Abstract
Copper-based substances have historically been shown to exhibit antimicrobial properties, but the mechanisms by which they interact with bacterial biofilms in pipeline systems remain unclear. This study investigates the effects of copper sulfate and copper nitrate on the growth, biofilm formation, and antibiotic resistance profiles of Escherichia coli and Enterococcus faecalis. Across a wide concentration range (0.00005–100 mg/mL), both salts demonstrated strong antimicrobial activity at higher concentrations, while sublethal levels produced more nuanced effects. Higher concentrations exhibited potent antimicrobial activity, while sublethal concentrations paradoxically enhanced biofilm resistance, particularly in E. faecalis. Growth kinetic assays and spectroscopic analysis were used to better understand how copper interacts with microbes on a biochemical and physical level. Surprisingly, prolonged exposure to sublethal copper concentrations altered the antibiogram profiles of E. faecalis, developing resistance to ceftazidime. The findings confirm the bimodal activity of copper salts as antimicrobial and biofilm-controlling agents, highlighting the critical need for precise concentration optimization in wastewater treatment. This current study contributes to the collective knowledge pool of metal–microbe interactions, shedding light on selecting materials for industrial and environmental applications.
1. Introduction
Choosing the most appropriate material for manufacturing water pipes that are both durable and non-toxic but still cost-effective is a challenging task in the wastewater industry. Commonly used pipe materials include concrete; metals such as stainless steel, copper, and ductile iron; and plastics such as polyvinyl chloride (PVC), cross-linked polyethylene (PEX), and high-density polyethylene (HDPE) [1]. Copper, a material often used in the construction industry for water transportation and refrigerant channeling in heating and cooling systems, stands out due to its unique properties. Its strength, reliable connections, and exceptional corrosion resistance make it a preferred choice for manufacturing wastewater pipes, and its UV resistance allows for above-ground installation [2]. While living organisms need low concentrations of copper because of its cofactor functions for many enzymes and metalloproteins, at higher concentrations, this metal can become toxic and inhibit the growth of microorganisms [3,4,5].
Copper ions’ antimicrobial properties can be explained by their ability to damage bacterial cell walls, obstruct metabolic activities, and cause oxidative stress, which results in cell death [6,7]. Its antimicrobial properties are mainly seen in the main biocidal form of copper, the cupric ion (Cu2+) [8], which forms when Cu is released through corrosion [6,9]. Because of this, the antimicrobial properties of copper can aid in reducing water pollution, particularly in household water supplies, that forms due to the infiltration of chemical and biological contaminants. Pathogens from human and animal waste, sewage overflows, agricultural runoffs, and industrial discharges primarily cause bacterial contamination in domestic water systems. Inadequate wastewater treatment and the leaching of contaminants into groundwater and surface water sources further contribute to the problem. This contamination introduces harmful bacteria, viruses, and parasites into water systems, which can infiltrate homes and pose serious health risks to inhabitants.
The most common pathogens include Enterococcus faecalis [10], Escherichia coli [11], Vibrio cholerae, and Salmonella typhi, and are responsible for numerous waterborne diseases [12,13]. Their presence affects water quality, leading to changes in taste and odor [14,15]. Biofilms, multicellular aggregates containing an extracellular matrix, make biological water contamination even more severe [16,17,18]. In various industry sectors, particularly water distribution and pipeline systems, these bacterial communities are challenging to eradicate since they can adhere to surfaces and form dense, protective matrices highly resistant to chemical and physical interventions [19,20]. Consequently, their formation leads to biofouling, a process in which their accumulation reduces the quality of fluid flow [21].
While earlier research has shown that copper sulfate and, to a lesser degree, copper nitrate have antimicrobial qualities, the majority of these studies have concentrated on the bactericidal effects of these substances alone or on a small number of pathogens. By directly comparing the two compounds over a broad concentration range, this study aims at filling a gap by evaluating not only their antibacterial properties but also their effects on biofilm formation and profiles of antibiotic resistance. This is especially important for pipeline and wastewater systems, where biofilm resilience and sublethal exposure are prevalent issues.
