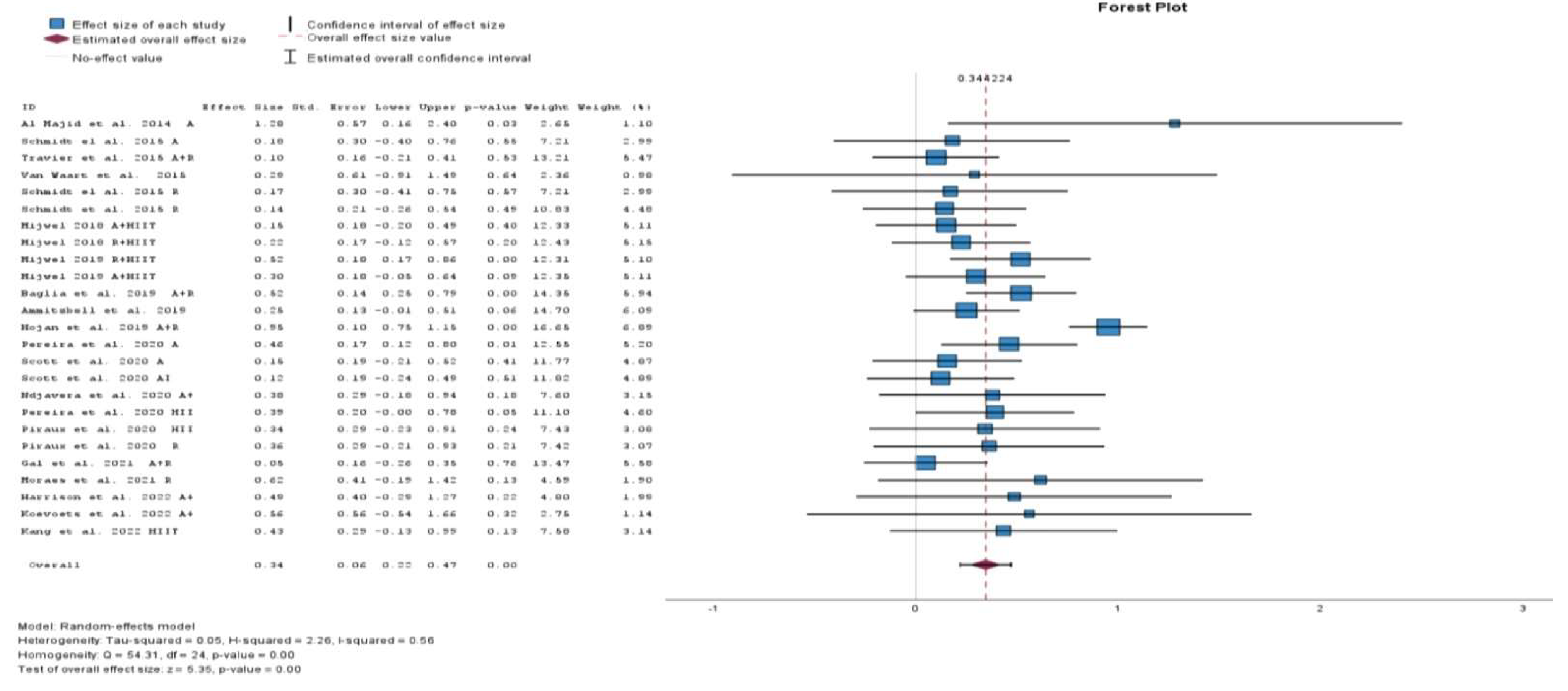
3.2.1. Meta-Analysis
3.2.2. Sensitivity and Subgroup Analyses
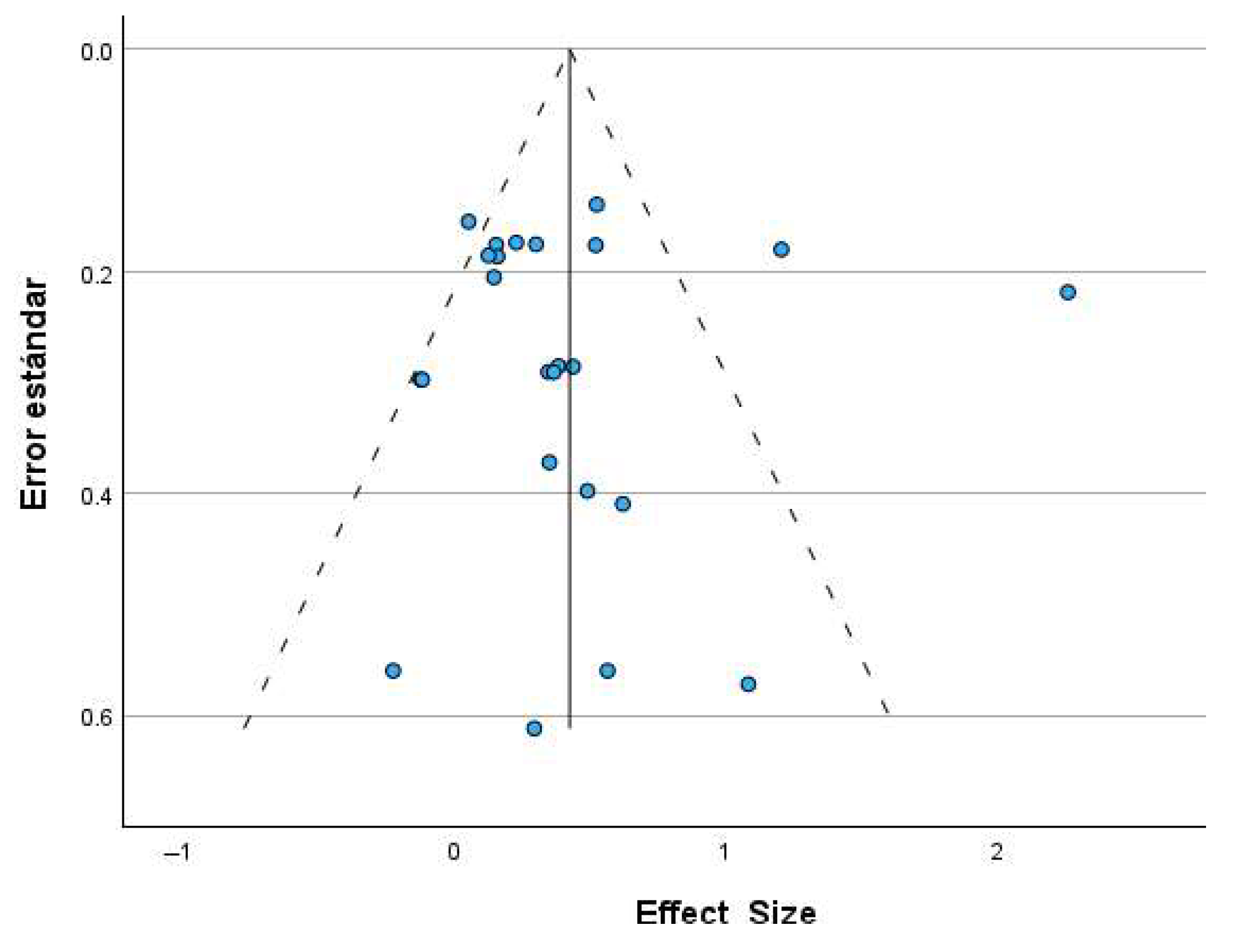
3.2.3. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; World Cancer Research Fund/American Institute for Cancer Research: London, UK, 2018; Continuous Update Project Expert Report 2018; Available online: http://www.dietandcancerreport.org (accessed on 15 March 2025).
