Adaptation of the PESTonFARM Model to Support Decision-Making and Planning of Local Implementation of the Sterile Insect Technique in the Control of Ceratitis capitata Flies (Diptera: Tephritidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. SIT Expansion—Model Operation
2.2. Demonstration of the Model’s Potential for Application as a Decision Support Tool
2.2.1. General Approach
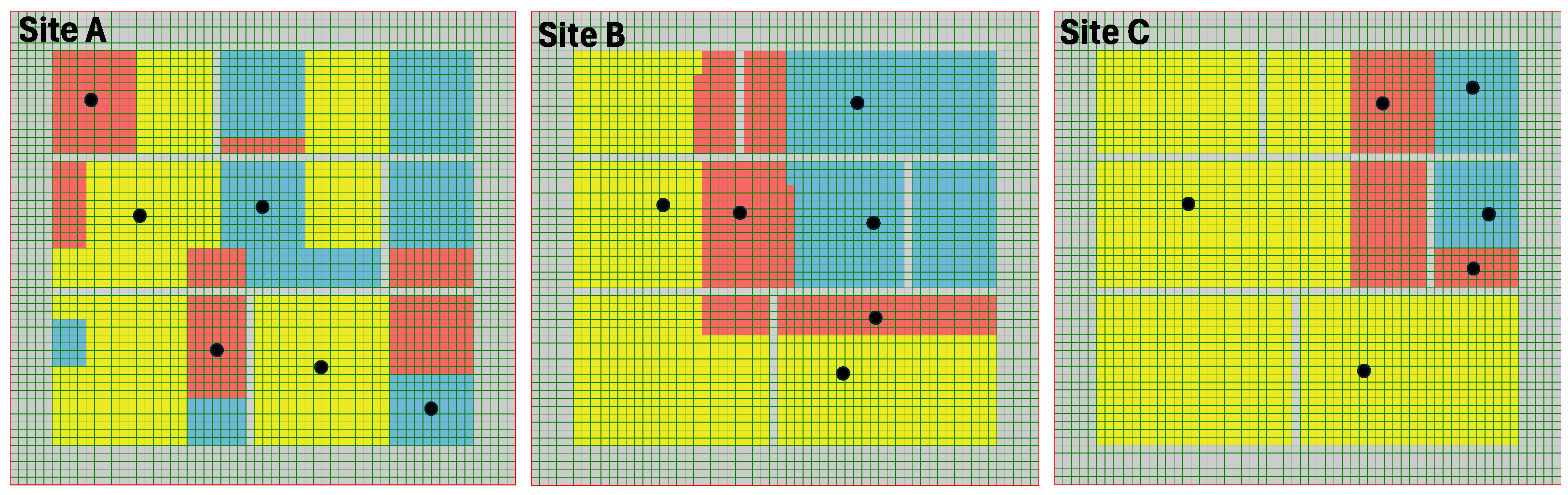
2.2.2. Fruit Growing Sites
2.2.3. Weather Data
2.2.4. Medfly “Starting” Population
2.2.5. Quality of Sterile Males
2.2.6. Medfly Behavior and Dispersal
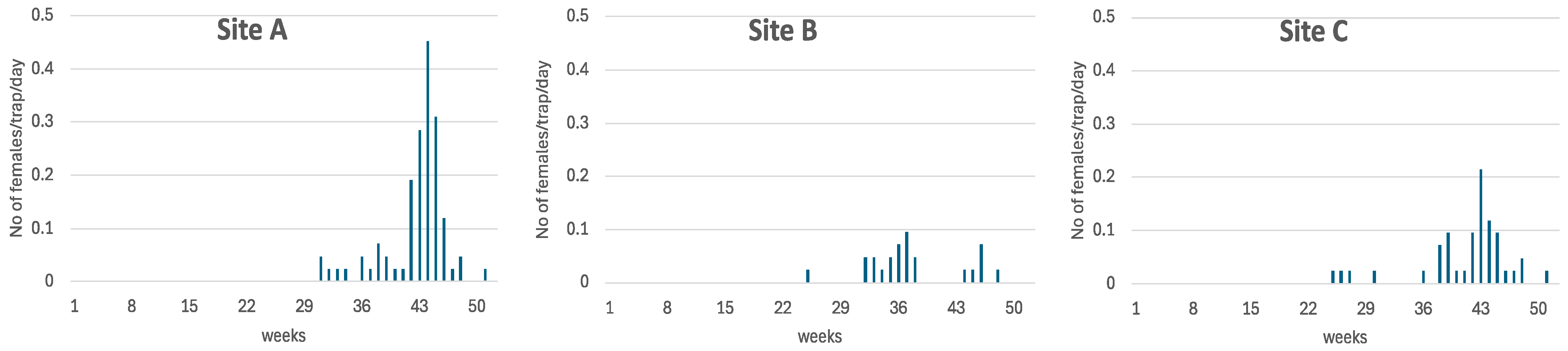
2.2.7. Medfly Monitoring
2.2.8. Simulated SIT Scenarios
- NO IPM—this simulation was used to illustrate the potential of the medfly population in local conditions in the absence of any control measures. The scenario served as a reference (control). The simulated results were also used to determine SIT-opportunity windows—periods and places in which the next cohorts of new SIT-vulnerable generations of adult medflies emerge. The established SIT opportunity windows were then used to set the timing and duration of the simulated SIT releases in all scenarios presented below.