2. Materials and Methods
2.1. Bacterial Strains
The following bacterial strains were tested: E. coli ATCC 14169, E. coli ATCC 25922, E. faecalis ATCC 19433, and E. faecalis ATCC 29212. The bacterial strains were cultured for 24 h at 37 °C on Mueller Hinton (MH) agar (Liofilchem Ltd., Roseto Degli Abruzzi, Italy). The bacterial suspension used in the research was 0.5 McFarland (equivalent to 1.5 × 108 CFU/mL) inoculum made in MH broth (Liofilchem Ltd., Roseto Degli Abruzzi, Italy). Then, 100 µL of the prepared 0.5 McFarland inoculum was diluted in 9.9 mL of MH broth.
2.2. Copper Salts
The copper salts used in this study were purchased from Sigma-Aldrich Steinheim, Germany. We used copper (II) sulfate pentahydrate (catalog no. C3036-250G) and copper (II) nitrate hydrate (catalog no. 229636-5G), both with a purity of ≥98%. Stock solutions were prepared at a concentration of 100 mg/mL in sterile distilled water and stored at room temperature until use in the experiments. No further purification was performed.
2.3. Agar-Well Diffusion Method
Using the agar-well diffusion method, four different concentrations of copper sulfate and copper nitrate were used to examine the antibacterial effect of copper salts. The initial concentration of the copper salt was 100 mg/mL. The McFarland of E. coli ATCC 14169, E. coli ATCC 25923, E. faecalis ATCC 19433, and E. faecalis ATCC 29212 was adjusted to 0.5 using MH broth (Sigma-Aldrich Steinheim, Germany) and plated onto Mueller Hinton agar (Sigma-Aldrich Steinheim, Germany) plates. Three holes were made in each agar plate for 80 µL of each copper salt dilution to be added. The plates were incubated at 37 °C for 24 h, after which the zones of inhibition were measured.
2.4. Biofilm Formation
The 0.5 McFarland of E. coli ATCC 14169, E. coli ATCC 25923, E. faecalis ATCC 19433, and E. faecalis ATCC 29212 was used to examine the effect of copper salts on biofilm formation. The initial copper sulfate and copper nitrate concentration was 100 mg/mL. Two-fold dilutions of each salt were diluted in Mueller Hinton broth (Sigma-Aldrich Steinheim, Germany), added to a 96-well microtiter plate, and mixed with each bacterial strain. The samples were incubated at 37 °C for 24 h, after which the absorbance was read on the ELISA plate reader (EPOCH, Agilent Technologies, Inc., Santa Clara, CA, USA) at 595 nm. Then, 0.1% of crystal violet was used for staining, and distilled water was used for washing the 96-well plates; 96% ethanol was added to each plate, and the absorbance was read at 595 nm using the ELISA plate reader (EPOCH, Agilent Technologies, Inc., Santa Clara, CA, USA). The following formula (Equation (1)) was used to calculate the optical density cut-off value (ODc):
ODc = average OD of negative control + 3× standard deviation of negative control.
The biofilm categories were determined by comparing the average OD of each sample with the calculated ODc value: OD ≤ ODc: non-adherent, ODc < OD ≤ 2× ODc: weakly adherent, 2× ODc < OD ≤ 4× ODc: moderately adherent, and 4× ODc < OD: strongly adherent [22].
