Enzymatic Degumming of Soybean Oil for Raw Material Preparation in BioFuel Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
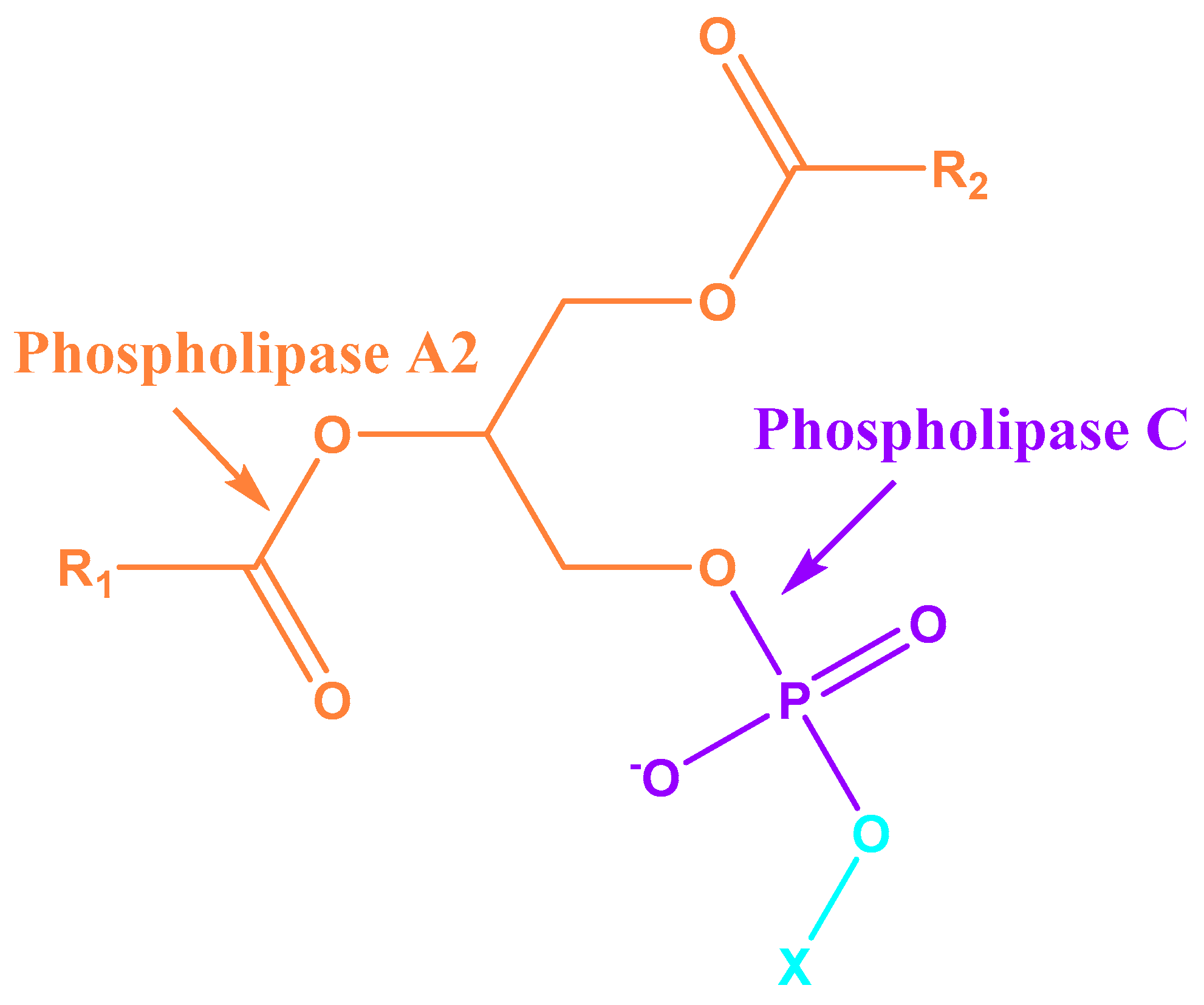
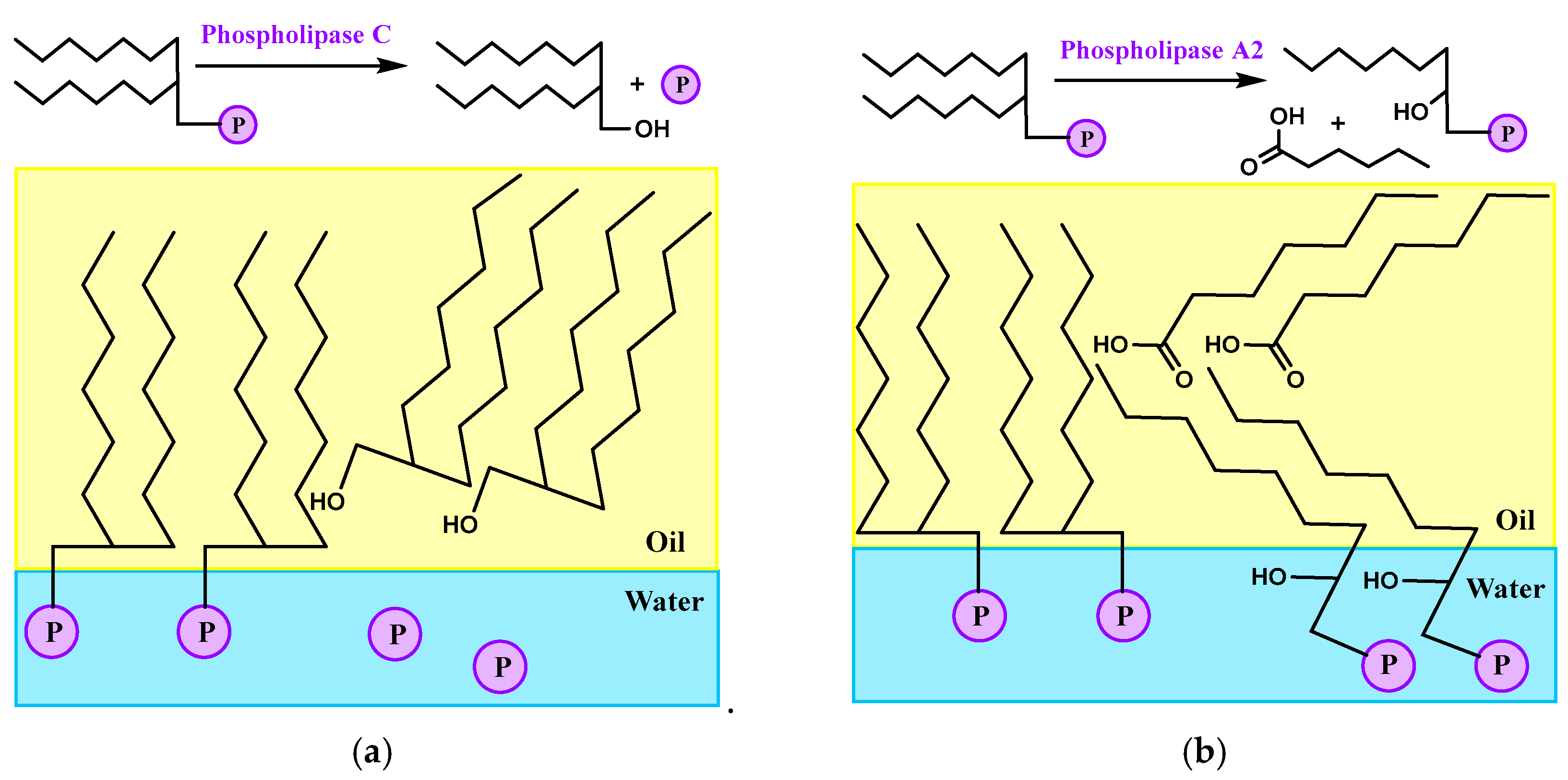
2.2.1. Enzymatic Reaction
2.2.2. Physical and Chemical Properties of Raw Materials/Products
2.2.3. GC-MS Analysis
2.2.4. FTIR Spectra Record