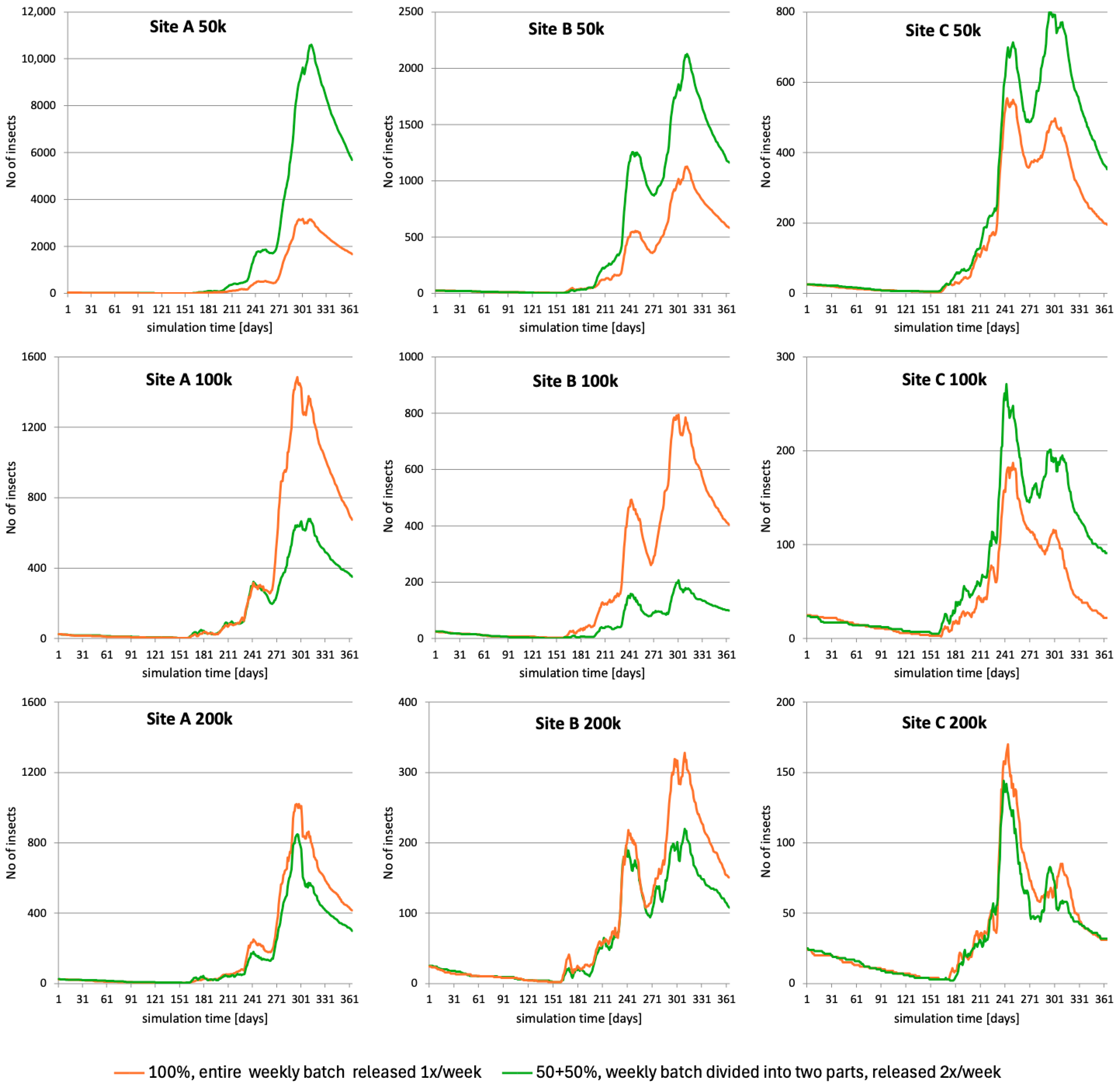
- Three SIT implementation scenarios with varying rates of releases of sterile males that emerged from a batch of 50,000, 100,000 or 200,000 of weekly supplied irradiated male pupae were used. All males released at once, in weekly intervals, were distributed uniformly by low-flying drones over all the plots that contain fruit trees.
- Three scenarios of bi-weekly release—the same numbers of weekly used batches of irradiated pupae (50,000, 100,000 or 200,000) were used, but the “base” weekly batch of pupae was divided into two parts, and the sterile males emerging from each part were released separately at set intervals of 3–4 days (twice a week).
