Application of Waste Tire Carbon for Iron-Containing Dust Reduction in Industrial Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
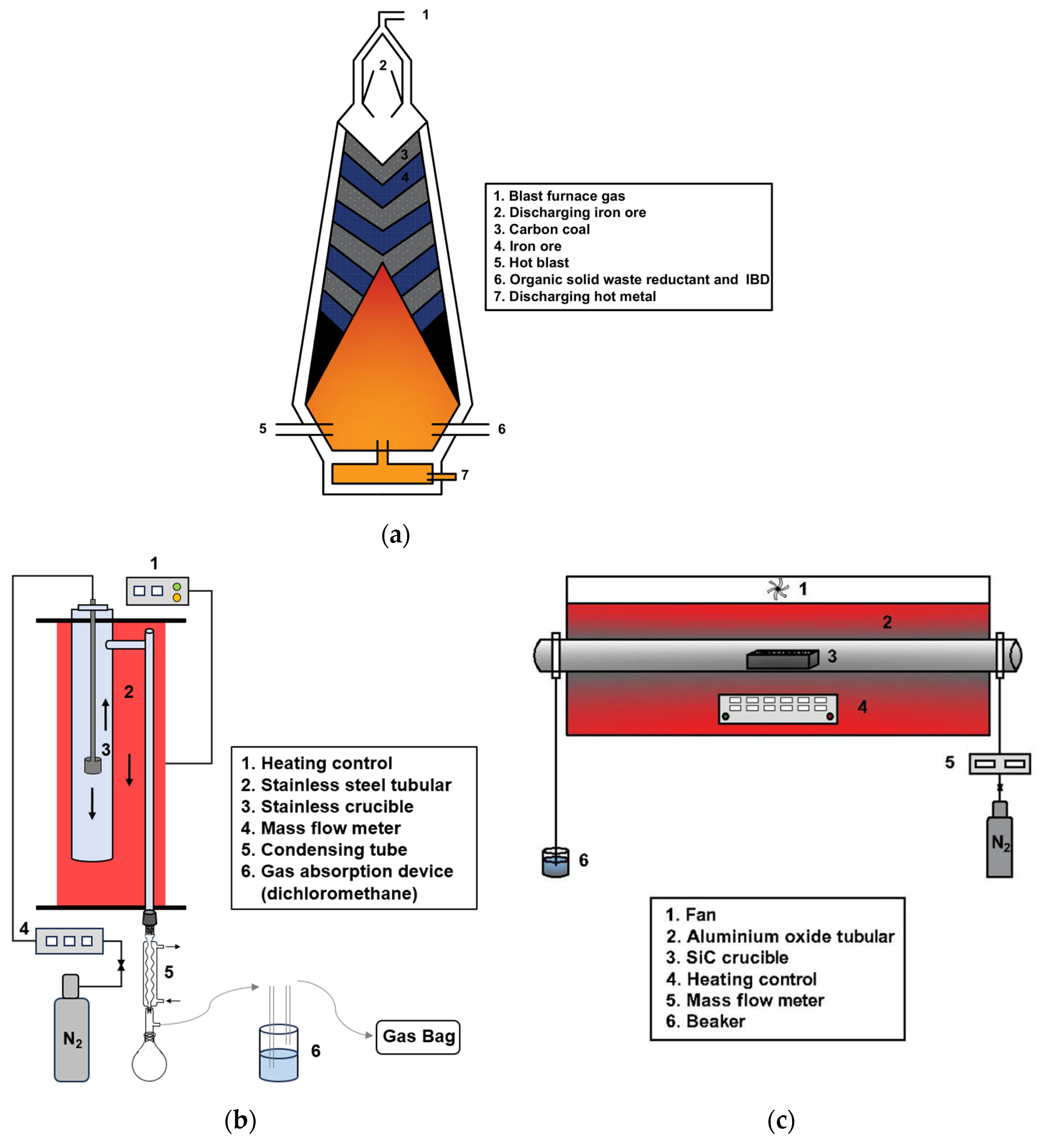
2.2. Experimental Equipment and Methods
2.3. Analytical Method
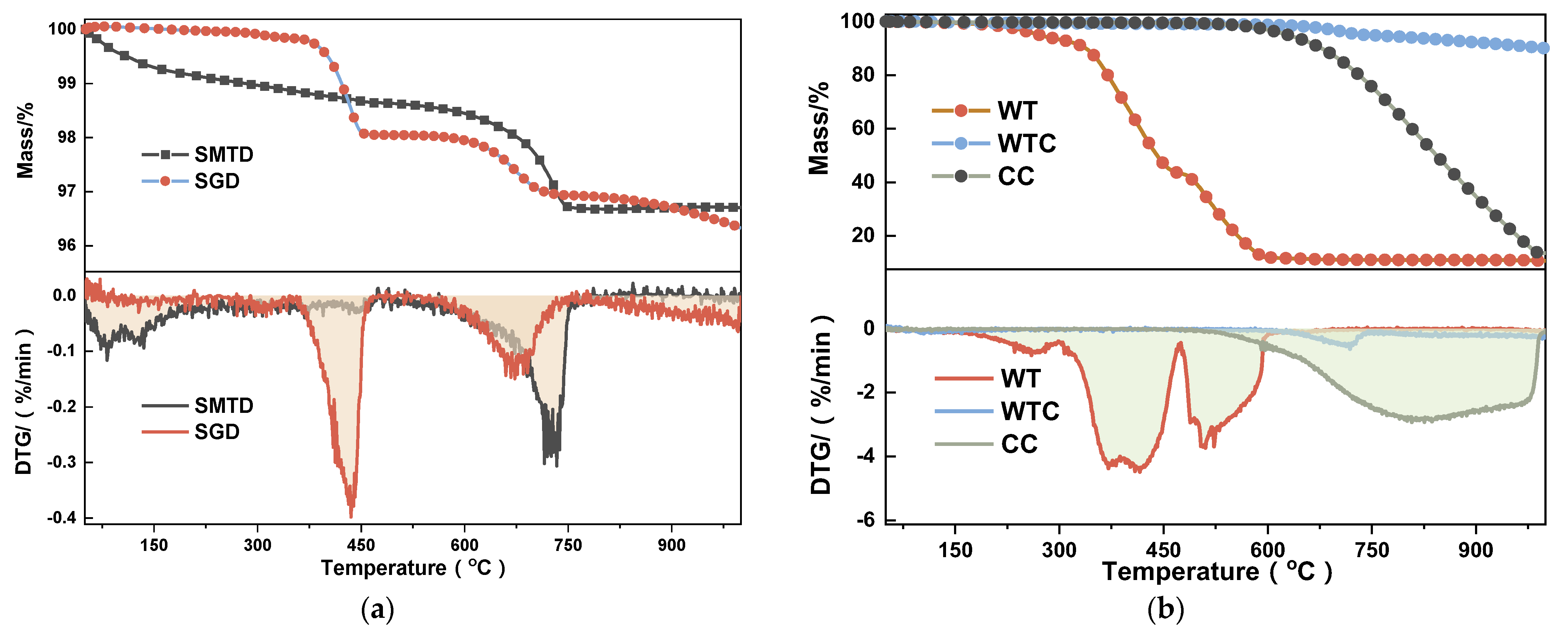
2.3.1. Thermal Analysis
2.3.2. GC Experiments
2.3.3. TCLP Experiments
3. Results
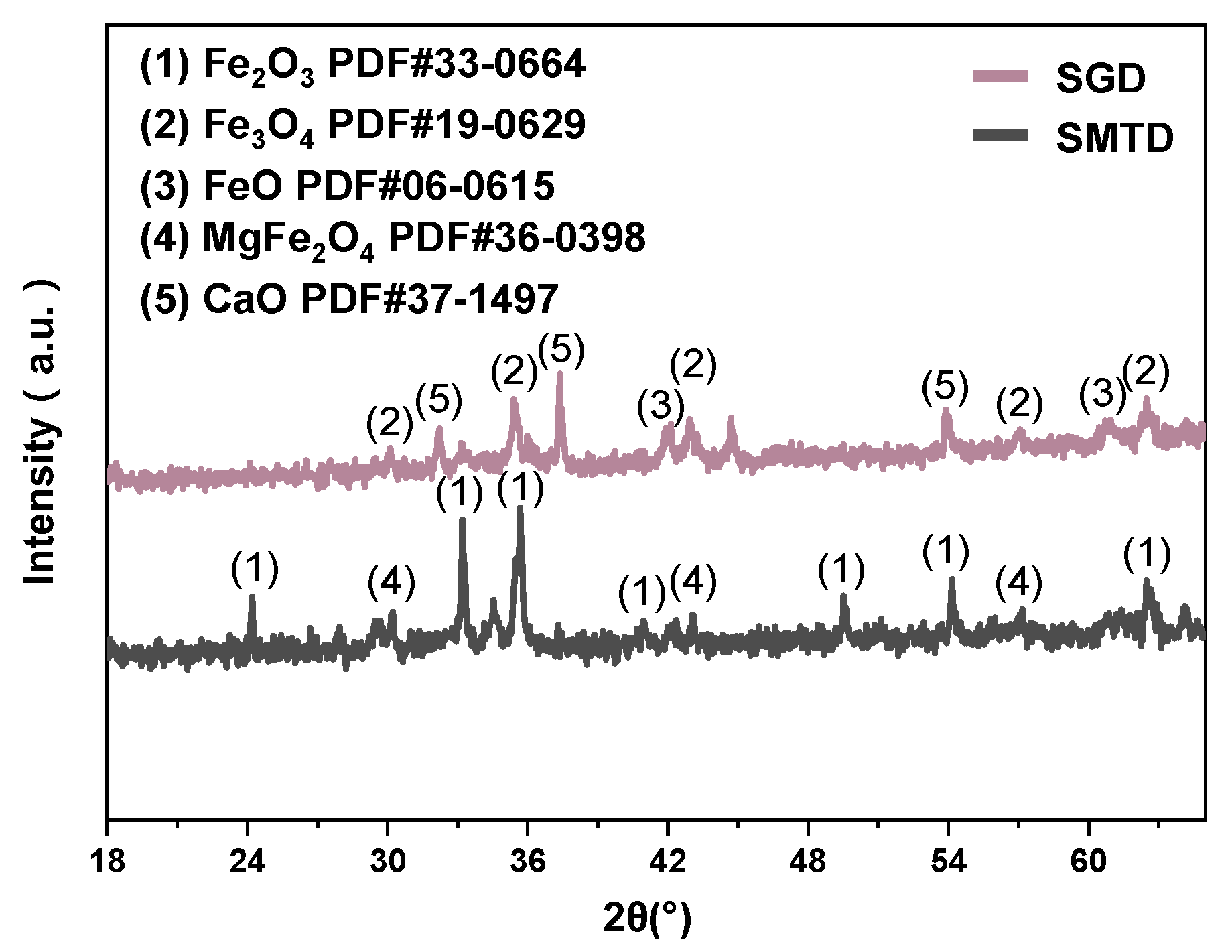
3.1. Characteristics of Raw Materials
3.2. Optimal Reaction Conditions for Iron Reduction
3.3. Comparison of Reduction Methods
3.4. Leaching of Heavy Metals
4. Discussion
- 1.
- Reduction conditions: The optimal conditions for reducing Fe to MFe are 1000 °C, with a mass ratio of SMTD to WTC of 2:1, and 0% O2, for a reaction time of 45 min. Under these conditions, the replacement of IBD with SGD ensures that all Fe phases detected by XRD are in the MFe form, without affecting Fe reduction under typical blast furnace oxygen injection rates of 0–8%;
- 2.
- Effects of reductants: The reduction efficiency follows the order: WTC > CC > WT. Although WTC has 20.66% lower carbon content than CC, its higher saturation, smaller TG weight loss, and a surface tar Ea of only 5.79 kJ/mol contribute to a faster reaction rate. Additionally, the carbon black structure is more three-dimensional;
- 3.