2.5. Antibiogram Assay
For this experiment, 15 antibiotic discs (Liofilchem Ltd., Roseto Degli Abruzzi, Italy) were used. The bacterial inoculums used for antibiotic susceptibility testing were taken from the 96-well plates of the biofilm formation assay. The positive control represented the untreated E. coli and E. faecalis strains. The tested strains included the bacterial inoculum growing with a subminimum concentration of copper salts. The positive control and the tested strains were adjusted to 0.5 McFarland and plated on Muller Hinton (Sigma-Aldrich Steinheim, Germany) agar plates. Then, antibiotic disks were placed on the same agar plates and incubated at 37 °C for 24 h. After the incubation period, the zones of inhibition were measured for each bacterial strain. The following antibiotic disks were used and their abbreviations: doxycycline 30 µg (DXT 30), amoxicillin 30 µg (AML 30), mezlocillin 75 µg (MEZ 75), cefuroxime 30 µg (CXM 30), ceftazidime 30 µg (CAZ 30), ceftriaxone 30 µg (CRO 30), ampicillin 2 µg (AMP 2), amoxicillin–clavulanic acid 30 µg (AUG 30), clavulanic acid 40 µg (CAL 40), ciprofloxacin 5 µg (CIP 5), gentamicin 30 µg (CN 30), gentamicin 10 µg (CN 10), kanamycin 30 µg (K 30), tobramycin 10 µg (TOB 10), and tetracycline 30 µg (TE 30).
2.6. Growth Curves
To further test the effect of subminimum concentrations of copper salts, the growth of E. coli and E. faecalis strains was measured over 48 h at 20 °C by measuring their absorbance at 595 nm using the ELISA plate reader (EPOCH, Agilent Technologies, Inc., Santa Clara, CA, USA). The untreated strains were compared to the strains growing in the presence of copper salts. All tested strains had an initial 0.5 McFarland. Ultimately, a growth curve with time (hours) on the x-axis and average absorbance at 595 nm on the y-axis was constructed.
2.7. Resazurin Assay
The McFarland of E. coli ATCC 14169, E. coli ATCC 25923, E. faecalis ATCC 19433, and E. faecalis ATCC 2921 was adjusted to 0.5 McFarland by diluting them in Tryptic Soy broth (Liofilchem Ltd., Roseto Degli Abruzzi, Italy). Then, 100 mg/mL of copper sulfate and copper nitrate were used as the initial concentration to test the effect of copper salts on bacterial growth. Two-fold dilutions of each salt were diluted in Mueller Hinton broth (Sigma-Aldrich Steinheim, Germany), added to a 96-well microtiter plate, and mixed with each bacterial strain. The samples were incubated at 37 °C for 24 h. Then, 60 µL of the resazurin dye was added to each well. The results were read on the ELISA plate reader (EPOCH, Agilent Technologies, Inc., Santa Clara, CA, USA) at 595 and 562 nm for two periods. The plates were first read immediately after adding the dye and after three hours of incubation. The plates were covered with aluminum foil during incubation and left in the incubator at 37 °C. The reduction percentage was calculated using Formula (2) and using the molar extinction coefficients for resazurin in Table 1:

Table 1.
The molar extinction coefficients for resazurin [23].
3. Results
3.1. Agar-Well Diffusion Method
The antibacterial effect of copper salts was examined by performing the agar-well diffusion assay, a standard method for evaluating the antimicrobial activity of substances. Both copper salts exhibited concentration-dependent inhibitory effects against the four pathogens (Figure 1). Significantly larger zones are seen at 75% and 100% concentrations, indicating a strong antibacterial activity.
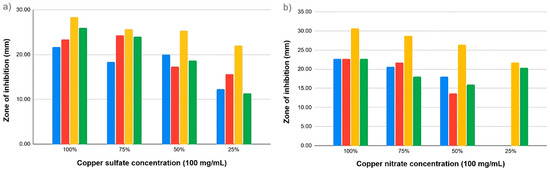
Figure 1.
Antibacterial potential of copper salts on different bacterial strains: E. coli ATCC 14169 (blue), E. coli ATCC 25923 (red), E. faecalis ATCC 19433 (yellow), and E. faecalis ATCC 29212 (green). The antibacterial effects were tested using (a) copper sulfate and (b) copper nitrate.