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Solin, L.J. Breast Conservation Treatment With Radiation: An Ongoing Success Story. J. Clin. Oncol. 2010, 28, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Vera Donoso, C.D. El tratamiento del cáncer de próstata: Presente y futuro, realidades y posibilidades. Actas Urol. Esp. 2007, 31, 575–579. [Google Scholar] [CrossRef]
- He, Y.; Tan, P.; He, M.; Hu, L.; Ai, J.; Yang, L.; Wei, Q. The primary treatment of prostate cancer with high-intensity focused ultrasound: A systematic review and meta-analysis. Medicine 2020, 99, e22610. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment. JAMA 2019, 321, 288. [Google Scholar] [CrossRef]
- Montero, A.; Hervás, A.; Morera, R.; Sancho, S.; Córdoba, S.; Corona, J.A.; Rodríguez, I.; Chajón, E.; Ramos, A. Control de síntomas crónicos: Efectos secundarios del tratamiento con Radioterapia y Quimioterapia. Oncología 2005, 28, 41–50. [Google Scholar] [CrossRef]
- Ruiz-Schutz, V.C.; Gomes, L.M.; Mariano, R.C.; de Almeida, D.V.; Pimenta, J.M.; Molin, G.Z.D.; Kater, F.R.; Yamamura, R.; Neto, N.F.C.; Maluf, F.C.; et al. Risk of fatigue and anemia in patients with advanced cancer treated with olaparib: A meta-analysis of randomized controlled trials. In Critical Reviews in Oncology/Hematology; Elsevier Ireland Ltd.: Amsterdam, The Netherlands, 2019; Volume 141, pp. 163–173. [Google Scholar]
- Schmidt, M.E.; Wiskemann, J.; Steindorf, K. Quality of life, problems, and needs of disease-free breast cancer survivors 5 years after diagnosis. Qual. Life Res. 2018, 27, 2077–2086. [Google Scholar] [CrossRef]
- Wefel, J.S.; Saleeba, A.K.; Buzdar, A.U.; Meyers, C.A. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 2010, 116, 3348–3356. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Kupelnick, B.; Miller, K.; Devine, D.; Lau, J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J. Natl. Cancer Inst. 2004, 32, 40–50. [Google Scholar] [CrossRef]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology. Cancer-Related Fatigue Version 2.2025. 2025. Available online: www.nccn.org (accessed on 21 April 2025).
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, P.B.; Hann, D.M.; Azzarello, L.M.; Horton, J.; Balducci, L.; Lyman, G.H. Fatigue in Women Receiving Adjuvant Chemotherapy for Breast Cancer: Characteristics, Course, and Correlates. J. Pain Symptom Manag. 1999, 18, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Hickok, J.T.; Roscoe, J.A.; Morrow, G.R.; Mustian, K.; Okunieff, P.; Bole, C.W. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer 2005, 104, 1772–1778. [Google Scholar] [CrossRef]
- Phillips, K.M.; Pinilla-Ibarz, J.; Sotomayor, E.; Lee, M.R.; Jim, H.S.; Small, B.J.; Sokol, L.; Lancet, J.; Tinsley, S.; Sweet, K.; et al. Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: A controlled comparison. Support. Care Cancer 2013, 21, 1097–1103. [Google Scholar] [CrossRef]
- Groenvold, M.; Petersen, M.A.; Idler, E.; Bjorner, J.B.; Fayers, P.M.; Mouridsen, H.T. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res. Treat. 2007, 105, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, A.; Barrett, S.; Haruna, F.; Mustian, K.; O’Donovan, A. The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: A systematic review and meta-analysis. Breast 2017, 32, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Cano-Uceda, A.; Pareja-García, P.; Sánchez-Rodríguez, E.; Fraguas-Ramos, D.; Martín-Álvarez, L.; Asencio-Vicente, R.; Rivero-de la Villa, A.; Pérez-Pérez, M.d.M.; Obispo-Portero, B.M.; Morales-Ruiz, L.; et al. Effects of a Short-Term Supervised Exercise Program in Women with Breast Cancer. Appl. Sci. 2024, 14, 6553. [Google Scholar] [CrossRef]
- Ramírez-vélez, R.; Zambom-ferraresi, F.; García-hermoso, A.; Kievisiene, J.; Rauckiene-michealsson, A.; Agostinis-sobrinho, C. Evidence-based exercise recommendations to improve mental wellbeing in women with breast cancer during active treatment: A systematic review and meta-analysis. Cancers 2021, 13, 264. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Schwartz, A.L.; Matthews, C.E.; Courneya, K.S.; Schmitz, K.H. Implementing the Exercise Guidelines for Cancer Survivors. J. Support. Oncol. 2012, 10, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.C.; Segal, R.J.; McKenzie, D.C.; Vallerand, J.R.; Morielli, A.R.; Mackey, J.R.; Gelmon, K.; Friedenreich, C.M.; Reid, R.D.; Courneya, K.S. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. Breast Cancer Res. Treat. 2016, 158, 497–507. [Google Scholar] [CrossRef]