- Effects of heating methods: The three heating methods (PH, FH, MH) have little impact on Fe reduction. Among them, the higher heating rate of FH results in a faster carbon gasification rate, making the FH residue more uniformly loose. MH exhibits a more concentrated reaction zone, leading to larger metal particle sizes;
- 4.
- TCLP results: Sufficient carbon thermal reduction significantly suppresses the leaching of Mg and Zn while promoting the leaching of Ca and Al. It also promotes the Cr leaching of 117.60%. The reduced residues require further processing to remove Cr to meet environmental safety standards.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMTD | sintering machine tail dust |
| SGD | steelmaking gravity dust |
| IBD | iron-bearing dust |
| CC | carbon coke |
| WT | waste tire |
| WTC | waste tire carbon |
| TFe | total iron |
| MFe | metallic iron |
| FH | flash heating group |
| PH | programmed heating group |
| MH | microwaved heating group |
| OO | the optimal group |
| CC-A | reduced group using CC as alternative material |
| WT-A | reduced group using WT as alternative material |
| SGD-A | reduced group using SGD as alternative material |
References
- Dianyin, Z.; Huadong, G.; Chun, X. The Principle of Recycled Utilization of Metallurgical Waste Slag. In Recycling and Utilization Technology of Metallurgical Waste Slag, 1st ed.; Kefa, C., Ed.; Chemical Industry Press Co., Ltd.: Beijing, China, 2017; p. 366. [Google Scholar]
- Canbin, L. Exploitation of Composite Binder Used for Cold-Pressed Carbon-Iron-Bearing Dust and Sludge Pellets and Its Mechanism. Master’s Thesis, Anhui University of Technology, Ma’anshan, China, 2022. [Google Scholar]
- Jinxia, Z.; Fusheng, N.; Zhishuai, X. Research on the separation technology of iron, carbon, and zinc from metallurgical iron-bearing dust in the steel industry. Min. Mach. 2014, 42, 97–102. [Google Scholar]
- Shang, H.; Li, H.; Wei, R.; Long, H.; Li, K.; Liu, W. Present situation and prospect of iron and steel dust and sludge utilization technology. Iron Steel 2019, 54, 9–17. [Google Scholar]
- Wang, S.Y.; Zhang, R.L.; Wu, A.J.; Zhang, W.; Yang, R.X.; Gao, Y.F.; Pan, W. Reduction of high-grade pyrolusite by pyrolyzed biomass in vacuum. J. Sustain. Metall. 2024, 10, 2621–2629. [Google Scholar] [CrossRef]
- Zhou, T.; Sun, Y.; Han, Y.; Li, Y. Hydrogen-based fluidization direct reduction of high purity iron concentrate: Experimental optimization and mechanism analysis. Miner. Eng. 2025, 227, 109273. [Google Scholar] [CrossRef]
- Zheng, H.; Li, B.; Zhou, H.; Wei, Y.; Wang, H. Dilution of copper slag under reduction of rubber seed oil. Chin. J. Process Eng. 2019, 19, 589–596. [Google Scholar]
- Wei, R.; Cang, D.; Bai, Y.; Huang, D.; Liu, X. Reduction characteristics of iron oxide by different biomass. J. lron Steel Res. 2020, 32, 186–194. [Google Scholar]
- CEIC. Available online: https://www.ceicdata.com/zh-hans/china/custeel-steel-factory-blast--electric-furnace-operating-rate-and-capacity-utilization-rate/cn-operating-rate-steel-factory-blast-furnace (accessed on 16 April 2025).