3.2. Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) for copper sulfate and copper nitrate was evaluated across four bacterial strains: E. coli ATCC 14169, E. coli ATCC 25923, E. faecalis ATCC 19433, and E. faecalis ATCC 29212. This assessment utilized a dilution series that spanned from 0.00005 mg/mL to 100 mg/mL. Both copper compounds demonstrated inhibitory effects on bacterial growth that were dependent on their concentration. For all bacterial strains examined, the MIC for both copper sulfate and copper nitrate was consistently found to be 1.56 mg/mL, at which point complete inhibition of visible bacterial growth occurred. There were no significant differences in MIC values noted between the E. coli and E. faecalis strains or between the two copper salts. The consistent MIC across the various species (see Figure 2) indicates a common susceptibility to copper ions among the strains tested. These findings underscore the potential for employing a uniform concentration of copper salts to suppress various bacterial contaminants within pipeline systems.
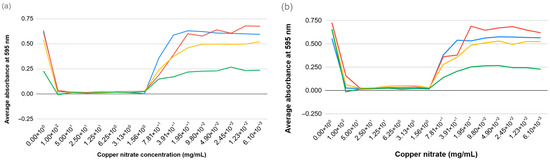
Figure 2.
Growth comparison of E. coli ATCC 14169 (blue), E. coli ATCC 25923 (red), E. faecalis ATCC 19433 (yellow), and E. faecalis ATCC 29212 (green) in the presence of (a) copper sulfate and (b) copper nitrate.
3.3. Effect on Biofilm Formation
To assess the impact of copper salts on biofilm development, strains of E. coli and E. faecalis were subjected to copper sulfate and copper nitrate within a concentration range of 0.00005–100 mg/mL. The extent of biofilm formation was measured and categorized into four classifications based on absorbance readings: non-adherent, weakly adherent, moderately adherent, and strongly adherent (see Figure 3 and Figure 4). At elevated concentrations, both types of copper salts markedly inhibited biofilm formation across all bacterial strains. Conversely, exposure to sublethal concentrations resulted in varied outcomes. Specifically, E. faecalis ATCC 29212 and E. faecalis ATCC 19433 showed an enhancement in biofilm strength, transitioning from moderately to strongly adherent classifications. These results indicate that while high levels of copper salts hinder biofilm formation, sublethal levels may provoke stress responses that promote biofilm production, especially in E. faecalis.
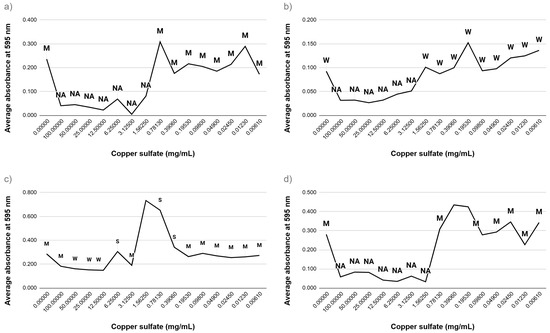
Figure 3.
Biofilm formation of bacteria in the presence of copper sulfate (a) E. coli ATCC 14169; (b) E. coli ATCC 25922; (c) E. faecalis ATCC 19433; (d) E. faecalis ATCC 29212. NA—non-adherent biofilm, W—weakly adherent biofilm, M—moderately adherent biofilm, S—strongly adherent biofilm.
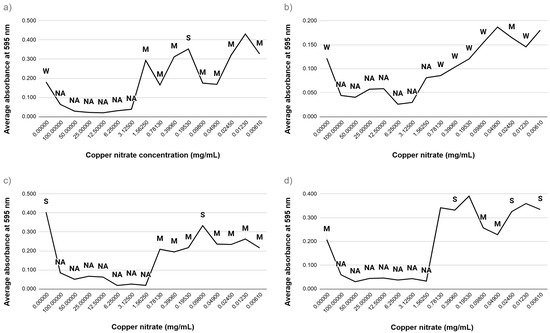
Figure 4.