- Ammitzbøll, G.; Kjær, T.K.; Johansen, C.; Lanng, C.; Andersen, E.W.; Kroman, N.; Zerahn, B.; Hyldegaard, O.; Bidstrup, P.E.; Dalton, S.O. Effect of progressive resistance training on health-related quality of life in the first year after breast cancer surgery–results from a randomized controlled trial. Acta Oncol. 2019, 58, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Midtgaard, J.; Hammer, N.M.; Andersen, C.; Larsen, A.; Bruun, D.M.; Jarden, M. Cancer survivors’ experience of exercise-based cancer rehabilitation-A meta-synthesis of qualitative research. Acta Oncol. 2015, 54, 609–617. [Google Scholar] [CrossRef]
- Gómez-Redondo, P.; Valenzuela, P.L.; Morales, J.S.; Ara, I.; Mañas, A. Supervised Versus Unsupervised Exercise for the Improvement of Physical Function and Well-Being Outcomes in Older Adults: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Sports Med. 2024, 54, 1877–1906. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2491–2507. [Google Scholar] [CrossRef]
- An, K.Y.; Morielli, A.R.; Kang, D.W.; Friedenreich, C.M.; McKenzie, D.C.; Gelmon, K.; Mackey, J.R.; Reid, R.D.; Courneya, K.S. Effects of exercise dose and type during breast cancer chemotherapy on longer-term patient-reported outcomes and health-related fitness: A randomized controlled trial. Int. J. Cancer 2020, 146, 150–160. [Google Scholar] [CrossRef]
- Harrison, M.R.; Davis, P.G.; Khouri, M.G.; Bartlett, D.B.; Gupta, R.T.; Armstrong, A.J.; McNamara, M.A.; Zhang, T.; Anand, M.; Onyenwoke, K.; et al. A randomized controlled trial comparing changes in fitness with or without supervised exercise in patients initiated on enzalutamide and androgen deprivation therapy for non-metastatic castration-sensitive prostate cancer (EXTEND). Prostate Cancer Prostatic Dis. 2022, 25, 58–64. [Google Scholar] [CrossRef]
- Kang, D.W.; Fairey, A.S.; Boulé, N.G.; Field, C.J.; Wharton, S.A.; Courneya, K.S. A Randomized Trial of the Effects of Exercise on Anxiety, Fear of Cancer Progression and Quality of Life in Prostate Cancer Patients on Active Surveillance. J. Urol. 2022, 207, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Mijwel, S.; Jervaeus, A.; Bolam, K.A.; Norrbom, J.; Bergh, J.; Rundqvist, H.; Wengström, Y. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J. Cancer Surviv. 2019, 13, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Reverte-Pagola, G.; Sánchez-Trigo, H.; Saxton, J.; Sañudo, B. Supervised and Non-Supervised Exercise Programs for the Management of Cancer-Related Fatigue in Women with Breast Cancer: A Systematic Review and Meta-Analysis. Cancer 2022, 14, 3428. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Ramírez-Vélez, R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: A systematic review and meta-analysis. BMC Cancer 2015, 15, 77. [Google Scholar] [CrossRef]
- Zhou, H.-J.; Wang, T.; Xu, Y.-Z.; Chen, Y.-N.; Deng, L.-J.; Wang, C.; Chen, J.-X.; Tan, J.-Y. Effects of exercise interventions on cancer-related fatigue in breast cancer patients: An overview of systematic reviews. Support. Care Cancer 2022, 30, 10421–10440. [Google Scholar] [CrossRef]
- VAN Vulpen, J.K.; Sweegers, M.G.; Peeters, P.H.M.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; Galvão, D.A.; Chinapaw, M.J.; Steindorf, K.; et al. Moderators of Exercise Effects on Cancer-related Fatigue: A Meta-analysis of Individual Patient Data. Med. Sci. Sports Exerc. 2020, 52, 303–314. [Google Scholar] [CrossRef]
- de Moura Ferraz Barbosa, M.P.; de Jesus, N.T.; Bergmann, A.; da Silva Alves Gomes, V.M.; Sacomori, C.; Dantas, D. Efficacy of supervised exercise on sleep of women who survived breast cancer: A systematic review with meta-analysis. J. Cancer Surviv. 2024, 52, 303–314. [Google Scholar] [CrossRef]
- Herranz-Gómez, A.; Cuenca-Martínez, F.; Suso-Martí, L.; Varangot-Reille, C.; Prades-Monfort, M.; Calatayud, J.; Casaña, J. Effectiveness of Therapeutic Exercise Models on Cancer-Related Fatigue in Patients with Cancer Undergoing Chemotherapy: A Systematic Review and Network Meta-analysis. Arch. Phys. Med. Rehabil. 2023, 104, 1331–1342. [Google Scholar] [CrossRef]