- Chaofeng, Y. Study on energy saving effect of oxygen-rich blast furnace. Shanxi Metall. 2024, 47, 199–201. [Google Scholar]
- Zhengming, Y.; Chou, Z.; Zheng, Z. Numerical Simulation of Blast Furnace Gas and Coke Oven Gas Combustion under Different Oxygen Enrichment Conditions. J. Eng. Therm. Energy Power 2022, 37, 84–91. [Google Scholar]
- Zhang, H.J.; Wang, J.S.; An, X.W.; Zuo, X.J.; Xue, Q.G. Reduction behavior of pellet under simulated oxygen blast furnace condition. J. Iron Steel Res. Int. 2015, 22, 115–120. [Google Scholar] [CrossRef]
- Fukushima, J.; Takizawa, H. In situ spectroscopic analysis of the carbothermal reduction process of iron oxides during microwave irradiation. Metals 2018, 8, 49. [Google Scholar] [CrossRef]
- Shengzhen, L.; Jin, Y.; Xiaoman, J.; Wei, L.; Xingmeng, Y. Research progress on the resource utilization of carbon black fromwaste tire pyrolysis. New Chem. Mater. 2024, 52, 231–236. [Google Scholar]
- Long, H.M.; Wei, R.F.; Li, N.; Zhou, D.; Meng, Q.M.; Li, J.X. Disposal of city combustible solid waste by blast furnace. Iron Steel 2018, 53, 1–9. [Google Scholar]
- Pyshyev, S.; Korchak, B.; Miroshnichenko, D.; Lebedev, V.; Yasinska, A.; Lypko, Y. Obtaining new materials from liquid pyrolysis products of used tires for waste valorization. Sustainability 2025, 17, 3919. [Google Scholar] [CrossRef]
- Chengkang, Y.; Chunlong, F.; Hexi, Z.; Xiangyang, X.; Long, D.; Lixin, Q.; Cheng, P.; Hongming, L. Pyrolytic carbon characteristics and iron oxidereduction ability of waste tire. Chin. Metall. 2025, 35, 1–14. [Google Scholar]
- Xue, P.; He, D.; Xu, A.; Yang, Q. Formation of MgFe2O4 and recycling of iron from modified BOF slag by magnetic separation. Iron Steel 2017, 52, 104–110. [Google Scholar]
- Hongyu, H. Study on the Mechanism of Material Metabolism and Emission Inventory of Iron-Containing Dust Sludge in the Process of Resource Extraction. Master’s Thesis, Central South University, Changsha, China, 2023. [Google Scholar]
- Zhiran, L.; Ding, L.; Jinmei, L. Analysis of issues and countermeasures in the recycling and utilization of iron-bearing dust in the steel industry. In Proceedings of the Annual Meeting of Chinese Society for Environmental Sciences, Kunming, China, 1 August 2013. [Google Scholar]
- Ning, S.; Jianghua, L.; Liang, Z. Determination of free calcium oxide in steel slag by thermogravimetry. Phys. Exam. Test. 2019, 37, 25–28. [Google Scholar]
- Xia, Y.; Yu, Y.; Cong, Z.; Shaobo, O.; Jun, W.; Liqing, L. Kinetic and thermodynamic characteristics in scrap tyres pyrolysis process and components analysis of pyrolysis oil. Low-Carbon Chem. Chem. Eng. 2023, 48, 36–45. [Google Scholar]
- Nisar, J.; Ali, G.; Shah, A.; Farooqi, Z.H.; Khan, R.A.; Iqbal, M.; Gul, M. Pyrolysis of waste tire rubber: A comparative kinetic study using different models. Energe Sources Part A 2020, 46, 12710–12720. [Google Scholar] [CrossRef]
- Fawei, L.; Fa, Z.; Jiantao, L.; Yahui, L.; Xuefeng, S.; Chengjun, X.; Wenchen, M.; Guanyi, Chen. Comparing pyrolysis characteristics of Daqing multi-source oil sludge. Chem. Ind. Eng. Prog. 2021, 40, 421–433. [Google Scholar]
- Ovčačíková, H.; Vlček, J.; Klárová, M.; Topinková, M. Metallurgy dusts as a pigment for glazes and engobes. Ceram. Int. 2017, 43, 7789–7796. [Google Scholar] [CrossRef]
- Guoliang, L.; Haiying, L.; Aimin, J. Research on collaborative treatment of fine particles in sintering smoke. Energy Conserv. 2024, 43, 70–72. [Google Scholar]