3. Results
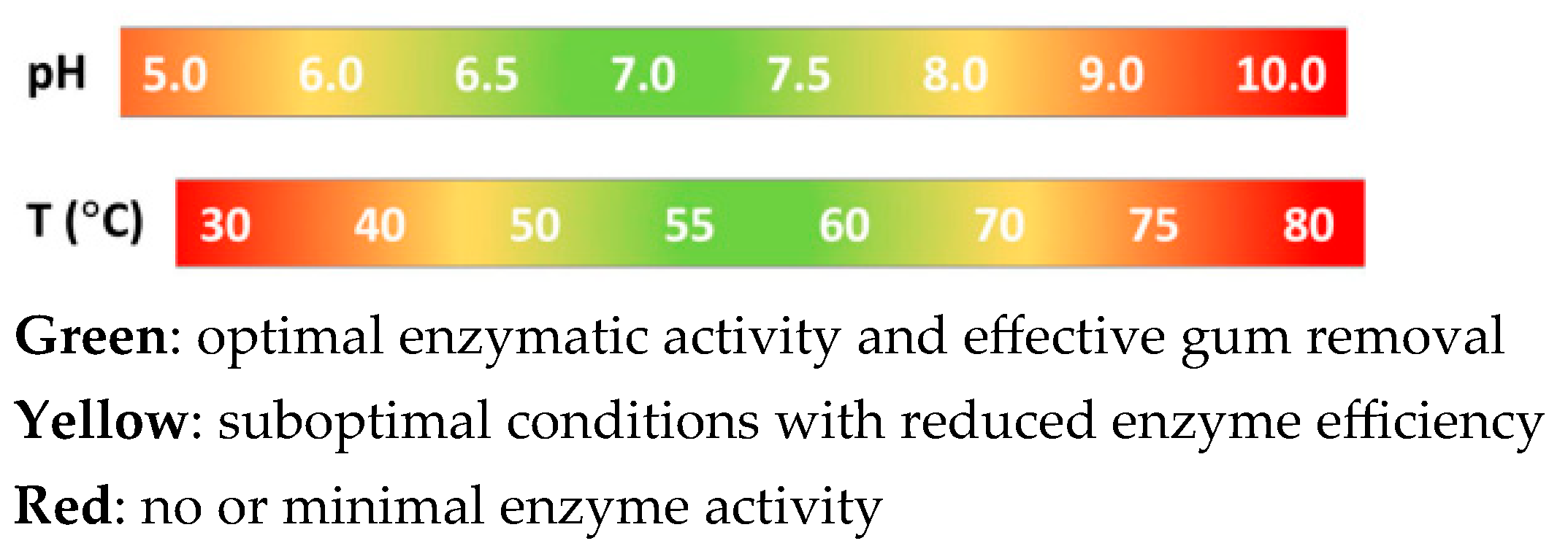
- Temperature: 50–70 °C, optimal for enzyme activity.
- pH value: 5.5–8.0, which ensures stable enzyme operation.
- Reaction duration: 1–5 h, depending on the quality of the starting material.
- Enzyme dose: 50–250 ppm, determined according to the concentration of phospholipids in the raw material.