- Hilfiker, R.; Meichtry, A.; Eicher, M.; Nilsson Balfe, L.; Knols, R.H.; Verra, M.L.; Taeymans, J. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: A systematic review incorporating an indirect-comparisons meta-analysis. Br. J. Sports Med. 2018, 52, 651–658. [Google Scholar] [CrossRef]
- Penna, G.B.; Otto, D.M.; da Silva, T.C.; Pedroni, A.S.; Macagnan, F.E. Physical rehabilitation for the management of cancer-related fatigue during cytotoxic treatment: A systematic review with meta-analysis. Support. Care Cancer 2023, 31, 129. [Google Scholar] [CrossRef]
- Gray, L.; Sindall, P.; Pearson, S.J. Does resistance training ameliorate cancer-related fatigue in cancer survivors? A systematic review with meta-analysis. Disabil. Rehabil. 2024, 46, 2213–2222. [Google Scholar] [CrossRef]
- Cramp, F.; Byron-Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2012, 11, CD006145. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 8, 336–341. [Google Scholar]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011); The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, 14898. [Google Scholar] [CrossRef]
- Sherrington, C.; Herbert, R.D.; Maher, C.G.; Moseley, A. PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man. Ther. 2000, 5, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Thalheimer, W.; Cook, S. How to Calculate Effect Sizes from Published Research: A Simplified Methodology. Work. Learn. Res. 2002, 1, 1–9. [Google Scholar]
- Sutton, A.J.; Sheldon, T.; Song, F.; Abrams, K.R.; Jones, D.R.; Sheldon, T.A.; Song, F. Methods for Meta-Analysis in Medical Research; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Systematic reviews in health care Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef]
- Al-Majid, S.; Wilson, L.D.; Rakovski, C.; Coburn, J.W. Effects of Exercise on Biobehavioral Outcomes of Fatigue During Cancer Treatment: Results of a Feasibility Study. Biol. Res. Nurs. 2015, 17, 40–48. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Wiskemann, J.; Armbrust, P.; Schneeweiss, A.; Ulrich, C.M.; Steindorf, K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. Int. J. Cancer 2015, 137, 471–480. [Google Scholar] [CrossRef]
- Schmidt, T.; Weisser, B.; Dürkop, J.; Jonat, W.; Van Mackelenbergh, M.; Röcken, C.; Mundhenke, C. Comparing endurance and resistance training with standard care during chemotherapy for patients with primary breast cancer. Anticancer Res. 2015, 35, 5623–5629. [Google Scholar]
- Travier, N.; Velthuis, M.J.; Bisschop, C.N.S.; Buijs, B.v.D.; Monninkhof, E.M.; Backx, F.; Los, M.; Erdkamp, F.; Bloemendal, H.J.; Rodenhuis, C.; et al. Effects of an 18-week exercise programme started early during breast cancer treatment: A randomised controlled trial. BMC Med. 2015, 13, 121. [Google Scholar] [CrossRef]
- van Waart, H.; Stuiver, M.M.; van Harten, W.H.; Geleijn, E.; Kieffer, J.M.; Buffart, L.M.; de Maaker-Berkhof, M.; Boven, E.; Schrama, J.; Geenen, M.M.; et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: Results of the PACES randomized clinical trial. J. Clin. Oncol. 2015, 33, 1918–1927. [Google Scholar] [CrossRef]
- Mijwel, S.; Backman, M.; Bolam, K.A.; Jervaeus, A.; Sundberg, C.J.; Margolin, S.; Browall, M.; Rundqvist, H.; Wengström, Y. Adding high-intensity interval training to conventional training modalities: Optimizing health-related outcomes during chemotherapy for breast cancer: The OptiTrain randomized controlled trial. Breast Cancer Res. Treat. 2018, 168, 79–93. [Google Scholar] [CrossRef]
- Baglia, M.L.; Lin, I.; Cartmel, B.; Sanft, T.; Ligibel, J.; Hershman, D.L.; Harrigan, M.; Ferrucci, L.M.; Li, F.; Irwin, M.L. Endocrine-related quality of life in a randomized trial of exercise on aromatase inhibitor–induced arthralgias in breast cancer survivors. Cancer 2019, 125, 2262–2271. [Google Scholar] [CrossRef]
- Hojan, K.; Milecki, P. Does regular physical exercises during radiotherapy influence fatigue and physical endurance in high-risk prostate cancer patients? Med. Rehabil. 2019, 23, 21–27. [Google Scholar] [CrossRef]