- Spot ground release—three scenarios where the sterile males emerging from 50,000, 100,000 or 200,000 of weekly supplied irradiated male pupae were released once a week on the ground at 100 fixed release points arranged into a grid spaced at approximately 50 m.
3. Results
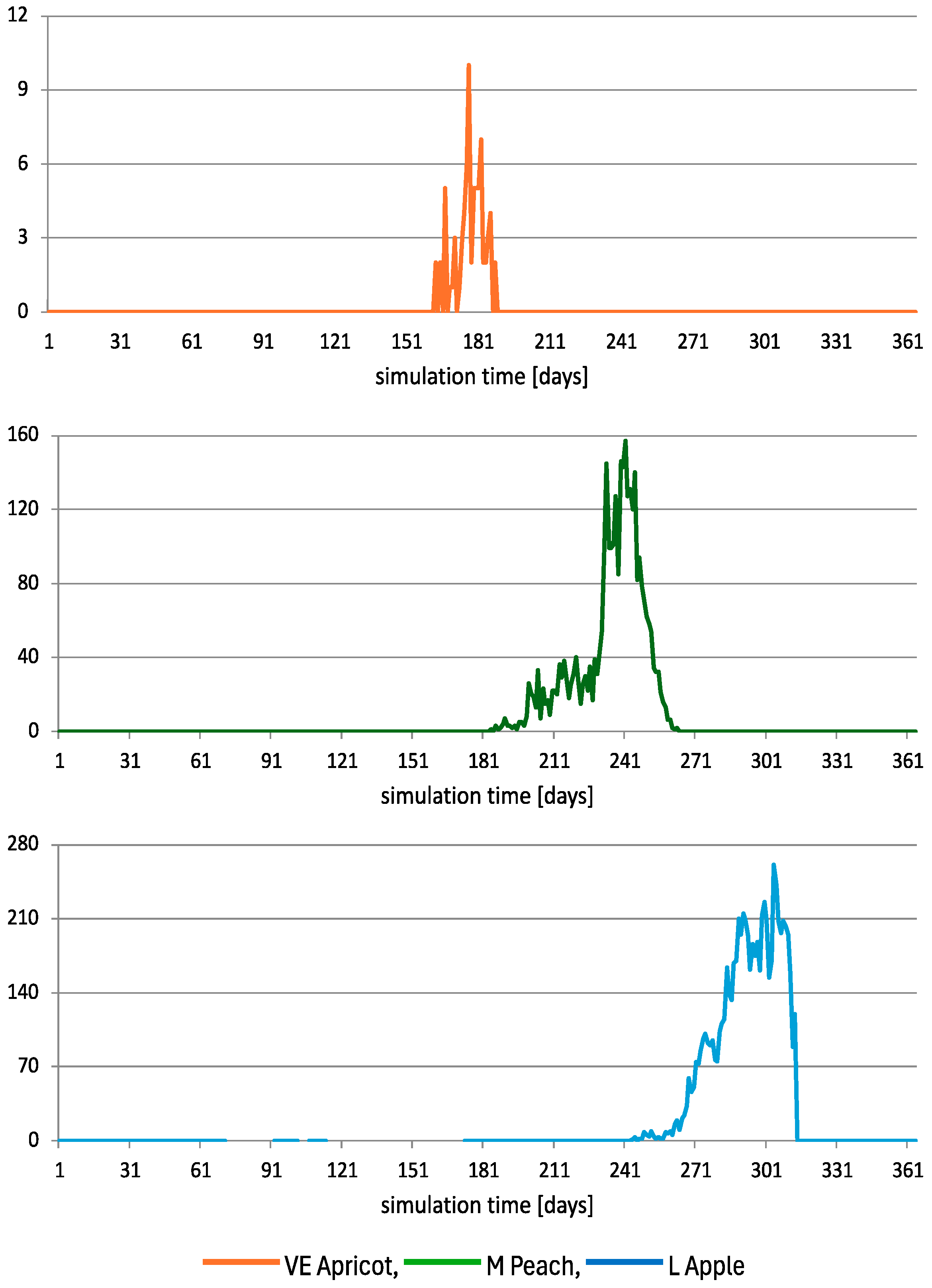
3.1. The Influence of Site Structure and Role of Seasonal Fruits in the Expansion of Medfly Population in the Absence of Any Control Measures
3.2. SIT Opportunity Windows—Timing and Patterns of Female Medfly Emergence in Successive Hosts
3.3. Detection of Founding Cohort Members
3.4. Outmigration
3.5. The Impact of Site Structure on the Effectiveness of SIT Implementation
3.5.1. Cohort Size
3.5.2. Release Frequency
3.5.3. Release Method
| Site | Release Rate | Release Method | ||
|---|---|---|---|---|
| Aerial 1/Week | Aerial 2/Week | Ground 1/Week | ||
| Site A | 0 (NO IPM) | 21,999 | ||
| 50 k | 4544 | 3707 | 9654 | |
| 100 k | 1245 | 1654 | 5057 | |
| 200 k | 499 | 1039 | 1935 | |
| Site B | 0 (NO IPM) | 3688 | ||
| 50 k | 1223 | 855 | 2374 | |
| 100 k | 427 | 143 | 684 | |
| 200 k | 311 | 147 | 1151 | |
| Site C | 0 (NO IPM) | 2538 | ||
| 50 k | 506 | 401 | 817 | |
| 100 k | 89 | 251 | 219 | |
| 200 k | 44 | 112 | 16 | |
| Site | Release Rate | Release Method | ||
|---|---|---|---|---|
| Aerial 1/Week | Aerial 2/Week | Ground 1/Week | ||
| Site A | 0 (NO IPM) | 15.2 | ||
| 50 k | 5.3 | 4.7 | 9.8 | |
| 100 k | 1.5 | 2.1 | 5.5 | |
| 200 k | 0.9 | 1.8 | 2.8 | |
| Site B | 0 (NO IPM) | 3.3 | ||
| 50 k | 1.4 | 1.0 | 2.7 | |
| 100 k | 0.6 | 0.2 | 0.9 | |
| 200 k | 0.5 | 0.3 | 1.6 | |
| Site C | 0 (NO IPM) | 7.3 | ||
| 50 k | 1.8 | 1.5 | 3.1 | |
| 100 k | 0.4 | 1.2 | 0.9 | |
| 200 k | 0.1 | 0.4 | 0.0 | |


4. Discussion
4.1. Study Area
4.2. Performance of Sterile Males After Their Release
4.3. Individual-Based Modelling Approach and Outline of Specific Features of the SIT-Enhanced PESTonFARM Model
4.4. Detection of Founding Cohort Members
4.5. Determining the Spatial and Temporal SIT Opportunity Window
4.6. Model Sensitivity—Ability to Capture and Quantify the Impact of Even Minor Differences in Terrain Topography on the Development of Medfly and Performance of SIT
4.7. Stochasticity of Very Small Invasive Propagules
4.8. Different Perspectives and Measures of Success of SIT Operation
5. Conclusions
- The SIT-enhanced PESTonFARM model reflects the influence of landscape structure on the behaviour of both wild female flies and released sterile males, and enables quantitative assessment of the effectiveness of different SIT scenarios.
- The model is sensitive enough to distinguish and quantify the effects of rather small spatial changes in local topography and fruit structure on medfly development and the effects of different SIT implementation approaches.
- The model simulates the development of both flies and fruits according to local annual weather patterns and can therefore generate locally relevant information on the optimal timing and spatial focus of local SIT operations.
- The use of the model enables informed decision-making and design of SIT implementation scenarios according to local conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourtzis, K.; Vreysen, M.J.B. Sterile Insect Technique (SIT) and Its Applications. Insects 2021, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Benedict, M.Q. Sterile insect technique: Lessons from the past. J. Med. Entomol. 2021, 58, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Hendrichs, J.; Robinson, A.S.; Cayol, J.P.; Enkerlin, W. Medfly areawide sterile insect technique programmes for prevention, suppression or eradication: The importance of mating behavior studies. Fla. Entomol. 2002, 85, 1–13. [Google Scholar] [CrossRef]