Biofilm formation of bacteria in the presence of copper nitrate (a) E. coli ATCC 14169; (b) E. coli ATCC 25922; (c) E. faecalis ATCC 19433; (d) E. faecalis ATCC 29212. NA—non-adherent biofilm, W—weakly adherent biofilm, M—moderately adherent biofilm, S—strongly adherent biofilm.
3.4. Antibiogram Susceptibility Profiles
The impact of sublethal exposure to copper salts on the antibiotic resistance of E. faecalis strains was assessed through disk diffusion assays involving 15 different antibiotics. The diameters of the zones of inhibition were measured and compared between untreated control strains and those subjected to treatments with copper sulfate and copper nitrate (see Table 2 and Table 3). A one-way ANOVA was conducted for each antibiotic to evaluate statistical variations among the three groups: control, copper sulfate-treated, and copper nitrate-treated strains. The results indicate no statistically significant differences (p > 0.05) for any of the antibiotics evaluated. The antibiotics that exhibited the lowest p-values—amoxicillin (AML, p = 0.16), ceftriaxone (CRO, p = 0.31), and mezlocillin (MEZ, p = 0.33)—suggested possible trends; however, these did not achieve statistical significance. Although ceftazidime (CAZ) exhibited a notable decrease in zone size and apparent resistance (as observed qualitatively), this change was not statistically significant (p = 0.41) in the ANOVA, attributed to variability and the small sample sizes. These results imply that while visual trends in susceptibility may be influenced by copper exposure, the current experimental conditions did not yield statistically significant alterations. Additional studies with larger sample sizes may be necessary to confirm the observed shifts in resistance.

Table 2.
The antibiotic susceptibility for E. coli.

Table 3.
The antibiotic susceptibility for E. faecalis.
3.5. Growth Dynamics Under Sublethal Stress
The growth patterns of E. coli and E. faecalis strains exposed to sublethal doses of copper salts were examined by measuring absorbance at 595 nm over 48 h (see Figure 5 and Figure 6). Between the 32nd and 42nd hours, E. faecalis strains displayed a delayed yet enhanced growth phase compared to untreated controls.
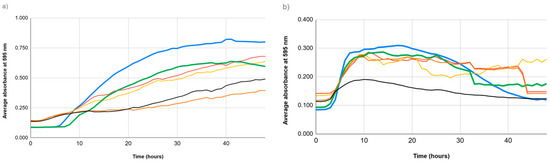
Figure 5.
Growth curves of bacterial strains exposed to copper salts over 48 h, measured as average absorbance at 595 nm. (a) Growth of E. coli strains (EC1 and EC2) under different conditions: alone (blue and green, respectively), with copper sulfate (red and orange, respectively), and with copper nitrate (yellow and black, respectively). (b) Growth of E. faecalis strains (EF1 and EF2) under different conditions: alone (blue and green, respectively), with copper sulfate (red and orange, respectively), and with copper nitrate (yellow and black, respectively).
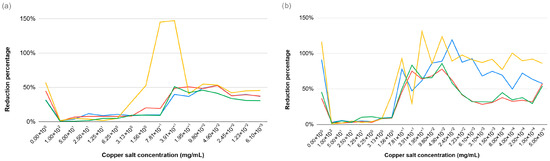
Figure 6.
(a) Resazurin assay results of E. coli ATCC 14169 with copper sulfate (blue), E. coli ATCC 25923 with copper sulfate (red), E. coli ATCC 14169 with copper nitrate (yellow), and E. coli ATCC 25923 with copper nitrate (green). (b) Resazurin assay results of E. faecalis ATCC 19433 with copper sulfate (blue), E. faecalis ATCC 29212 with copper sulfate (red), E. faecalis ATCC 19433 with copper nitrate (yellow), and E. faecalis ATCC 29212 with copper nitrate (green).