3.1. Effect of Enzyme Dose on Degumming Efficiency
3.2. The Effect of Temperature on the Enzymatic Degumming Process
3.3. Determining the Optimal pH Value
3.4. Effect of Water Dosage on Degumming Efficiency
3.5. Study of Enzymatic Degumming Duration on Degumming Efficiency
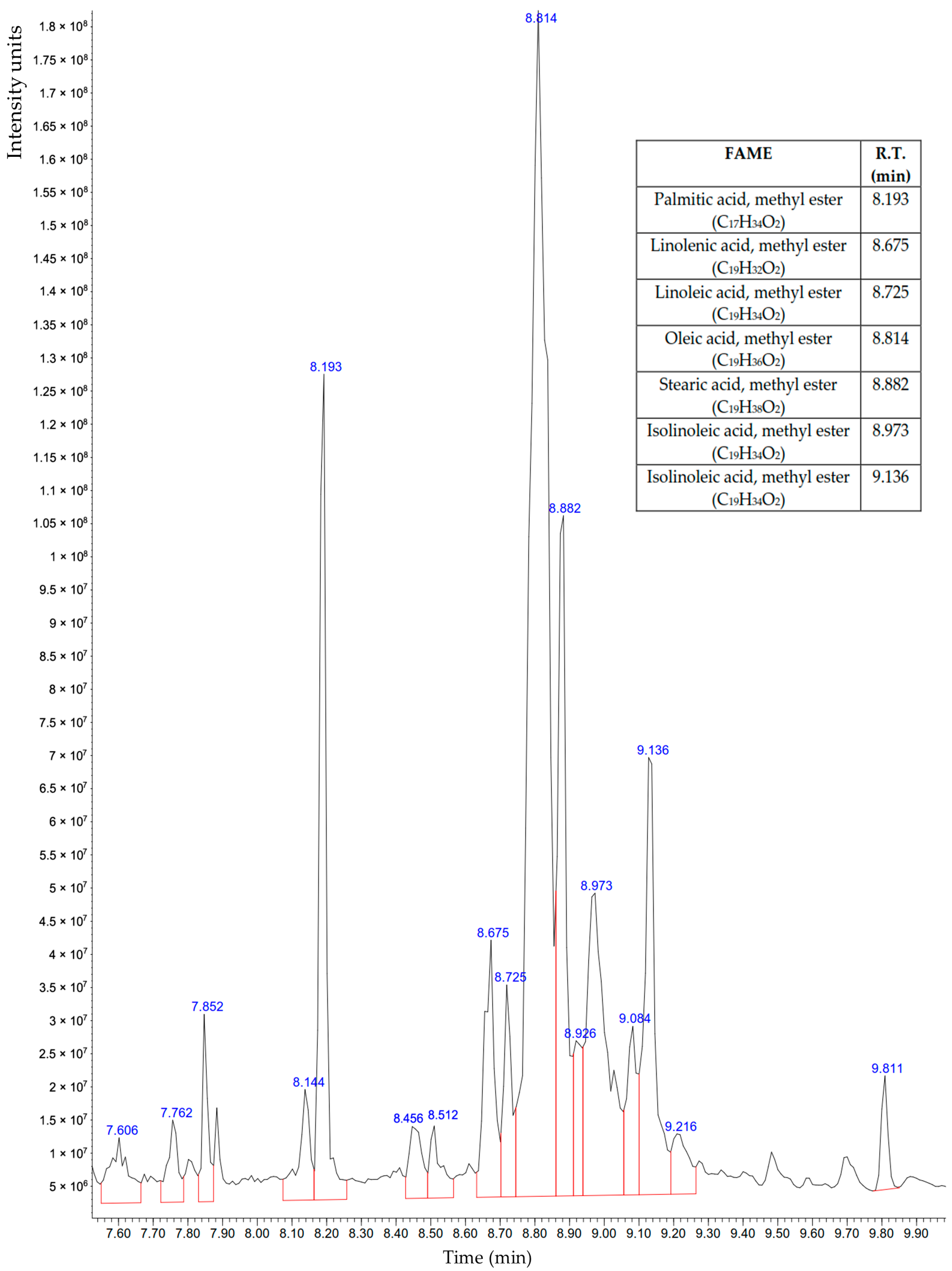
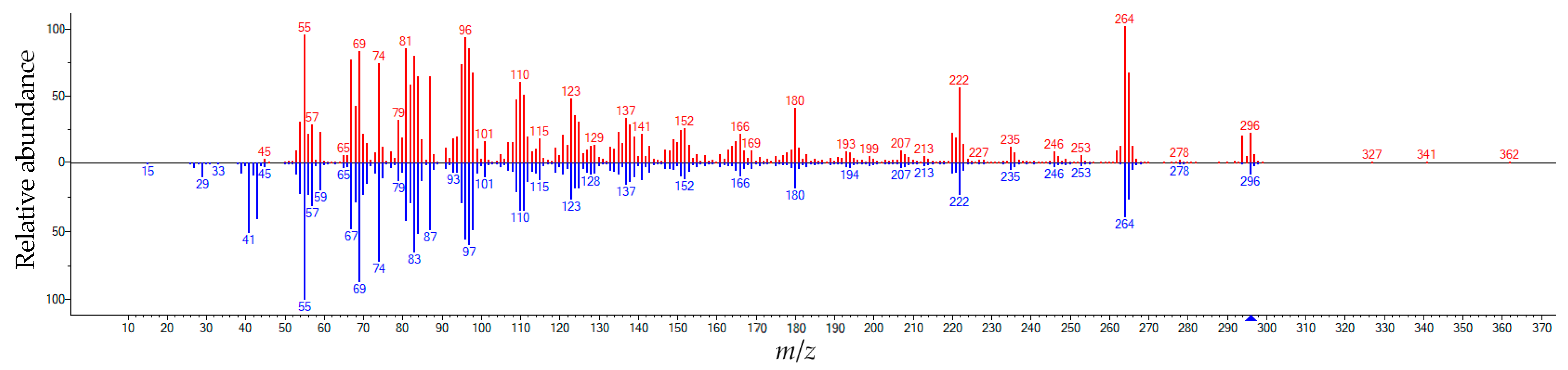
3.6. Analysis of the Transesterification Product by GC-MS
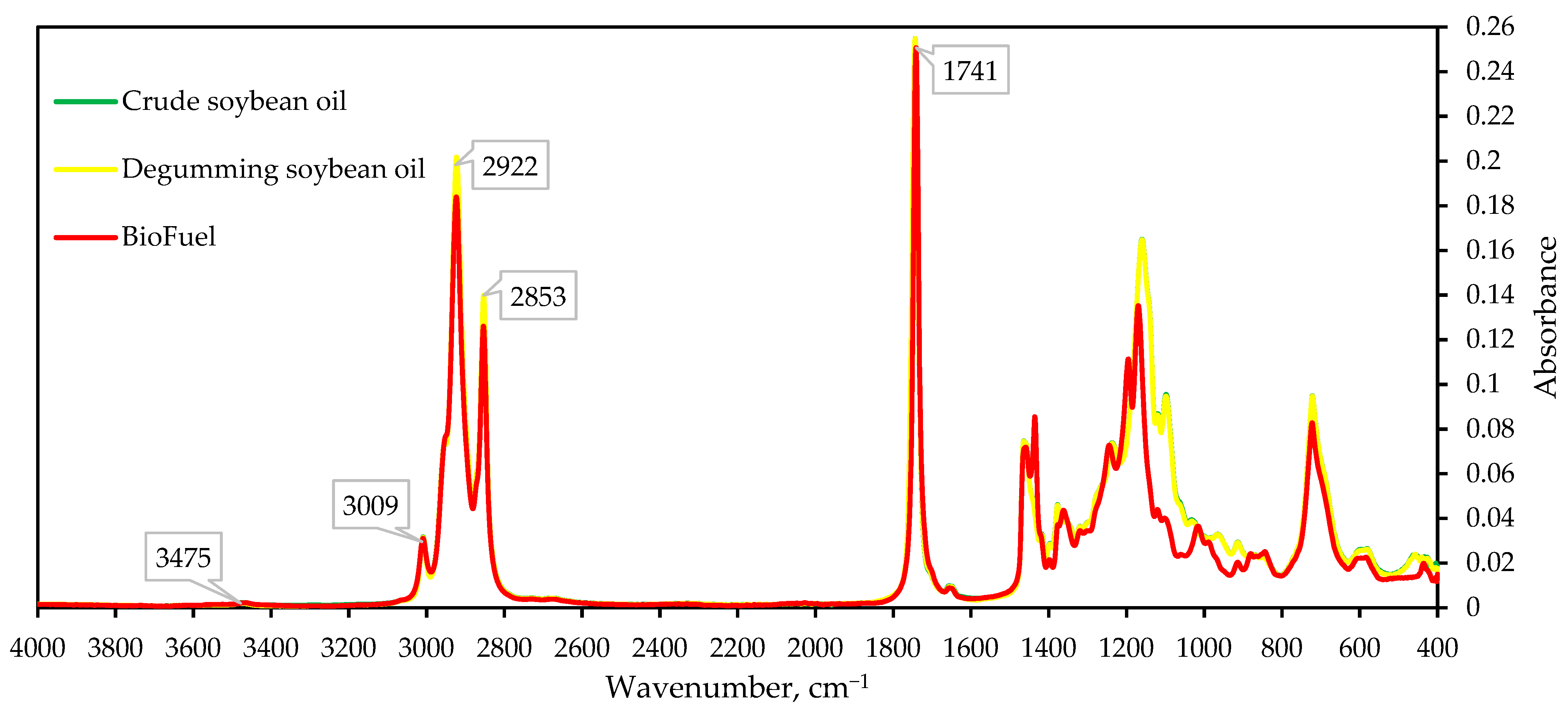
3.7. FTIR Spectral Analysis of Crude Soybean Oil, Enzymatically Degummed Soybean Oil, and the Resulting Biofuel
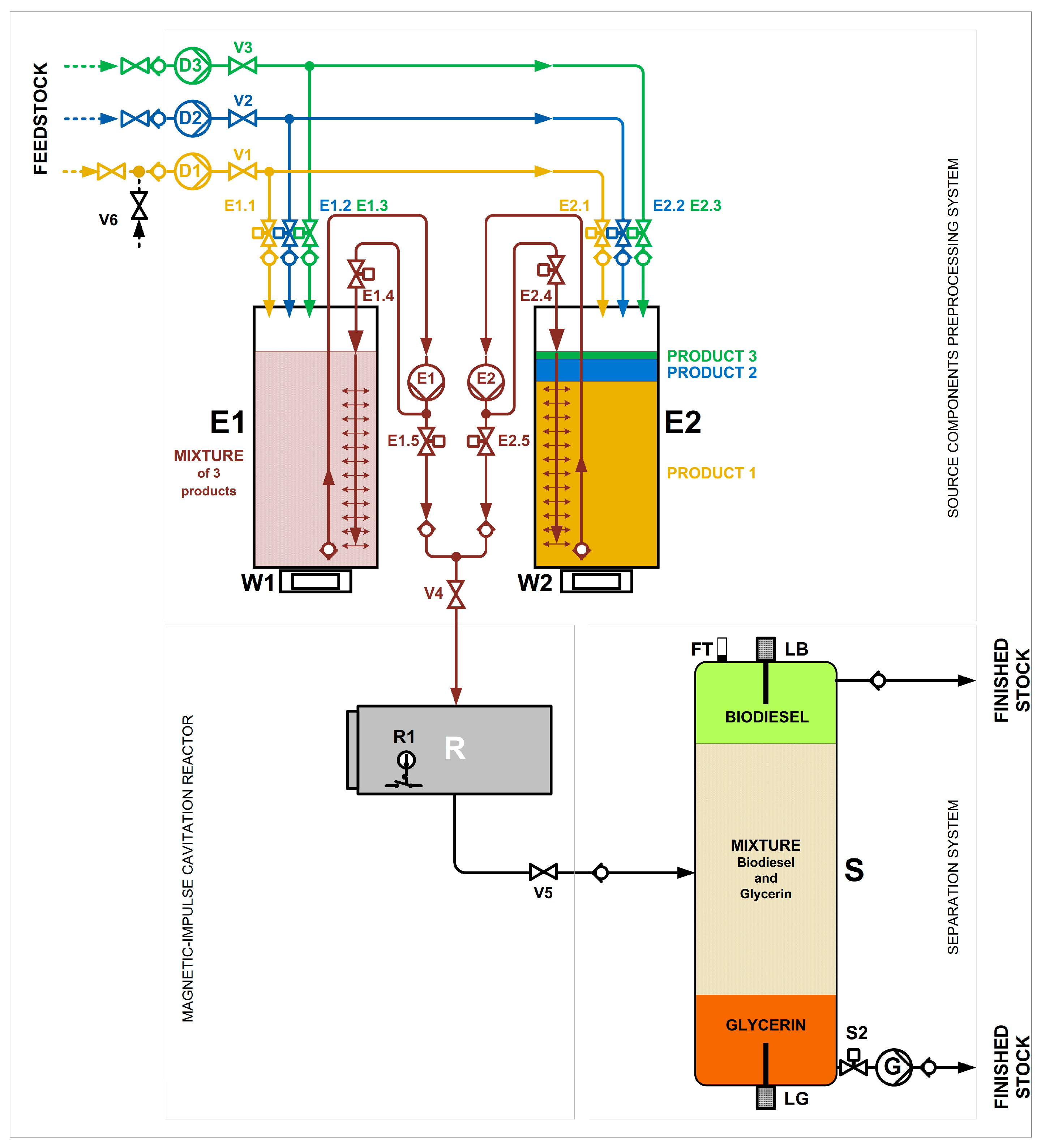
3.8. Pilot Industrial Implementation
- (1)
- enzymatic degumming of crude soybean oil;
- (2)
- production of BioFuel from degumming soybean oil (using a cavitation unit BIOTRON-R 1000 (CT Systems Corporation, 2910-567 Setúbal, Portugal)).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAME | Fatty acid methyl ester |
| FFA | Free fatty acids |
| FTIR | Fourier-transform infrared spectroscopy |
| GC-MS | Gas Chromatography Mass Spectrometry |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PI | Phosphatidylinositol |
| PL-A2 | Phospholipase A2 |
| PL-C | Phospholipase C |
| PL | Phospholipids FFA |
References
- Dijkstra, A.J. Enzymatic degumming. Eur. J. Lipid Sci. Technol. 2010, 112, 1178–1189. [Google Scholar] [CrossRef]