- CABI (Centre for Agriculture and Bioscience International). Invasive Species Compendium. Ceratitis capitata (Mediterranean Fruit Fly). 2020. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.12367 (accessed on 16 May 2025). [CrossRef]
- Szyniszewska, A.M.; Tatem, A.J. Global assessment of seasonal potential distribution of Mediterranean Fruit Fly, Ceratitis capitata (Diptera: Tephritidae). PLoS ONE 2014, 9, e111582. [Google Scholar] [CrossRef]
- Liquido, N.J.; Cunningham, R.T.; Nakagawa, S. Host plants of Mediterranean fruit fly (Diptera: Tephritidae) on the Island of Hawaii (1949–1985 survey). J. Econ. Entomol. 1990, 83, 1863–1878. [Google Scholar] [CrossRef]
- Morales, P.; Cermeli, M.; Godoy, F.; Salas, B. A list of Mediterranean fruit fly Ceratitis capitata Wiedemann (Diptera: Teph-ritidae) host plants based on the records of INIA-CENIAP Museum of Insects of Agricultural Interest. Entomotropica 2004, 19, 51–54. [Google Scholar]
- De Longo, O.; Colombo, A.; Gomez-Riera, P.; Bartolucci, A.; Tan, K.H. The use of massive SIT for the control of the Medfly, Ceratitis capitata (Wied.), Strain SEIB 6–96, in Mendoza, Argentina. In Proceedings of the International Conference On Area-Wide Control Of Insect Pests And The Fifth International Symposium On Fruit Flies Of Economic Importance, Penang, Malaysia, 28 May–5 June 1998. [Google Scholar]
- Zavala-López, J.L.; Marte-Diaz, G.; Martínez-Pujols, F. Successful area-wide eradication of the invading Mediterranean fruit fly in the Dominican Republic. In Area-Wide Integrated Pest Management; Hendrichs, J., Pereira, R., Vreysen, J.R., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 519–537. [Google Scholar]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 2021; pp. 1–1216. [Google Scholar] [CrossRef]
- Enkerlin, W.; Gutiérrez-Ruelas, J.M.; Cortes, A.V.; Roldan, E.C.; Midgarden, D.; Lira, E.; López, J.L.Z.; Hendrichs, J.; Liedo, P.; Arriaga, F.J.T. Area freedom in Mexico from Mediterranean fruit fly (Diptera: Tephritidae): A review of over 30 years of a successful containment program using an integrated area-wide SIT approach. Fla. Entomol. 2015, 98, 665–681. [Google Scholar] [CrossRef]
- Bazelet, C.S.; Leo, A.; Lowe, P.; Johnson, R.; Usnick, S. A Multifaceted approach to domestic management of invasive fruit flies in the United States. In Management of Fruit Flies in the Americas; Garcia, F.R.M., Ed.; Springer: Cham, Switzerland, 2024; pp. 445–460. [Google Scholar]
- Bingham, R.R.; Hoffman, K.M.; Gilbert, A.J.; Leathers, J.W. California’s Insect Trapping Guide: Safeguarding a Multi-Billion-Dollar Agricultural Industry. Am. Entomol. 2020, 66, 40–47. [Google Scholar] [CrossRef]
- Bjeliš, M.; Popović, L.; Marušić, I.; Gakić, S.; Buljubašić, I.; Ivanović, A.; Arnaut, P.; Cardoso-Pereira, R. Suppression of Medfly by Sterile Insect Technique (SIT) in Neretva River Valley of Croatia. In Proceedings of the Regional Symposium on the Management of Fruit Flies in Near East Countries, Hammamet, Tunisia, 6–8 November 2012; p. 53. [Google Scholar]
- Bjeliš, M.; Popović, L.; Deak, S.; Buljubašić, I.; Ivanović, A.; Arnaut, P.; Pereira, R. Suppression of Mediterranean Fruit Fly by SIT over the 4000 ha of fruit orchards in Neretva River Valley. Zb. Pred. Ref. 2013, 11, 101–108. [Google Scholar]
- Plá, I.; García de Oteyza, J.; Tur, C.; Martínez, M.Á.; Laurín, M.C.; Alonso, E.; Martinez, M.; Martin, A.; Sanchis, R.; Navarro, M.C.; et al. Sterile Insect Technique programme against Mediterranean Fruit Fly in the Valencian Community (Spain). Insects 2021, 12, 415. [Google Scholar] [CrossRef]