- Ndjavera, W.; Orange, S.T.; O’DOherty, A.F.; Leicht, A.S.; Rochester, M.; Mills, R.; Saxton, J.M. Exercise-induced attenuation of treatment side-effects in patients with newly diagnosed prostate cancer beginning androgen-deprivation therapy: A randomised controlled trial. BJU Int. 2019, 125, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Eliécer, P.-R.J.; Geesel, P.-F.D.; Ricardo, P.-R.; Pedro, P.-R.; Ximena, V.-B.; Alberto, C.-P.Y. Fatiga asociada al cáncer de mama luego de un programa de entrenamiento. Acta Méd. Costarric. 2020, 62, 18–25. [Google Scholar]
- Piraux, E.; Caty, G.; Renard, L.; Vancraeynest, D.; Tombal, B.; Geets, X.; Reychler, G. Effects of high-intensity interval training compared with resistance training in prostate cancer patients undergoing radiotherapy: A randomized controlled trial. Prostate Cancer Prostatic Dis. 2021, 24, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Thomas, S.M.; Peppercorn, J.M.; Herndon, J.E., 2nd; Douglas, P.S.; Khouri, M.G.; Dang, C.T.; Yu, A.F.; Catalina, D.; Ciolino, C.; et al. Effects of Exercise Therapy Dosing Schedule on Impaired Cardiorespiratory Fitness in Patients with Primary Breast Cancer: A Randomized Controlled Trial. Circulation 2020, 141, 560–570. [Google Scholar] [CrossRef]
- Gal, R.; Monninkhof, E.M.; van Gils, C.H.; Groenwold, R.H.H.; Elias, S.G.; van den Bongard, D.H.J.G.; Peeters, P.H.M.; Verkooijen, H.M.; May, A.M. Effects of exercise in breast cancer patients: Implications of the trials within cohorts (TwiCs) design in the UMBRELLA Fit trial. Breast Cancer Res. Treat. 2021, 190, 89–101. [Google Scholar] [CrossRef]
- Moraes, R.F.; Ferreira-Júnior, J.B.; Marques, V.A.; Vieira, A.; Lira, C.A.B.; Campos, M.H.; Freitas-Junior, R.; Rahal, R.M.S.; Gentil, P.; Vieira, C.A. Resistance training, fatigue, quality of life, anxiety in breast cancer survivors. J. Strength Cond. Res. 2021, 35, 1350–1356. [Google Scholar] [CrossRef]
- Koevoets, E.W.; Schagen, S.B.; de Ruiter, M.B.; Geerlings, M.I.; Witlox, L.; van der Wall, E.; Stuiver, M.M.; Sonke, G.S.; Velthuis, M.J.; Jobsen, J.J.; et al. Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: A randomized controlled trial (PAM study). Breast Cancer Res. 2022, 24, 36. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Strasser, B.; Steindorf, K.; Wiskemann, J.; Ulrich, C.M. Impact of resistance training in cancer survivors: A meta-analysis. Med. Sci. Sports Exerc. 2013, 45, 2080–2090. [Google Scholar] [CrossRef]
- Champ, C.E.; Carpenter, D.J.; Diaz, A.K.; Rosenberg, J.; Ackerson, B.G.; Hyde, P.N. Resistance Training for Patients with Cancer: A Conceptual Framework for Maximizing Strength, Power, Functional Mobility, and Body Composition to Optimize Health and Outcomes. Sports Med. 2023, 53, 75–89. [Google Scholar] [CrossRef]
- Farley, M.J.; Boytar, A.N.; Adlard, K.N.; Salisbury, C.E.; Hart, N.H.; Schaumberg, M.A.; Jenkins, D.G.; Skinner, T.L. Interleukin-15 and high-intensity exercise: Relationship with inflammation, body composition and fitness in cancer survivors. J. Physiol. 2024, 602, 5203–5215. [Google Scholar] [CrossRef] [PubMed]
- Toohey, K.; Pumpa, K.; McKune, A.; Cooke, J.; Welvaert, M.; Northey, J.; Quinlan, C.; Semple, S. The impact of high-intensity interval training exercise on breast cancer survivors: A pilot study to explore fitness, cardiac regulation and biomarkers of the stress systems. BMC Cancer 2020, 20, 787. [Google Scholar] [CrossRef] [PubMed]
- Reljic, D.; Herrmann, H.J.; Jakobs, B.; Dieterich, W.; Mougiakakos, D.; Neurath, M.F.; Zopf, Y. Feasibility, Safety, and Preliminary Efficacy of Very Low-Volume Interval Training in Advanced Cancer Patients. Med. Sci. Sports Exerc. 2022, 54, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, K.; Schneider, J.; Sprave, T.; Wiskemann, J.; Rosenberger, F. Feasibility of Two High-Intensity Interval Training Protocols in Cancer Survivors. Med. Sci. Sports Exerc. 2019, 51, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Wender, C.L.A.; Manninen, M.; O’Connor, P.J. The Effect of Chronic Exercise on Energy and Fatigue States: A Systematic Review and Meta-Analysis of Randomized Trials. Front. Psychol. 2022, 13, 907637. [Google Scholar] [CrossRef] [PubMed]
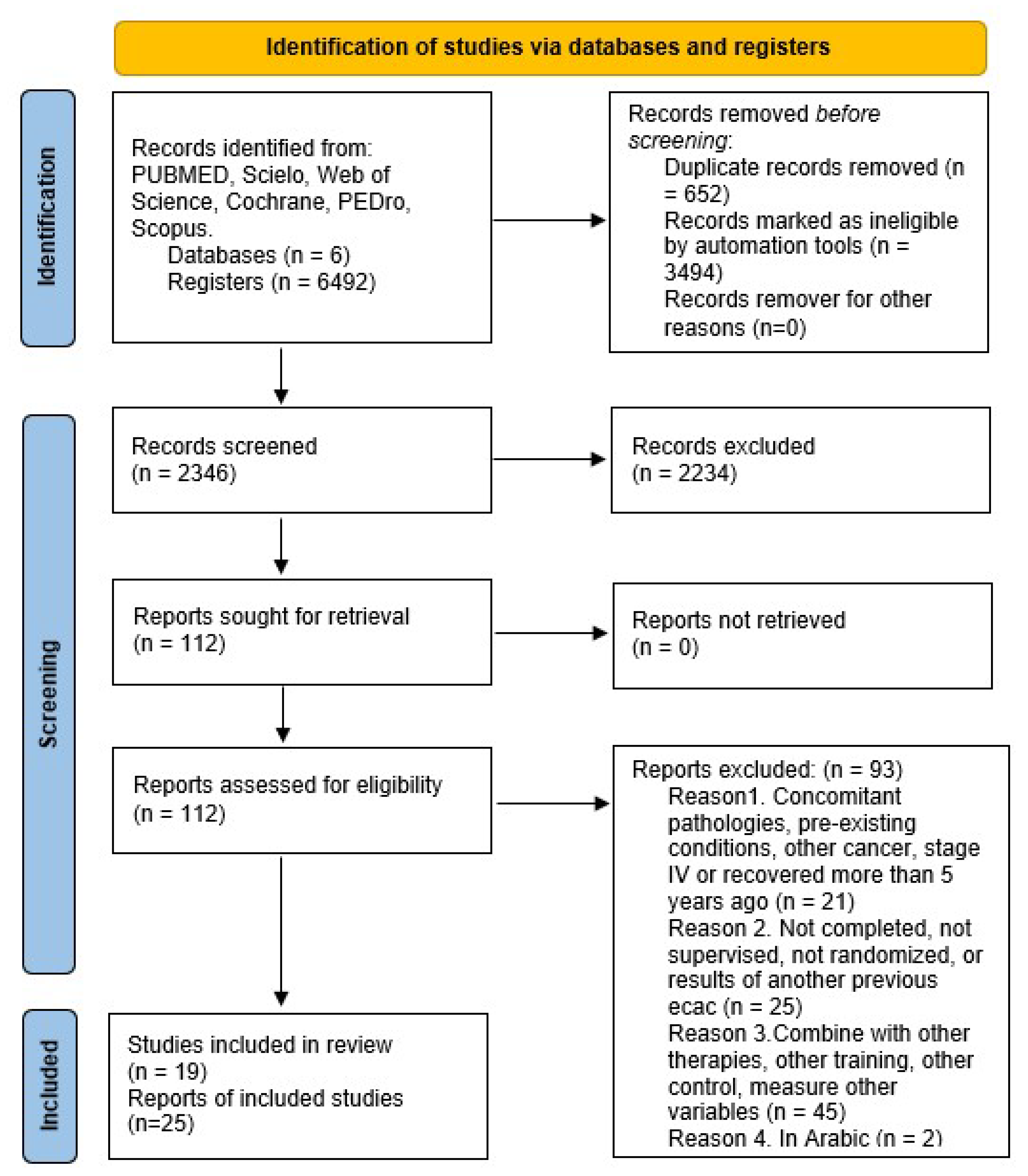
| Search Strategy | Natural Terms and Equations | Results Obtained |
|---|---|---|
| Pubmed | ||
| #1 | (“Breast cancer” [Title/Abstract] OR “Prostate cancer” [Title/Abstract]) | |
| #2 | (“supervised exercise” [Title/Abstract] OR “strength” [Title/Abstract] OR “aerobic” [Title/Abstract] OR “resistance” [Title/Abstract] OR “stretching” [Title/Abstract])) | |