- Yang, P.; Wu, Y.; Jiang, S.; Zheng, Z.; Hou, Z.; Mu, D.; Xiao, W.; Jiang, S.; Yang, Y.H. Effective Expression of the Serratia marcescens Phospholipase A1 Gene in Escherichia coli BL21(DE3), Enzyme Characterization, and Crude Rapeseed Oil Degumming via a Free Enzyme Approach. Front. Bioeng. Biotechnol. 2019, 7, 272. [Google Scholar] [CrossRef]
- Yu, D.; Ma, Y.; Xue, S.J.; Jiang, L.; Shi, J. Characterization of immobilized phospholipase A1 on magnetic nanoparticles for oil degumming application. LWT-Food Sci. Technol. 2013, 50, 519–525. [Google Scholar] [CrossRef]
- Ambrosewicz-Walacik, M.; Tańska, M.; Rotkiewicz, D. Phospholipids of rapeseeds and rapeseed oils: Factors determining their content and technological significance—A review. Food Rev. Int. 2015, 31, 385–400. [Google Scholar] [CrossRef]
- dos Passos, R.M.; da Silva, R.M.; de Almeida Pontes, P.V.; Morgano, M.A.; Meirelles, A.J.; Stevens, C.V.; Ferreira, M.C.; Sampaio, K.A. Phospholipase cocktail: A new degumming technique for crude soybean oil. LWT 2022, 159, 113197. [Google Scholar] [CrossRef]
- Chen, L.; Gao, Y.; He, M.; Liu, Y.; Teng, F.; Li, Y. Magnetic nanoparticles-immobilized phospholipase LM and phospholipase 3G: Preparation, characterization, and application on soybean crude oil degumming. Int. J. Biol. Macromol. 2024, 279 Pt 3, 135368. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.D.; Farr, W.E.; Wan, P.J. Introduction to Fats and Oils Technology; AOCS Press: Champaign, IL, USA, 2000; 618p. [Google Scholar]