- Duarte, F.; Caro, A.; Delgado, S.; Asfennato, A.; López, L.; Hernández, F.; Calvo, M.V. Sterile insect technique (sit) effectiveness to control Ceratitis capitata (Diptera: Tephritidae) and medfly catches in two mass trapping layouts. Int. J. Pest Manag. 2022, 68, 402–413. [Google Scholar] [CrossRef]
- Yazid, J.B.; Chafik, Z.; Bibi, I.; Kharmach, E. Effectiveness of sterile insect technique for medfly (Ceratitis capitata, Wiedemann, 1824) control in citrus orchards of Moulouya perimeter North East of Morocco. ISPEC J. Agric. Sci. 2020, 4, 405–421. [Google Scholar] [CrossRef]
- Colacci, M.; Tabilio, M.R.; Lolletti, D.; Ferrante, P.; Ceccaroli, C.; Bernabei, G.; Sciarretta, A. Spatio temporal association between sterile and wild males in a Ceratitis capitata SIT programme. Sci. Rep. 2025, 15, 19584. [Google Scholar] [CrossRef] [PubMed]
- Charbonnel, E.; Ouvrard, D.; Benoit, L.; Chapuis, M.P. Identifying the specific status and geographical origin of European incursions of an invasive and cryptic pest. In Proceedings of the ANSES Scientific and Doctoral Days, Maisons-Alfort, France, 2–3 October 2023. [Google Scholar]
- Nugnes, F.; Russo, E.; Viggiani, G.; Bernardo, U. First record of an invasive fruit fly belonging to Bactrocera dorsalis complex (Diptera: Tephritidae) in Europe. Insects 2018, 9, 182. [Google Scholar] [CrossRef]
- Nugnes, F.; Carbone, C.; Ascolese, R.; Miele, F.; Pica, F.; Palmieri, A.; Griffo, R.V.; Bernardo, U. The enemy is already inside! Bactrocera dorsalis is a serious threat to European orchards and crops. Entomol. Gen. 2024, 44, 1243–1251. [Google Scholar] [CrossRef]
- Barclay, H.J. Mathematical models for using Sterile Insects. In Sterile Insect Technique, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 201–244. [Google Scholar] [CrossRef]
- Lux, S.A. PESTonFARM—stochastic model of on-farm insect behaviour and their response to IPM interventions. J. Appl. Entomol. 2014, 138, 458–467. [Google Scholar] [CrossRef]
- Lux, S.A. Individual-based modeling approach to assessment of the impacts of landscape complexity and climate on dispersion, detectability and fate of incipient Medfly populations. Front. Physiol. 2018, 8, 1121. [Google Scholar] [CrossRef] [PubMed]
- Colacci, M.; Lux, S.A.; Sciarretta, A. Use of the PESTonFARM model for the management of Ceratitis capitata (Diptera: Tephritidae) populations. In Proceedings of the Abstarct Book of the XX International Pant Protection Congress, Athens, Greece, 1–5 July 2024. [Google Scholar]
- Lux, S.A.; Wnuk, A.; Vogt, H.; Belien, T.; Spornberger, A.; Studnicki, M. Validation of individual-based Markov-like stochastic process model of insect behavior and a “virtual farm” concept for enhancement of site-specific IPM. Front. Physiol. 2016, 7, 363. [Google Scholar] [CrossRef]
- Andress, E.; Walters, I.; del Toro, M.; Shelly, T. Release-Recapture of Sterile Male Mediterranean Fruit Flies (Diptera: Tephritidae) in Southern California. Proc. Hawaii. Entomol. Soc. 2013, 45, 11–29. [Google Scholar] [CrossRef]
- Barry, J.D.; Dowell, R.V.; Morse, J.G. Comparison of Two Sterile Mediterranean Fruit Fly (Diptera: Tephritidae) Strains Released in California’s Preventative Release Program. J. Econ. Entomol. 2002, 95, 936–944. [Google Scholar] [CrossRef]