- Jianfang, W. Study on the Synergistic Cementing Mechanism of CaO/SiO2/Al2O3/CaSO4·2H2O/Fe2O3/MgO Multi-Component Blending and the Co-Blending of Multi-Solid Waste. Ph.D. Dissertation, Shanxi University, Taiyuan, China, 2023. [Google Scholar]
- Danuka, W.W.M.D.; Timo, F.; Mamdouh, O. The reduction reaction behavior of steelmaking dusts with lignin under different atmospheres. Materials 2024, 17, 3106. [Google Scholar] [CrossRef]
- Kontoyannis, C.G. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analysis 2000, 125, 251–255. [Google Scholar] [CrossRef]
- Edward, L.E.M.; Danny, C.K.K.; Gordon, M. Production of active carbons from waste tires—A review. Carbon 2004, 42, 2789–2805. [Google Scholar]
- Chen, G.; Sun, B.; Li, J.; Lin, F.; Xiang, L.; Yan, B. Products distribution and pollutants releasing characteristics during pyrolysis of waste tires under different thermal process. J. Hazard. Mater. 2022, 424, 127351. [Google Scholar] [CrossRef] [PubMed]
- Miao, L. Experimental Study on Migration Characteristics of Nitrogen, Sulfur and Chlorine During Pyrolysis of Waste Tire. Master’s Thesis, Tianjin University, Tianjin, China, 2020. [Google Scholar]
- Menares, T.; Herrera, J.; Romero, R.; Osorio, P.; Arteaga-Pérez, L.E. Waste tires pyrolysis kinetics and reaction mechanisms explained by TGA and Py-GC/MS under kinetically-controlled regime. Waste Manag. 2020, 102, 21–29. [Google Scholar] [CrossRef]
- Dan, P.; Siguang, H.; Guanming, Z.; Tao, Y.; Wenzheng, M.; Shulian, L.; Shaoming, F.; Shide, W. Research progress on recycle utilization of pyrolytic carbon from waste tires. J. Light Ind. 2022, 37, 119–126. [Google Scholar]
- Einara, B.M.; Daniel, T.P.; João, A.D.C. Energetic valorization of waste tires. Renew. Sustain. Energy Rev. 2017, 68, 306–315. [Google Scholar]
- Pingan, H. Study on Preparation of Carbon Black by Pyrolysis of Waste Tire and Ball Milling Modification. Mater’s Thesis, Zhejiang University, Xiamen, China, 2022. [Google Scholar]
- Martínez, J.D.; Murillo, R.; García, T.; Arauzo, I. Thermodynamic analysis for syngas production from volatiles released in waste tire pyrolysis. Energy Convers. Manag. 2014, 81, 338–353. [Google Scholar] [CrossRef]
- Boyu, Q. Kinetic Analysis of Waste Tire Pyrolysis. Mater’s Thesis, Dalian University of Technology, Dalian, China, 2021. [Google Scholar]
- Minbo, C. Misunderstanding about the correlation coefficient R in statistical data processing. Chemistry 2011, 74, 387–395. [Google Scholar]
- Haiyang, W.; Jianlaing, Z.; Guangwei, W. Feasibility research of injection of BF dust into melter gasifier of COREX. Iron Steel 2017, 52, 29–34. [Google Scholar]
- Baetzold, R.C.; Somorjai, G.A. Preexponential factors in surface reactions. J. Catal. 1976, 45, 94–105. [Google Scholar] [CrossRef]
- Xuesong, W.; Jiaqin, Y.; Xiaoxi, W.; Lemei, Y.; Bing, H. Preparation of nano-MgFe2O4 by microemulsion-precipitation method and its characterization. Bull. Chin. Ceram. Soc. 2001, 3, 58–61. [Google Scholar]
- Zengbin, W. Study on Characteristics and Adsorption Performance of Non-Activated Tire-Based Pyrolytic Char. Mater’s Thesis, Suzhou University of Science and Technology, Suzhou, China, 2014. [Google Scholar]
- Jin, J. Kinetics and Mechanisms of Coke and Iron Ore on the Coupling Reaction and Their Evaluating Indexes. Ph.D. Dissertation, University of Science and Technology Liaoning, Anshan, China, 2023. [Google Scholar]
- Changwei, L.; Jikun, W. Direct reduction of zinc sulfide concentrate for zinc volatilization. Nonferrous Met. 1995, 4, 5–6+4. [Google Scholar]
- En, T. Fundamental Research to Obtain Nugget Iron from Iron-Bearing Solid Waste Through Rotary Hearth Furnace. Ph.D. Dissertation, Wuhan University of Science and Technology, Wuhan, China, 2019. [Google Scholar]