| #3 | (“Fatigue” [Title/Abstract]) | |
| #4 | #1 AND #2 AND #3 | 848 |
| Scielo | ||
| #1 | (ab:(“Breast cancer” OR “Prostate cancer”)) | |
| #2 | (ab:(“ Supervised exercise” OR ab: “strength” OR ab:”aerobic” OR ab:”resistance” OR ab:”stretching”)) | |
| #3 | (ab:(“Fatigue”)) | |
| #4 | #1 AND #2 AND #3 | 5 |
| Web of Science | ||
| #1 | (“Breast cancer” OR “Prostate cancer”) | |
| #2 | (“supervised exercise” OR aerobic OR strength OR resistance OR strecthing) | |
| #3 | (“Fatigue”) | |
| #4 | Tittle/abstract: #1 AND #2 AND #3 | 1215 |
| Cochrane | ||
| #1 | (“Breast cancer” OR “Prostate cancer”) | |
| #2 | (“supervised exercise” OR aerobic OR strength OR resistance OR stretching) | |
| #3 | (“Fatigue”) | |
| #4 | Tittle/Abstract/Keywords #1 AND #2 AND #3 | 1945 |
| Scopus | ||
| #1 | (TITLE-ABS-KEY (“breast cancer”) OR TITLE-ABS-KEY (“Prostate cancer”)) | |
| #2 | (TITLE-ABS-KEY (“supervised exercise”) OR TITLE-ABS-KEY (aerobic) OR TITLE-ABS-KEY(strength) OR TITLE-ABS-KEY(resistance) OR TITLE-ABS-KEY(stretching)) | |
| #3 | (TITLE-ABS-KEY(“Fatigue”) | |
| #4 | #1 AND #2 AND #3 | 2407 |
| PEDro | ||
| Subdiscipline | Oncology | |
| Body Part | #1 Chest #2 Perineum or genito urinary system | |
| Therapy | #3 fitness training #4 strength training | |
| #1 + #3; #1 + #4; #2 + #3; #2 + #4 | 72 | |
| TOTAL | Pubmed +Scielo + Web of Science + Cochrane + Scopus + PEDro | 6492 |
| Author/Year | Country | Cancer Type | Sample Size | Age (SD) | Sex | Intervention Characteristics | Duration of Intervention | Measurement Tool | Results |
|---|---|---|---|---|---|---|---|---|---|
| Al-Majid et al., 2015 [55] | USA | Breast | IG: 7 CG: 7 | IG: 47.9 (10.4) CG: 52.7 (10.7) | W | IG: AT CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 12 weeks 2 days/week | PFS | IG Pre: 3.0 ± 0.7 Post: 3.0 ± 0.8 CG Pre: 0.8 ± 0.5 Post: 4.6 ± 0.9 |
| Schmidt et al., 2015 [56] | Germany | Breast | IG: 49 CG: 46 ITT | IG: 52.2 (9.9) CG: 53.3 (10.2) | W | IG: RT CG: Active Structured Controls (stretching) | 12 weeks 2 days/week | FAQ | IG Pre: 36.4 ± 19.2 Post: 36.1 ± 20.6 CG Pre: 41.0 ± 21.1 Post: 44.8 ± 21.0 |
| Schmidt et al., 2015 [57] | Germany | Breast | GRT: 21 GET: 20 CG: 26 | RT: 53 (12.55) ET: 56 (10.15) CG: 54 (11.19) | W | RT: RT ET: AT CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 12 weeks 2 days/week | MFI | GRT Pre: 9.25 ± 3.09 Post: 10.55 ± 3.22 GET Pre: 8.76 ± 4.31 Post: 12.35 ± 4.37 CG Pre: 9.54 ± 3.35 Post: 12.38 ± 3.50 |
| Travier et al., 2015 [58] | Netherlands | Breast | IG: 102 CG: 102 | IG: 49.7 (8.2) CG: 49.5 (7.9) | W | IG: Mixed CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 18 weeks 2 days/week | MFI | IG Pre: 10.1 ± 4.3 Δ change IC95% 1.9 [1.0 to 2.8] CG Pre: 10.6 ± 4.1 Δ change IC95% 2.3 [1.4 to 3.3] |
| Van Waart et al., 2015 [59] | Netherlands | Breast | IG: 76 CG: 77 | IG: 49.9 (8.4) CG: 51.6 (8.8) | W | CI: Mixed CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 20 weeks 2 days/week | MFI y FQL | IG Pre: 10.6 ± 4.1 Post: 13.1 ± 3.9 CG Pre: 11.7 ± 4.4 Post: 14.7 ± 4.2 |
| Mijwel et al., 2018 [60] | Sweden | Breast | GRT/HIIT: 74 GAT/HIIT: 72 CG: 60 | RT/HIIT: 52.7 (10.3) AT/HIIT: 54.4 (10.3) CG: 52.6 (10.2) | W | GRT/HIIT + RT GAT/HIIT +AT CG: Usual Care with General Recommendations (ACSM, WHO, healthy lifestyle advice) | 16 weeks 2 days/week | PFS | GRT/HIIT Pre: 3.09 ± 3.17 Post: 3.16 ± 2.9 GAT/HIIT Pre: 2.10 ± 2.63 Post: 3.16 ± 2.6 CG Pre: 2.30 ± 2.81 Post: 3.94 ± 2.95 |
| Ammitzbøll et al., 2019 [29] | Denmark | Breast | IG: 82 CG: 76 | IG: 53 (33–73) CG: 52 (30–74) | W | IG: RT CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 20 weeks 2 days/week | FACIT-F | IG Δ change IC95% 2.71 [−0.1 to 5.5] CG Δ change IC95% 0.00 Reference |
| Baglia et al., 2019 [61] | USA | Breast | IG: 61 CG: 60 | IG: 61.2 (7.09 CG: 62.0 (7.0) | W | IG: RT + AT CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 52 weeks 2 days/week | Facit-F | IG Pre: 37.9 ± 10.6 Post: 43.6 ± 10.6 CG Pre: 36.2 ± 10.8 Post: 36.7 ± 10.8 |
| Mijwel et al., 2019 [36] | Sweden | Breast | GRT/HIIT: 74 GAT/HIIT: 72 CG: 60 | GRT/HIIT: 52.7 (10.3) GAT/HIIT: 54.4 (10.3) CG: 52.6 (10.2) | W | RT: HIIT + RT AT HIIT: HIIT + AT CG: Usual Care with General Recommendations (ACSM, WHO, healthy lifestyle advice) | 16 weeks 2 days/week | PFS | GRT/HIIT Pre: 3.09 ± 3.17 Post: 3.12 ± 3.03 GAT/HIIT Pre: 2.10 ± 2.63 Post: 3.18 ± 2.77 CG Pre: 2.30 ± 2.81 Post: 3.98 ± 3.05 |