- Lee, Y.Y.; Tang, T.K.; Phuah, E.T.; Lai, O.M. Vegetable Oil Refining. In Recent Advances in Edible Fats and Oils Technology; Springer Nature Singapore Pte Ltd.: Singapore, 2022. [Google Scholar] [CrossRef]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Cipolatti, E.P.; Manoel, E.A.; Pastore, C.; di Bitonto, L.; Hanelt, D.; Nitbani, F.O.; et al. Has the time finally come for green oleochemicals and biodiesel production using large-scale enzyme technologies? Current status and new developments. Biotechnol. Adv. 2023, 69, 108275. [Google Scholar] [CrossRef] [PubMed]
- Khamies, M.; Hagar, M.; Kassem, T.S.; Moustafa, A.H.E. Case study of chemical and enzymatic degumming processes in soybean oil production at an industrial plant. Sci. Rep. 2024, 14, 4064. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Nyam, K.L. Refining of edible oils. In Lipids and Edible Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 213–241. [Google Scholar] [CrossRef]
- O’Brien, R.D. Soybean oil purification. In Soybeans; AOCS Press: Cambridge, MA, USA, 2008; pp. 377–408. Available online: https://www.sciencedirect.com/science/article/pii/B9781893997646500159 (accessed on 5 May 2025).