- Duarte, F.; Calvo, M.V.; Delgado, S.; Bartolucci, S.; Asfennato, A.; Borges, A.; Scatoni, I.; García, F.M. Release-Recapture Test of Dispersal and Survival of Sterile Males on Ceratitis capitata (Diptera: Tephritidae). Neotrop. Entomol. 2020, 49, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Gavriel, S.; Gazit, Y.; Leach, A.; Mumford, J.; Yuval, B. Spatial patterns of sterile Mediterranean fruit fly dispersal. Entomol. Exp. Appl. 2012, 142, 17–26. [Google Scholar] [CrossRef]
- Meats, A.; Smallridge, C.J. Short- and long-range dispersal of medfly, Ceratitis capitata (Dipt., Tephritidae), and its invasive potential. J. Appl. Entomol. 2007, 131, 518–523. [Google Scholar] [CrossRef]
- Paranhos, B.J.; Papadopoulos, N.T.; McInnis, D.; Gava, C.; Lopes, F.S.C.; Morelli, E.; Malavasi, A. Field Dispersal and Survival of Sterile Medfly Males Aromatically Treated with Ginger Root Oil. Environ. Entomol. 2010, 39, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Plant, R.C.; Cunningham, R.T. Analyses of the Dispersal of Sterile Mediterranean Fruit Flies (Diptera: Tephritidae) Released from Point Source. Environ. Entomol. 1991, 20, 1493–1503. [Google Scholar] [CrossRef]
- Shelly, T.E.; Whittier, T.S. Mating Competitiveness of Sterile Male Mediterranean Fruit Flies (Diptera: Tephritidae) in Male-Only Releases. Ann. Entomol. Soc. Am. 1996, 89, 754–758. [Google Scholar] [CrossRef]
- Shelly, T.E.; Edu, J.; Pahio, E. Lack of an irradiation effect on the mating performance of mass-reared males on the Mediterranean fruit fly. Fla. Entomol 2005, 88, 547–548. [Google Scholar] [CrossRef]
- Virginio, J.F.; Gomez, M.; Pinto, A.M.; Aniely, G.G.; Paranhos, B.J.; Gava, C.A.T.; Caceres, C.; Walder, J.M.M. Male sexual competitiveness of two Ceratitis capitata strains, tsl Vienna 8 and OX3864A transgenics, in field cage conditions. Entomol. Exp. Appl. 2017, 164, 318–326. [Google Scholar] [CrossRef]
- Goldshtein, E.; Gazit, Y.; Hetzroni, A.; Timar, D.; Rosenfeld, L.; Grinshpon, Y.; Cohen, Y. Long-term auto-matic trap data reveal factors affecting diurnal flight patterns of the Mediterranean Fruit fly. J. Appl. Entomol. 2021, 145, 427–439. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Carey, J.R.; Katsoyannos, B.I.; Kouloussis, N.A. Overwintering of Ceratitis capitata (Diptera: Tephritidae) in Northern Greece. Ann. Entomol. Soc. Am. 1996, 89, 526–534. [Google Scholar] [CrossRef]
- Jang, E.B. Physiology of mating behavior in Mediterranean fruit fly (Diptera: Tephritidae: Chemorecep- tion and male accessory gland fluids in female post-mating behavior. Fla. Entomol. 2002, 85, 89–93. [Google Scholar] [CrossRef]
- Dumont, Y.; Oliva, C.F. On the impact of re-mating and residual fertility on the Sterile Insect Technique efficacy: Case study with the medfly, Ceratitis capitata. PLoS Comput. Biol. 2024, 20, e1012052. [Google Scholar] [CrossRef] [PubMed]
- Barclay, H.J.; Matlock, R.; Gilchrist, S.; Suckling, D.M.; Reyes, J.; Enkerlin, W.R.; Vreysen, M.J.B. A conceptual model for assessing the minimum size area for an area-wide integrated pest management program. Int. J. Agron. 2011, 2011, 409328. [Google Scholar] [CrossRef][Green Version]
- Diouf, E.G.; Brévault, T.; Ndiaye, S.; Faye, E.; Chailleux, A.; Diatta, P.; Piou, C. An agent-based model to simulate the boosted Sterile Insect Technique for fruit fly management. Ecol. Model. 2022, 468, 109951. [Google Scholar] [CrossRef]
- Manoukis, N.C.; Hoffman, K. An agent-based Simulation of extirpation of Ceratitis capitata applied to invasions in California. J. Pest Sci. 2014, 87, 39–51. [Google Scholar] [CrossRef]
- FAO/IAEA. Guidelines for the Use of Mathematics in Operational Area-Wide Integrated Pest Management Programmes Using the Sterile Insect Technique with a Special Focus on Tephritid Fruit Flies; Barclay, H.L., Enkerlin, W.R., Manoukis, N.C., Reyes-Flores, J., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; 95p. [Google Scholar]