| Hojan et al., 2020 [62] | Poland | Prostate | IG: 36 CG: 36 ITT | IG: 65.7 (6.2) CG: 67.9 (4.9) | M | IG: Mixed CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 8 weeks 5 days/week | FACT-F | IG Pre: 113.4 ± 3.5 Post: 117.9 ± 9.7 CG Pre: 112.9 ± 3.9 Post: 81.5 ± 9.7 |
| Ndjavera et al., 2020 [63] | UK | Prostate | IG: 24 CG: 26 ITT | IG: 71.4 (5.4) CG: 72.5 (4.2) | M | IG: Mixed CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 12 weeks 2 days/week | Facit-F | IG Pre: 41.8 ± 10.2 Post: 41.8 ± 11.2 CG Pre: 42.9 ± 8.4 Post: 38.5 ± 11.9 |
| Pereira et al., 2020 [64] | Mexico | Breast | GMICT: 80 GHIIT: 70 CG: 66 | GMICT: 51 (4) GHIIT: 55 (5) CG: 53 (7) | W | MICT: Mixed HIIT: HIIT + RT CG: Usual Care with General Recommendations (ACSM, WHO, healthy lifestyle advice) | 36 weeks 3 days/week | FACT-F | GMICT Pre: 18.6 ± 9.5 Post: 8.0 ± 4.2 GHIIT Pre: 20.4 ± 5.6 Post: 5.1 ± 3.6 CG Pre: 16.6 ± 5.6 Post: 16.9 ± 4.6 |
| Piraux et al., 2020 [65] | Belgium | Prostate | GHIIT: 24 GRES: 24 CG: 24 | GHIIT: 67.4 (8.9) GRES: 67.9 (7.1) CG: 71.9 (8.1) | M | HIIT: HIIT RT: RT CG: Usual Care with General Recommendations (ACSM, WHO, healthy lifestyle advice) | 5–8 weeks 3 days/week | Facit-F | GHIIT Pre: 43.1 ± 6.9 Post: 42.1 ± 10.3 GRES Pre: 41.2 ± 7.7 Post: 40.5 ± 9.8 CG Pre: 41.1 ± 9.0 Post: 40.5 ± 9.8 |
| Scott et al., 2020 [66] | USA | Breast | GLET: 58 GNLET: 59 CG: 57 | GLET: 58 (9) GNLET: 59 (9) CG: 58 (9) | W | LET: AT NLET: AT + HIIT CG: Active Structured Controls (stretching) | 16 weeks 3–4 days/week | Facit-F | GLET Pre: 36.7 ± 11.9 Post: 39.5 ± 12.2 GNLET Pre: 42.8 ± 8.9 Post: 44.8 ± 9.0 CG Pre: 39.6 ± 10.9 Post: 39.9 ± 10.7 |
| Gal et al., 2021 [67] | Netherlands | Breast | IG: 68 CG: 114 | IG: 58.0 (9.8) CG: 58.3 (9.5) | W | IG: Mixed CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 12 weeks 2 days/week | MFI | IG Pre: 11.6 ± 4.7 Δ change IC95% −1.0 [−1.8 to −0.1] CG Pre: 10.6 ± 4.3 Δ change IC95% −0.3 [−1.0 to 0.4] |
| Moraes et al., 2021 [68] | Brazil | Breast | IG: 13 CG: 13 | IG: 55.0 (5.8) CG: 54.3 (5.2) | W | IG: RT CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 8 weeks 1 day/week | PFS | IG Pre: 5.1 ± 2.7 Post: 2.3 ± 1.4 CG Pre: 3.9 ± 2.0 Post: 3.0 ± 2.4 |
| Harrison et al., 2022 [34] | USA | Prostate | IG: 13 CG: 13 | IG: 65.7 (8.1) CG: 64.4 (8.3) | M | IG: Mixed CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 16 weeks 3 days/week | Facit-F | IG vs. CC Δ change IC95% +4.0 [−3.2 to 11.1] |
| Kang et al., 2022 [35] | Canada | Prostate | IG: 26 CG: 26 | IG-CG: 63.4 (7.1) | M | IG: HIIT CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 12 weeks 3 days/week | FACT-F | IG Pre: 43.6 ± 6.6 Post: 46.0 ± 4.3 CG Pre: 45.3 ± 4.7 Post: 44.6 ± 6.0 |
| Koevoets et al., 2022 [69] | Netherlands | Breast | IG: 91 CG: 90 | IG: 52.1 (8.6) CG: 52.5 (8.7) | W | IG: Mixed CG: Passive Usual Care (no exercise/maintain habitual activity/no specific advice) | 6 months 4 days/week | MFI | IG vs. CC Δ change IC95% +2.22 [1.11 to 3.32] |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Madjid et al., 2015 [55] | + | + | - | + | - | - | - | + | - | + | + | 5 |
| Schmidt et al., 2015 [56] | + | + | + | - | - | - | - | - | - | + | + | 5 |
| Schmidt et al., 2015 [57] | + | + | - | + | - | - | - | + | + | + | + | 6 |
| Travier et al., 2015 [58] | + | + | + | + | - | - | + | + | + | + | + | 8 |
| Van Waart et al., 2015 [59] | + | + | - | + | - | - | - | + | + | + | + | 6 |
| Mijwel et al., 2018 [60] | + | + | - | + | - | - | - | - | - | + | + | 4 |
| Ammitzbøll et al., 2019 [29] | + | + | - | + | - | - | - | - | + | + | + | 5 |
| Baglia et al., 2019 [61] | + | + | + | + | - | - | - | + | + | + | + | 7 |
| Mijwel et al., 2019 [36] | + | + | - | + | - | - | - | + | + | + | + | 6 |
| Hojan 2020 [62] | + | + | + | + | - | - | - | + | + | + | + | 7 |
| Ndjavera et al., 2020 [63] | + | + | + | + | - | - | + | - | + | + | + | 7 |
| Pereira et al., 2020 [64] | + | + | + | + | - | - | + | - | - | + | + | 6 |
| Piraux et al., 2020 [65] | - | + | - | + | - | - | - | + | + | + | + | 6 |
| Scott et al., 2020 [66] | - | + | - | + | - | - | - | + | + | + | + | 6 |