- Ma, Y.; Shi, L.; Liu, Y.; Lu, Q. Effects of Neutralization, decoloration, and deodorization on polycyclic aromatic hydrocarbons during laboratory-scale oil refining process. J. Chem. 2017, 2017, 7824761. [Google Scholar] [CrossRef]
- Cerminati, S.; Paoletti, L.; Aguirre, A.; Peirú, S.; Menzella, H.G.; Castelli, M.E. Industrial uses of phospholipases: Current state and future applications. Appl. Microbiol. Biotechnol. 2019, 103, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.; Hung, Y.T. Advances in treatment of vegetable oil refining wastes. Environ. Nat. Resour. Eng. 2021, 19, 325–375. [Google Scholar] [CrossRef]
- Casado, V.; Martín, D.; Torres, C.; Reglero, G. Phospholipases in food industry: A review. Lipases Phospholipases Methods Protoc. 2012, 861, 495–523. [Google Scholar] [CrossRef]
- Sousa, R.R.d.; dos Santos, M.M.; Medeiros, M.W.R.; Manoel, E.A.; Berenguer-Murcia, Á.; Freire, D.M.G.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Immobilized Lipases in the Synthesis of Short-Chain Esters: An Overview of Constraints and Perspectives. Catalysts 2025, 15, 375. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.N.; Uversky, V.N. Enzymology: Early insights. In Structure and Intrinsic Disorder in Enzymology; Academic Press: Cambridge, MA, USA, 2023; pp. 1–29. [Google Scholar] [CrossRef]
- DSTU EN ISO 3675:2012; Crude Petroleum and Liquid Petroleum Products. Laboratory Determination of Density. Hydrometer Method (EN ISO 3675). Ministry of Economic Development of Ukraine: Kyiv, Ukraine, 2012.
- DSTU EN ISO 3104:2022; Petroleum Products. Transparent and Opaque Liquids. Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity (EN ISO 3104). SE “Ukrainian Scientific Research and Training Center for Standardization, Certification and Quality”: Kyiv, Ukraine, 2022.
- DSTU ISO 2719:2006; Determination of Flash Point. Pensky. Martens Closed CUP Method (ISO 2719). Ukrainian Research Institute of Oils and Fats: Kharkiv, Ukraine, 2006.
- DSTU ISO 20846:2009; Petroleum Products. Determination of Sulfur Content of Automotive Fuels. Ultraviolet Fluorescence Method (ISO 20846). Ukrainian Research Institute of Oil Refining Industry "MASMA": Kyiv, Ukraine, 2009.
- DSTU 7082:2009; Vegetable Oils. Methods for Determination of Mass Concentration Phosphorated Content (Ukrainian Standard). Technical Committee “Oils, Fats and Products of Their Processing”: Kyiv, Ukraine, 2009.
- DSTU EN ISO 8534:2019; Animal and Vegetable Fats and Oils. Determination of Water Content Karl Fischer Method (Pyridine Free) (EN ISO 8534). SE “Ukrainian Scientific Research and Training Center for Standardization, Certification and Quality”: Kyiv, Ukraine, 2019.
- DSTU EN ISO 2160:2012; Petroleum Products. Corrosiveness to Copper. Copper Strip Test (EN ISO 2160). Ukrainian Research Institute of Oil Refining Industry “MASMA”: Kyiv, Ukraine, 2012.
- DSTU EN 14104:2009; Fat and Oil Derivates. Fatty Acid Methyl Ester (FAME). Determination of ACID value (EN 14104). Kharkiv Petro Vasylenko National Technical University of Agriculture: Kharkiv, Ukraine, 2009.
- DSTU EN ISO 3961:2019; Animal and Vegetable Fats and Oils. Determination of Iodine Value (EN ISO 3961). Spanish Association for Standardization: UNE: Madrid, Spain, 2007.
- DSTU EN 14103:2009; Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Ester and Linolenic Acid Methyl Ester Contents (EN 14103). Kharkiv National Technical University of Agriculture: Kharkiv, Ukraine, 2009.
- DSTU EN 14110:2009; Fat and Oil Derivatives. Fatty Acid Methyl Esters. Determination of Methanol Content (EN 14110). National University of Life and Environmental Sciences of Ukraine: Kyiv, Ukraine, 2009.
- DSTU EN 14105:2009; Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Free and Total Glycerol and Mono-, Di-, Triglyceride Contents (EN 14105). Kharkiv Petro Vasylenko National Technical University of Agriculture: Kharkiv, Ukraine, 2009.