3.6. Resazurin Assay
The resazurin assay was performed to evaluate the effect of copper salts across a concentration range of 0.00005–100 mg/mL on the metabolic activity of E. coli and E. faecalis strains. Higher concentrations reduced metabolic activity with sublethal doses causing sustained bacterial activity (see Figure 6).
4. Discussion
This research illustrates that both copper sulfate and copper nitrate present antimicrobial effects against E. coli and E. faecalis, which are significant indicators of fecal pollution in aquatic environments, in a dose-dependent manner. At elevated concentrations, these copper compounds effectively inhibited bacterial proliferation and diminished biofilm development. Conversely, sublethal doses resulted in unexpected outcomes, enhancing biofilm resilience and modifying antibiotic resistance profiles, especially in E. faecalis.
The agar-well diffusion tests validated the strong antibacterial characteristics of the copper salts, showing that inhibition zones expanded in proportion to the concentration applied. These findings are consistent with earlier research indicating that copper can disrupt cellular membranes, produce reactive oxygen species, and interfere with critical enzymatic functions [12,20]. The minimum inhibitory concentration (MIC) for all examined strains consistently measured at 1.56 mg/mL, highlighting copper’s broad-spectrum effectiveness across various bacterial types. This suggests that a single well-defined concentration could be useful against multiple microbial contaminants in pipeline systems, potentially streamlining dosage approaches in industrial contexts. However, biofilm assays indicated a more complex interaction. While elevated concentrations were effective in hindering biofilm formation, sublethal levels, particularly in the range of 0.39 to 0.78 mg/mL, led to enhanced biofilm strength in E. faecalis. This phenomenon may represent an adaptive mechanism involving stress-induced increases in extracellular polymeric substances or the activation of genes associated with biofilm survival [20,24]. These insights are particularly pertinent given the ongoing challenge of biofouling in water systems, where inadequate antimicrobial dosing might worsen contamination issues instead of mitigating them. The profiles of antibiotic susceptibility offered additional insights regarding the risks associated with copper exposure. While descriptive analysis indicated a noteworthy emergence of resistance—most notably, the appearance of ceftazidime resistance in E. faecalis ATCC 29212—statistical evaluation through one-way ANOVA did not establish significance for any antibiotic (p > 0.05). This inconsistency may stem from substantial variability between groups or a lack of statistical power, suggesting the need for further research with larger sample sizes. Nonetheless, the qualitative shift in resistance patterns aligns with previous studies indicating that metal stress can co-select for antibiotic resistance through shared regulatory mechanisms or closely situated resistance genes [7]. These results underscore the dual function of copper salts in managing microbial populations. While high concentrations serve as effective biocides, prolonged exposure to sublethal levels could inadvertently foster resistance and support biofilm formation, complicating long-term management strategies. This bimodal behavior highlights the necessity of precise concentration regulation in practical applications, particularly concerning wastewater treatment and pipeline infrastructure. Our observations align with recent studies suggesting that exposure to sublethal concentrations of metal salts can enhance biofilm resilience and trigger adaptive resistance responses [17,19]. This work expands on our previous findings regarding the impact of iron salts on microbial communities in comparable pipeline systems [23]. In both papers, similar trends are observed: high concentrations of the metal inhibited biofilm formation and microbial growth, while lower concentrations induced stress-adaptive responses by E. coli and E. faecalis, and shifts in antibiotic resistance. The findings from both studies indicate that transition metals like iron and copper exert dose-dependent and sometimes paradoxical effects on microbial physiology. This highlights the importance of optimized dosage strategies to exploit the antimicrobial effects of the metal ions while reducing the risk of unwanted resistance development or biofilm fortification.