- Guojun, M.; Yibiao, J.; Dabin, Y. Production of Si-Al-Fe alloy with carbothermal reduction of coal fly ash. Adv. Mater. Res. 2011, 1335, 1808–1811. [Google Scholar]
- Yanhua, Z. Study on Phases Formation and Crystallization Kinetics of Non-Equilibrium Solidified Alloys. Ph.D. Dissertation, Tianjin University, Tianjin, China, 2008. [Google Scholar]
- Liu, L.; Kuang, S.; Guo, B.; Yu, A. Optimization of ironmaking blast furnace operations using an integrated mathematical model. Chem. Ing. Tech. 2022, 95, 219–233. [Google Scholar] [CrossRef]
- Zhucheng, H.; Jianjia, H.; Yixin, L.; Yang, S. Study on direct reduction of iron concentrate by biomass. J. Cent. South Univ. 2023, 54, 1230–1239. [Google Scholar]
- Hammam, A.; Li, Y.; Nie, H.; Zan, L.; Ding, W.; Ge, Y.; Li, M.; Omran, M.; Yu, Y. Isothermal and non-isothermal reduction behaviors of iron ore compacts in pure hydrogen atmosphere and kinetic analysis. Mining Metall. Explor. 2021, 38, 81–93. [Google Scholar] [CrossRef]
- Xiaoming, L.; Yi, L.; Xiangdong, X. Particle growth characteristics of metallic iron in direct coal-based reduction of nickel slag. Iron Steel 2020, 55, 104–109. [Google Scholar]
- Sun, Y.S.; Han, Y.X.; Gao, P.; Wang, Z.H.; Ren, D.Z. Recovery of iron from high phosphorus oolitic iron ore using coal-based reduction followed by magnetic separation. Int. J. Min. Met. Mater. 2013, 20, 411–419. [Google Scholar] [CrossRef]
- Ye, Q.; Peng, Z.; Li, G.; Lee, J.; Liu, Y.; Liu, M.; Wang, L.; Rao, M.; Zhang, Y.; Jiang, T. Microwave-assisted reduction of electric arc furnace dust with biochar: An examination of transition of heating mechanism. ACS Sustain. Chem. Eng. 2019, 7, 9515–9524. [Google Scholar] [CrossRef]
- Liyong, X. Kinetics Study on Decomposition and Reduction Behavior of In-Flight Fine Iron Ore Particles. Ph.D. Dissertation, Northeastern University, Shenyang, China, 2020. [Google Scholar]
- Mahedi, M.; Cetin, B. Carbonation based leaching assessment of recycled concrete aggregates. Chemosphere 2020, 250, 126307. [Google Scholar] [CrossRef] [PubMed]
- Zhaoming, L.; Jinping, F. Volatilization behavior and microstructure properties of Mg, Zn in AZ31 during vacuum melt. J. North Univ. China Nat. Sci. Ed. 2007, 05, 462–466. [Google Scholar]
- Zhiwei, M. Zinc Leach Residue Treated by Carbothermal Reduction and High Value Utilization of ZnO Smoke. Master’s Thesis, Lanzhou University of Technology, Lanzhou, China, 2020. [Google Scholar]
- Xixian, W. Activity calculation of each component in Al Si Fe ternary system. Chin. J. Nonferrous Met. 1999, 3, 627–630. [Google Scholar]
- Qirun, Y.; Bengen, G.; Yongchun, Z.; Junying, Z. Carbothermal reduction of Si-Al-Fe-Ca quaternary system in a high-silica coal. J. Fuel Chem. Technol. 2017, 45, 1296–1302. [Google Scholar]
- Habibi, H.; Piruzian, D.; Shakibania, S.; Pourkarimi, Z.; Mokmeli, M. The effect of carbothermal reduction on the physical and chemical separation of the red mud components. Miner. Eng. 2021, 173, 107–216. [Google Scholar] [CrossRef]
- Hou, X.; Shi, Y.; Wang, X.; Tang, Y.; Wu, M.; Zhan, H. Selective leaching of inert mineral product and the RO phase in steel slag with acetum to improve total Fe content. Materials 2022, 15, 1242. [Google Scholar] [CrossRef]
- GB 5058.3-2007; Identification Standards for Hazardous Wastes. Chinese Academy of Environmental Sciences: Beijing, China, 2007. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/gthw/wxfwjbffbz/200705/W020120104532752182600.pdf (accessed on 16 April 2025).