- Ye, Z.; Qiao, X.; Luo, Z.; Hu, C.; Liu, L.; He, D. Optimization and comparison of water degumming and phospholipase C degumming for rapeseed oil. CyTA-J. Food 2016, 14, 604–612. [Google Scholar] [CrossRef][Green Version]
- Mueller, U.; Pascal, G.; Olempska-Beer, Z.; Leblanc, J.; Meyland, M.I. Phospholipase C Expressed in Pichia pastoris. In Safety Evaluation of Certain Food Additives, Proceedings of the Sixty-Ninth Meeting of the FAO/WHO Expert Committee on Food Additives (JECFA), Rome, Italy, 17–26 June 2008; WHO Food Additives Series; WHO: Geneva, Switzerland, 2009; Volume 60, pp. 107–115. [Google Scholar]
- DSTU 6081:2009; Automotive Fuels. Fatty Acid Methyl Esters of Fat and Oil for DIESEL Engines. Technical Requirements (Ukrainian Standard). National University of Life and Environmental Sciences of Ukraine: Kyiv, Ukraine, 2009.
- DSM. Annual Report 2022. Koninklijke DSM N.V. 2022. Available online: https://www.dsm.com/corporate/about/business/highlights/annual-reports.html (accessed on 16 July 2025).
- Gunstone, F.D.; Harwood, J.L. The Lipid Handbook with CD-ROM; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]

| Properties | Units of Measurement | Value | Methods |
|---|---|---|---|
| Density at 15 °C | kg/m3 | 922 | [20] |
| Kinematic viscosity at 40 °C | mm2/s | 30.7 | [21] |
| Flash point | °C | 345 | [22] |
| Sulphur content | ppm | 15 | [23] |
| Phosphorus content | ppm | 421 | [24] |
| Water content | ppm | 200 | [25] |
| Copper strip test (3 h at 50 °C) | - | withstands class 1 | [26] |
| Acid value | mg KOH/g | 0.49 | [27] |
| Iodine value | g I2/100 g | 39.0 | [28] |
| Dosage of Purifine® 3G Enzyme Complex (ppm) | Phosphorous Content (ppm) | FFA (Acid Value, mg KOH/g) |
|---|---|---|
| 50 | 12.0 | 0.67 |
| 100 | 9.5 | 0.85 |
| 150 | 8.7 | 1.17 |
| 200 | 8.3 | 1.59 |
| 250 | 7.8 | 2.15 |
| Enzymatic Degumming Temperature (°C) | Phosphorous Content (ppm) | FFA (Acid Value, mg KOH/g) |
|---|---|---|
| 50–52 | 35.0 | 0.61 |
| 53–55 | 15.0 | 0.79 |
| 56–58 | 10.0 | 0.86 |
| 59–61 | 10.2 | 0.82 |
| 62–64 | 18.1 | 0.80 |
| 65–67 | 52.3 | 0.80 |
| 68–70 | 128.4 | 0.79 |
| pH Value | Phosphorous Content (ppm) | FFA (Acid Value, mg KOH/g) |
|---|---|---|
| 6.3–6.5 | 26.2 | 0.59 |
| 6.6–6.8 | 12.1 | 0.81 |
| 6.9–7.1 | 9.5 | 0.88 |
| 7.2–7.4 | 19.2 | 0.85 |
| 7.5–7.7 | 37.5 | 0.71 |
| Dosage of Water (%) | Phosphorous Content (ppm) | FFA (Acid Value, mg KOH/g) |
|---|---|---|
| 0.50 | 254.2 | 0.59 |
| 1.00 | 171.9 | 0.67 |
| 1.50 | 56.2 | 1.07 |
| 2.00 | 10.0 | 0.93 |
| 2.50 | 9.1 | 0.87 |
| 3.00 | 9.0 | 0.85 |
| 3.50 | 8.8 | 0.81 |
| 4.00 | 8.8 | 0.78 |
| 4.50 | 8.7 | 0.74 |
| 5.00 | 8.6 | 0.73 |
| Enzymatic Degumming Time (min) | Phosphorous Content (ppm) | FFA (Acid Value, mg KOH/g) |
|---|---|---|
| 30 | 178.2 | 0.58 |
| 60 | 59.2 | 0.65 |
| 90 | 25.3 | 0.73 |
| 120 | 9.2 | 0.84 |
| 150 | 9.0 | 0.95 |
| 180 | 8.8 | 1.18 |
| 210 | 8.5 | 1.47 |
| 240 | 8.4 | 1.95 |
| 270 | 8.1 | 2.49 |
| 300 | 8.0 | 3.18 |
| FAME | R.T. (min) | Contents (% of Total) | Characteristic Fragments with Masses m/z |
|---|---|---|---|
| Palmitic acid, methyl ester (C17H34O2) | 8.193 | 9.808 | 74; 87; 43; 55; 41; 143; 75; 57; 69; 227 |
| Linolenic acid, methyl ester (C19H32O2) | 8.675 | 4.448 | 79; 67; 95; 81; 41; 55; 108; 93; 80; 94 |
| Linoleic acid, methyl ester (C19H34O2) | 8.725 | 2.808 | 67; 81; 41; 55; 95; 43; 82; 79; 96; 68 |
| Oleic acid, methyl ester (C19H36O2) | 8.814 | 35.651 | 55; 69; 74; 83; 97; 96; 84; 41; 98; 87 |
| Stearic acid, methyl ester (C19H38O2) | 8.882 | 10.075 | 74; 87; 298; 143; 255; 75; 43; 55; 199; 57 |
| Isolinoleic acid, methyl ester (C19H34O2) | 8.973 | 10.686 | 67; 81; 95; 82; 55; 41; 96; 68; 79; 294 |
| Isolinoleic acid, methyl ester (C19H34O2) | 9.136 | 8.207 | 67; 81; 95; 82; 55; 41; 96; 68; 79; 294 |