- Barclay, H.J.; Hendrichs, J. Modeling trapping of Fruit Flies for detection, suppression, or eradication. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Springer: Berlin/Heidelberg, Germany, 2014; pp. 379–420. [Google Scholar] [CrossRef]
- Bali, E.M.D.; Rodovitis, V.G.; Verykouki, E.; Terblanche, J.S.; Carey, J.R.; Papadopoulos, N.T. Factors affecting detection and trapping efficacy of Mediterranean fruit flies. Pest Manag. Sci. 2025, 81, 3548–3556. [Google Scholar] [CrossRef] [PubMed]
- Meats, A. Fruit fly detection programs: The potentials and limitations of trap arrays. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Springer: Berlin/Heidelberg, Germany, 2014; pp. 253–275. [Google Scholar] [CrossRef]
- Bjeliš, M.; Tavra, I.; Strikić, F.; Stojić, M.; Nestel, D. Invasion of Ceratitis capitata W. (Diptera, Tephritidae) from coastal to inland areas of Dalmatia Region of Croatia: E-Traps as an improved detection tool. In IOBC-WPRS Citrus Working Group Meeting; IOBC-Global: Zürich, Switzerland, 2022; pp. 66–72. [Google Scholar]
- Čizmović, M.; Pereira, R. Population dynamics of the Mediterranean Fruit Fly in Montenegro. Int. J. Insect Sci. 2013, 5, IJIS.S12964. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Katsoyannos, B.I.; Carey, J.R.; Kouloussis, N.A. Seasonal and annual occurrence of the Mediterranean Fruit Fly (Diptera: Tephritidae) in Northern Greece. Ann. Entomol. Soc. Am. 2001, 94, 41–50. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Katsoyannos, B.I.; Kouloussis, N.A.; Economopoulos, A.P.; Carey, J.R. Effect of adult age, food, and time of day on sexual calling incidence of wild and mass-reared Ceratitis capitata males. Entomol. Exp. Appl. 1998, 89, 175–182. [Google Scholar] [CrossRef]
- Colacci, M.; Sciarretta, A.; Lolletti, D.; Bernabei, G.; Moraiti, C.A.; Papadogiorgou, G.D.; Rodovitis, V.G.; Papachristos, D.P.; Milonas, P.; Antonatos, S.; et al. Naturally Abscised Fruitlets as a Potential Breeding Resource for Early Spring Buildup of Medfly Populations in Temperate Regions. Agronomy 2024, 14, 1882. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Katsoyannos, B.I.; Nestel, D. Spatial autocorrelation analysis of a Ceratitis capitata (Diptera: Tephritidae) adult population in a mixed deciduous fruit orchard in Northern Greece. Environ. Entomol. 2003, 32, 319–326. [Google Scholar] [CrossRef]
- Szymańska, E.J.; Rysz, M. Determinants of changes in fruit production in farms in areas with a fragmented agrarian structure. Agriculture 2022, 12, 1767. [Google Scholar] [CrossRef]
- Carey, J.R.; Papadopoulos, N.T.; Müller, H.-G.; Katsoyannos, B.I.; Kouloussis, N.A.; Wang, J.-L.; Wachter, K.; Yu, W.; Liedo, P. Age Structure Changes and Extraordinary Lifespan in Wild Medfly Populations. Aging Cell 2008, 7, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Kouloussis, N.A.; Papadopoulos, N.T.; Katsoyannos, B.I.; Müller, H.-G.; Wang, J.-L.; Su, Y.-R.; Molleman, F.; Carey, J.R. Seasonal trends in Ceratitis capitata reproductive potential derived from live-caught females in Greece. Entomol. Exp. Appl. 2011, 140, 181–188. [Google Scholar] [CrossRef][Green Version]
- Nestel, D.; Katsoyannos, B.; Nemny-Lavy, E.; Mendel, Z.; Papadopoulos, N.; Barnes, B.N. Spatial analysis of Medfly populations in heterogeneous landscapes. In Proceedings of the 6th International Symposium on Fruit Flies of Economic Importance, Stellenbosch, South Africa, 6–10 May 2004; pp. 35–43. [Google Scholar]