A limitation of this research is its emphasis on only two bacterial species in a controlled laboratory setting. Although E. coli and E. faecalis serve as important indicator organisms, future studies should incorporate mixed-species biofilms and environmental isolates to more accurately represent natural microbial communities. Furthermore, mechanistic investigations utilizing transcriptomic or proteomic analyses would elucidate the molecular mechanisms behind copper-induced resistance and biofilm modulation.
5. Conclusions
This study demonstrates the antimicrobial and antibiofilm potential of copper sulfate and copper nitrate against E. coli and E. faecalis, two common contaminants in pipeline systems. Copper salts exhibited clear dose-dependent effects, with concentrations between 1.56 and 100 mg/mL resulting in significant inhibition of bacterial growth and biofilm formation. For instance, at 100 mg/mL, complete growth inhibition was observed in both bacterial species. However, sublethal concentrations in the range of 0.39–0.78 mg/mL paradoxically enhanced biofilm strength in E. faecalis, shifting its classification from moderately to strongly adherent. Regarding antibiotic susceptibility profiles, copper salts did not significantly alter antibiotic resistance profiles for most results, but a change in resistance to ceftazidime was observed in E. faecalis. The observed potential for increased antibiotic susceptibility or resistance in specific cases underscores the need to manage copper usage carefully. Because of this, this paper further highlights the need to find the optimal concentration of copper salts that are effective, non-toxic, and compliant with water quality standards. To determine whether sodium nitrate or sodium sulfate contributes independently to the inhibition of bacterial growth or the modulation of biofilms, future research could include additional controls employing these anions. Moreover, since nanoparticles have demonstrated encouraging antimicrobial qualities, this study could be expanded in the future by contrasting the efficacy of alternative copper forms, such as copper nanoparticles, with conventional copper salts. Additionally, transcriptomic or proteomic analyses could be used to better understand the mechanisms behind the development of resistance and the antimicrobial effects induced by copper. Future studies could also incorporate surface visualization techniques such as 2D/3D mesh plotting or SEM imaging to investigate how copper salts alter biofilm architecture at the structural level.
Author Contributions
Conceptualization, M.A. and A.E.S.; methodology, N.C., M.L. and S.D.; validation, A.E.S., A.P. and M.H.; formal analysis, N.C.; investigation, N.C., M.L. and S.D.; data curation, N.C., M.L. and S.D.; writing—original draft preparation, N.C. and M.L.; writing—review and editing, M.A., A.P. and M.H.; visualization, N.C.; supervision, A.E.S. and M.A.; project administration, A.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
The research that has produced this research paper has been financially supported by the European Union through the project Introducing Student Research Mobilities to BH Unis—INSTREAM, implemented by a consortium led by International Burch University, but does not necessarily represent the official position of the European Union or International Burch University and is the sole responsibility of the author.
Conflicts of Interest
Author Andrzej Piątkowski was employed by the company ALLINS LLC LP. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Burlingame, G.A.; Anselme, C. Advances in Taste-and-Odor Treatment and Control; AR Foundation: Denver, CO, USA, 1995. [Google Scholar]
- Trevors, J.T.; Cotter, C.M. Antibiotic resistance and metal tolerance of bacteria isolated from soil. J. Ind. Microbiol. Biotechnol. 1990, 6, 77. [Google Scholar] [CrossRef]