| Gal et al., 2021 [67] | + | + | - | + | - | - | - | - | + | + | + | 5 |
| Moraes et al., 2021 [68] | + | + | - | + | - | - | - | + | + | + | + | 6 |
| Harrison et al., 2022 [34] | - | + | - | + | - | - | + | + | - | + | + | 6 |
| Kang et al., 2022 [35] | + | + | - | + | - | - | - | + | + | + | + | 6 |
| Koevoets et al., 2022 [69] | - | + | - | + | - | - | - | + | + | + | + | 6 |
| Author, Year | ES | LL | UL | I2 |
|---|---|---|---|---|
| Ammitzbøll et al., 2019 [29] R | 0.35 | 0.22 | 0.48 | 56.7 |
| Harrison et al., 2022 [34] C | 0.34 | 0.21 | 0.47 | 55.8 |
| Kang et al., 2022 [35] H | 0.34 | 0.21 | 0.47 | 57.6 |
| Mijwel 2019 [36] R + HIIT | 0.33 | 0.20 | 0.47 | 57.2 |
| Mijwel 2019 [36] A + HIIT | 0.35 | 0.21 | 0.48 | 57.4 |
| Al Majid et al., 2015 [55] A | 0.33 | 0.20 | 0.45 | 55.7 |
| Schmidt el al. 2015 [56] A | 0.34 | 0.22 | 0.47 | 57.3 |
| Schmidt el al. 2015 [57] R | 0.35 | 0.22 | 0.48 | 57.2 |
| Schmidt et al., 2015 [57] R | 0.35 | 0.22 | 0.48 | 56.5 |
| Travier et al., 2015 [58] C | 0.36 | 0.23 | 0.49 | 54.8 |
| Van Waart et al., 2015 [59] C | 0.34 | 0.22 | 0.47 | 57.6 |
| Mijwel 2018 [60] A + HIIT | 0.35 | 0.22 | 0.48 | 56.1 |
| Mijwel 2018 [60] R + HIIT | 0.35 | 0.21 | 0.48 | 56.9 |
| Baglia et al., 2019 [61] C | 0.33 | 0.20 | 0.46 | 56.9 |
| Hojan et al., 2019 [62] C | 0.28 | 0.19 | 0.36 | 0.0 |
| Ndjavera et al., 2020 [63] C | 0.34 | 0.21 | 0.47 | 57.7 |
| Pereira et al., 2020 [64] A | 0.33 | 0.20 | 0.47 | 57.5 |
| Pereira et al., 2020 [64] H | 0.34 | 0.20 | 0.47 | 59.5 |
| Piraux et al., 2020 [65] H | 0.34 | 0.21 | 0.47 | 57.6 |
| Piraux et al., 2020 [65] R | 0.34 | 0.21 | 0.47 | 57.6 |
| Scott et al., 2020 [66] A C | 0.35 | 0.22 | 0.48 | 56.3 |
| Scott et al., 2020 A I [66] | 0.35 | 0.22 | 0.48 | 55.9 |
| Gal et al., 2021 [67] C | 0.36 | 0.23 | 0.48 | 53.3 |
| Moraes et al., 2021 [68] R | 0.33 | 0.21 | 0.46 | 57.4 |
| Koevoets et al., 2022 [69] C | 0.34 | 0.21 | 0.47 | 57.6 |
| Exercise | ES | LL | UL | I2 |
|---|---|---|---|---|
| Endurance | 0.258 | 0.052 | 0.518 | 26.4 |
| Combined | 0.421 | 0.095 | 0.747 | 80.0 |
| HIIT | 0.321 | 0.175 | 0.468 | 0.0 |
| Resistance | 0.250 | 0.062 | 0.439 | 0.0 |
| Duration | ES | LL | UL | I2 |
|---|---|---|---|---|
| ≤12 weeks | 0.407 | 0.131 | 0.682 | 72.7 |
| >12–24 weeks | 0.234 | 0.131 | 0.682 | 0.0 |
| >24 weeks | 0.474 | 0.290 | 0.679 | 0.0 |
| Gender | ES | LL | UL | I2 |
|---|---|---|---|---|
| Men | 0.555 | 0.254 | 0.855 | 55.0 |
| Women | 0.274 | 0.190 | 0.362 | 0.0 |
| Cancer Type | ES | LL | UL | I2 |
|---|---|---|---|---|
| Prostate | 0.555 | 0.254 | 0.855 | 55.0 |
| Breast | 0.274 | 0.186 | 0.362 | 0.0 |
| Exercise vs. Control Group | Coefficient | p-Value |
|---|---|---|
| 0.284 | 0.672 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Uceda, A.; García-Fernández, P.; Peuyadé-Rueda, B.; Cañuelo-Marquez, A.M.; Solís-Mencía, C.; Lucio-Allende, C.; De Sousa-De Sousa, L.; Maté-Muñoz, J.L. From Evidence to Practice: A Systematic Review and Meta-Analysis on the Effects of Supervised Exercise on Fatigue in Breast and Prostate Cancer Survivors. Appl. Sci. 2025, 15, 8399. https://doi.org/10.3390/app15158399
Cano-Uceda A, García-Fernández P, Peuyadé-Rueda B, Cañuelo-Marquez AM, Solís-Mencía C, Lucio-Allende C, De Sousa-De Sousa L, Maté-Muñoz JL. From Evidence to Practice: A Systematic Review and Meta-Analysis on the Effects of Supervised Exercise on Fatigue in Breast and Prostate Cancer Survivors. Applied Sciences. 2025; 15(15):8399. https://doi.org/10.3390/app15158399
Chicago/Turabian StyleCano-Uceda, Arturo, Pablo García-Fernández, Blanca Peuyadé-Rueda, Ana María Cañuelo-Marquez, Cristian Solís-Mencía, Carmen Lucio-Allende, Luis De Sousa-De Sousa, and José Luis Maté-Muñoz. 2025. "From Evidence to Practice: A Systematic Review and Meta-Analysis on the Effects of Supervised Exercise on Fatigue in Breast and Prostate Cancer Survivors" Applied Sciences 15, no. 15: 8399. https://doi.org/10.3390/app15158399
APA StyleCano-Uceda, A., García-Fernández, P., Peuyadé-Rueda, B., Cañuelo-Marquez, A. M., Solís-Mencía, C., Lucio-Allende, C., De Sousa-De Sousa, L., & Maté-Muñoz, J. L. (2025). From Evidence to Practice: A Systematic Review and Meta-Analysis on the Effects of Supervised Exercise on Fatigue in Breast and Prostate Cancer Survivors. Applied Sciences, 15(15), 8399. https://doi.org/10.3390/app15158399