- Sciarretta, A.; Trematerra, P. Spatio-Temporal distribution of Ceratitis capitata population in a heterogeneous landscape in Central Italy. J. Appl. Entomol. 2011, 135, 241–251. [Google Scholar] [CrossRef]
- Sciarretta, A.; Tabilio, M.R.; Lampazzi, E.; Ceccaroli, C.; Colacci, M.; Trematerra, P. Analysis of the Mediterranean Fruit Fly [Ceratitis capitata (Wiedemann)] Spatio-Temporal Distribution in Relation to Sex and Female Mating Status for Precision IPM. PLoS ONE 2018, 13, e0195097. [Google Scholar] [CrossRef]
- Drolet, D.; Locke, A. Relative importance of propagule size and propagule number for establishment of non-indigenous species: A stochastic simulation study. Aquat. Invasions 2016, 11, 101–110. [Google Scholar] [CrossRef]
- Fisher, K.T.; Hill, A.R.; Sproul, A.N. Eradication of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) in Carnarvon, Western Australia. Aust. J. Entomol. 1985, 24, 207–208. [Google Scholar] [CrossRef]


| Sterile Male Quality Parameter | Assumed Value | Number of Individuals |
|---|---|---|
| PUPAE: base cohort of irradiated male pupae | 1000 | |
| ADULT males: emergence rate | 85% | 850 |
| Active fliers at the release time | 85% | 723 |
| Time to maturity post-release | 2 days | |
| Daily mortality rate in the field (assumed 50% survival after 4 days) | 20% | |
| Effective fliers surviving in the field until maturity | 462 | |
| Mature fliers joining wild male calling groups (leks) | 90% | 416 |
| Sterile to wild mating competitiveness | 30% | |
| Effectively competitive sterile males from 1000 irradiated pupae | 125 | |
| Female re-mating chance (mating with a sterile and then wild male) | 5% |
| Site A | Site B | Site C | |
|---|---|---|---|
| Eggs | |||
| VE apricot | 543 | 371 | 471 |
| M peach | 29,049 | 20,603 | 20,262 |
| L apple | 945,083 | 120,421 | 95,796 |
| TOTAL | 974,675 | 141,395 | 116,529 |
| Larvae | |||
| VE apricot | 434 | 294 | 365 |
| M peach | 24,584 | 17,432 | 17,210 |
| L apple | 771,713 | 98,477 | 78,384 |
| TOTAL | 796,731 | 116,203 | 95,959 |
| Pupae | |||
| VE apricot | 192 | 141 | 172 |
| M peach | 9649 | 7022 | 6917 |
| L apple | 279,335 | 36,088 | 28,478 |
| TOTAL | 289,176 | 43,251 | 35,567 |
| Adult females | |||
| VE apricot | 133 | 75 | 115 |
| M peach | 4293 | 3045 | 3066 |
| L apple | 52,278 | 7976 | 5945 |
| TOTAL | 56,704 | 11,096 | 9126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lux, S.A.; Colacci, M. Adaptation of the PESTonFARM Model to Support Decision-Making and Planning of Local Implementation of the Sterile Insect Technique in the Control of Ceratitis capitata Flies (Diptera: Tephritidae). Appl. Sci. 2025, 15, 6694. https://doi.org/10.3390/app15126694
Lux SA, Colacci M. Adaptation of the PESTonFARM Model to Support Decision-Making and Planning of Local Implementation of the Sterile Insect Technique in the Control of Ceratitis capitata Flies (Diptera: Tephritidae). Applied Sciences. 2025; 15(12):6694. https://doi.org/10.3390/app15126694
Chicago/Turabian StyleLux, Slawomir Antoni, and Marco Colacci. 2025. "Adaptation of the PESTonFARM Model to Support Decision-Making and Planning of Local Implementation of the Sterile Insect Technique in the Control of Ceratitis capitata Flies (Diptera: Tephritidae)" Applied Sciences 15, no. 12: 6694. https://doi.org/10.3390/app15126694
APA StyleLux, S. A., & Colacci, M. (2025). Adaptation of the PESTonFARM Model to Support Decision-Making and Planning of Local Implementation of the Sterile Insect Technique in the Control of Ceratitis capitata Flies (Diptera: Tephritidae). Applied Sciences, 15(12), 6694. https://doi.org/10.3390/app15126694