| Observation | Frequency, cm−1 | ||
|---|---|---|---|
| Crude Soybean Oil | Degummed Soybean Oil | BioFuel | |
| –OH | 3475.00 | 3475.46 | 3464.00 |
| –C=C–H | 3008.93 | 3008.93 | 3009.00 |
| –CH2 | 2922.00 | 2922.81 | 2923.49 |
| –CH2 | 2853.00 | 2853.27 | 2853.70 |
| C=O | 1741.30 | 1741.35 | 1741.38 |
| –CH2 | 1463.96 | 1463.97 | 1459.29 |
| C(O)–O–CH3 asσ | - | - | 1435.62 |
| C(O)–O–CH3 sσ | - | - | 1361.78 |
| C(O)–O– | - | - | 1241.02 |
| P=O | 1237.08 | 1237.15 | - |
| C–O | - | - | 1195.43 |
| C–O | 1159.97 | 1160.00 | 1169.58 |
| P=O | 1144.24 | 1144.24 | - |
| P=O | 1118.77 | 1119.22 | - |
| P–O | 1099.38 | 1099.92 | - |
| P–O | 1032.31 | 1032.89 | - |
| -CH2 | 719.93 | 720.93 | 722.59 |
| Process Parameter | Units of Measurement | Value |
|---|---|---|
| 1. The stage of enzymatic degumming | ||
| Enzyme | - | Purifine® 3G enzyme complex |
| Enzyme content | Ppm | 100 |
| Temperature | °C | 56–58 |
| pH value | - | 6.9–7.1 |
| Dosage of water | wt. % | 2.5 |
| Time | Min | 120 |
| 2. The stage of biodiesel production by cavitation | ||
| Catalyst | - | 32% solution of potassium methoxide (CH3OK) in methanol |
| Content catalyst | wt. % | 3.9 |
| Degumming Soybean Oil | wt. % | 86.6 |
| CH3OH (Methanol) | wt. % | 9.5 |
| Cavitation temperature | °C | 80 |
| Properties | Units of Measurement | Value | Methods | Requirements for Biofuels According to Ukrainian Regulations [30] | ||
|---|---|---|---|---|---|---|
| Crude Soybean Oil | Degummed Soybean Oil | BioFuel | ||||
| Content of fatty acids methyl esters | wt. % | - | - | 97.2 | [29] | ≥96.5 |
| Density at 15 °C | kg/m3 | 922 | 917 | 865 | [20] | 860–900 |
| Kinematic viscosity at 40 °C | mm2/s | 30.7 | 24.1 | 4.1 | [21] | 3.5–5.0 |
| Flash point | °C | 345 | 326 | 156 | [22] | ≥120 |
| Sulphur content | ppm | 15 | 12 | 7 | [23] | ≤10 |
| Phosphorus content | ppm | 421 | 9.2 | 3.9 | [24] | ≤10 |
| Water content | ppm | 200 | 500 | 430 | [25] | ≤500 |
| Copper strip test (3 h at 50 °C) | - | withstands class 1 | [26] | withstands class 1 | ||
| Acid value | mg KOH/g | 0.49 | 0.83 | 0.46 | [27] | ≤0.5 |
| Iodine value | g I2/100 g | 39.0 | 53.4 | 80.7 | [28] | ≤120 |
| Linoleic acid methyl ester content | wt. % | - | - | 2.808 | [29] | ≤12 |
| Methanol content | wt. % | - | - | 0.05 | [30] | ≤0.2 |
| Monoglycerides content | wt. % | - | - | 0.4 | [31] | ≤0.8 |
| Diglycerides content | wt. % | - | - | 0.08 | [31] | ≤0.2 |
| Triglycerides content | wt. % | - | - | 0.18 | [31] | ≤0.2 |
| Free glycerol content | wt. % | - | - | 0.008 | [31] | ≤0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polovkovych, S.; Karkhut, A.; Gunka, V.; Blikharskyy, Y.; Nebesnyi, R.; Khomyak, S.; Selejdak, J.; Blikharskyy, Z. Enzymatic Degumming of Soybean Oil for Raw Material Preparation in BioFuel Production. Appl. Sci. 2025, 15, 8371. https://doi.org/10.3390/app15158371
Polovkovych S, Karkhut A, Gunka V, Blikharskyy Y, Nebesnyi R, Khomyak S, Selejdak J, Blikharskyy Z. Enzymatic Degumming of Soybean Oil for Raw Material Preparation in BioFuel Production. Applied Sciences. 2025; 15(15):8371. https://doi.org/10.3390/app15158371
Chicago/Turabian StylePolovkovych, Sviatoslav, Andriy Karkhut, Volodymyr Gunka, Yaroslav Blikharskyy, Roman Nebesnyi, Semen Khomyak, Jacek Selejdak, and Zinoviy Blikharskyy. 2025. "Enzymatic Degumming of Soybean Oil for Raw Material Preparation in BioFuel Production" Applied Sciences 15, no. 15: 8371. https://doi.org/10.3390/app15158371
APA StylePolovkovych, S., Karkhut, A., Gunka, V., Blikharskyy, Y., Nebesnyi, R., Khomyak, S., Selejdak, J., & Blikharskyy, Z. (2025). Enzymatic Degumming of Soybean Oil for Raw Material Preparation in BioFuel Production. Applied Sciences, 15(15), 8371. https://doi.org/10.3390/app15158371