- Liu, Z.; Stout, J.E.; Tedesco, L.; Boldin, M.; Hwang, C.; Diven, W.F.; Yu, V.L. Controlled evaluation of copper–silver ionization in eradicating Legionella pneumophila from a hospital water distribution system. J. Infect. Dis. 1994, 169, 919–922. [Google Scholar] [CrossRef]
- Videla, H.A.; Characklis, W.G. Biofouling and microbially influenced corrosion. Int. Biodeterior. Biodegrad. 1992, 29, 195–212. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Szewzyk, U.; Szewzyk, R.; Manz, W.; Schleifer, K.H. Microbiological safety of drinking water. Annu. Rev. Microbiol. 2000, 54, 81–127. [Google Scholar] [CrossRef]
- Iversen, A.; Kühn, I.; Rahman, M.; Franklin, A.; Burman, L.G.; Olsson-Liljequist, B.; Möllby, R. Evidence for transmission between humans and the environment. Environ. Microbiol. 2004, 6, 55–63. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Miettinen, I.T.; Keinänen, M.M.; Kekki, T.K.; Laine, O.; Hirvonen, A.; Martikainen, P.J. Microbiology and chemical quality of water. Water Res. 2004, 38, 3769–3779. [Google Scholar] [CrossRef]
- Silhan, J.; Corfitzen, C.B.; Albrechtsen, H.J. Monitoring microbiological water quality. Water Sci. Technol. 2006, 54, 49–56. [Google Scholar] [CrossRef]
- Aguirre, J.S.; Pin, C.; Rodriguez, M.R.; Garcia de Fernando, G.D. Behavior of Listeria monocytogenes in biofilms. Appl. Environ. Microbiol. 2009, 75, 6992–6998. [Google Scholar] [CrossRef]
- Cabral, J.P. Water microbiology. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of copper in biofilm formation. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef] [PubMed]
- Cowle, M.W.; Babatunde, A.O.; Bockelmann-Evans, B.N. The frictional resistance induced by bacterial based biofouling in drainage pipelines. Environ. Technol. Rev. 2017, 55, 269–283. [Google Scholar] [CrossRef]
- Gomes, I.; Simões, L.; Simões, M. Biofilms in drinking water systems. RSC Adv. 2019, 9, 32184–32199. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, C.; Shao, B.; Fu, L.; Yu, J.; Qiang, Z.; Chen, J. Residual antibiotics in water. Sci. Total Environ. 2020, 723, 138160. [Google Scholar] [CrossRef]
- Arthur, E.K.; Gikunoo, E.; Agyemang, F.O.; Azeko, S.T.; Andrews, A.; Twenewaa, A. Evaluation of microbial quality. J. Sci. Technol. 2020, 5, 29–37. [Google Scholar]
- Hemdan, B.A.; Azab El-Liethy, M.; El-Taweel, G.E. Biofilm resistance and water disinfection. Water Environ. Res. 2020, 92, 2155–2164. [Google Scholar] [CrossRef]
- Danilova, T.; Danilina, G.; Adzhieva, A.A.; Vostrova, E.I.; Zhukhovitskii, V.G.; Cheknev, S.B. Immune modulation by biofilms. Bull. Exp. Biol. Med. 2020, 169, 648–651. [Google Scholar] [CrossRef]
- Al-Abdan, M.A.; Bin-Jumah, M.N.; Alarifi, S. Exploration of cadmium silica nanoparticles on bioaccumulation, oxidative stress, and carcinogenic potentiates in Oreochromis mossambicus L. Oxid. Med. Cell. Longev. 2020, 2020, 5407159. [Google Scholar] [CrossRef]
- Festa, R.A.; Thiele, D.J. Copper homeostasis in microbial pathogens. PLoS Pathog. 2012, 8, e1002887. [Google Scholar] [CrossRef]
- Aguilera, A.; Souza-Egipsy, V.; San Martín-Úriz, P.; Amils, R. Microbial communities in extreme environments. Aquat. Toxicol. 2008, 88, 257–263. [Google Scholar] [CrossRef]
- Panda, P.S.; Chaudhary, U.; Dube, S.K. Study on Pseudomonas in water systems. Indian J. Pathol. Microbiol. 2016, 59, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Deumić, S.; El Sayed, A.; Hsino, M.; Glamočak, A.; Crnčević, N.; Avdić, M. Investigating the Effect of Iron Salts on E. coli and E. faecalis Biofilm Formation in Water Distribution Pipelines. Water 2025, 17, 886. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: A microbial lifestyle. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).