- Shuai, C. Study on the Preparation of Oily Sludge-Based Geopolymerand Solidification/Stabilization of Cd2+, Cu2+ and Cr3+. Master’s Thesis, Anhui University of Technology, Ma’anshan, China, 2023. [Google Scholar]
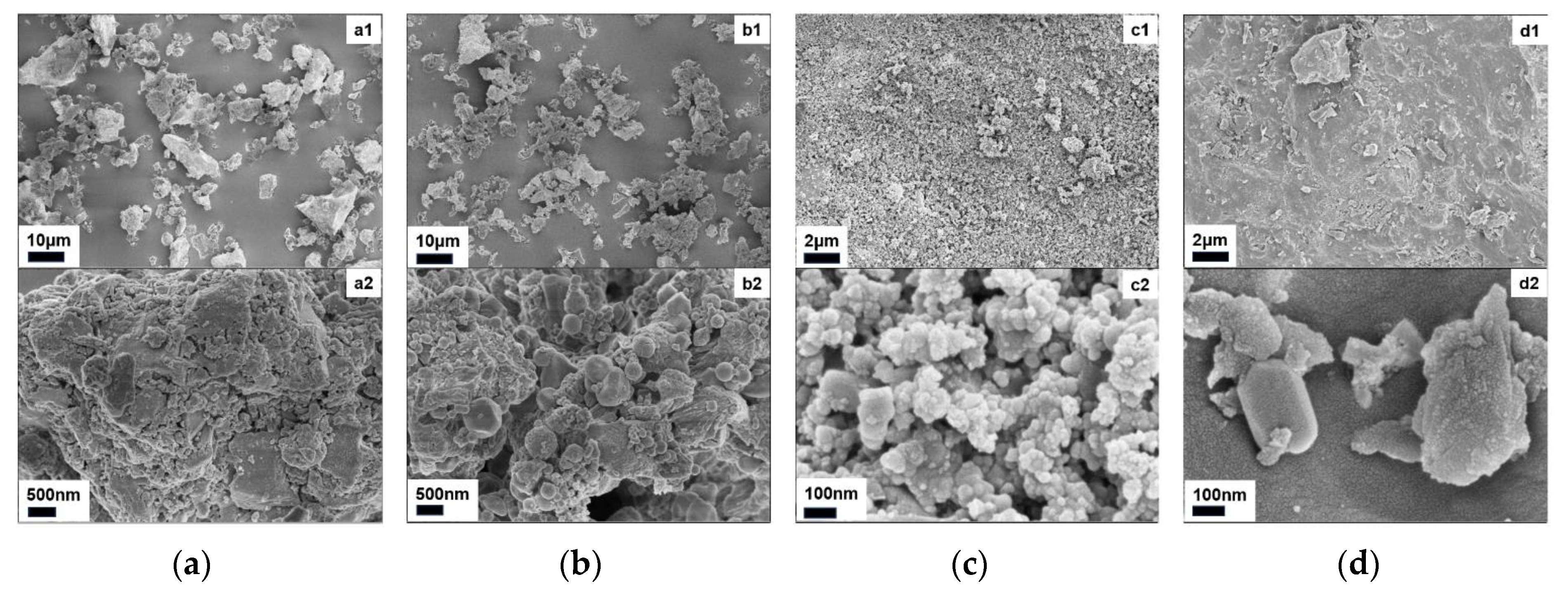


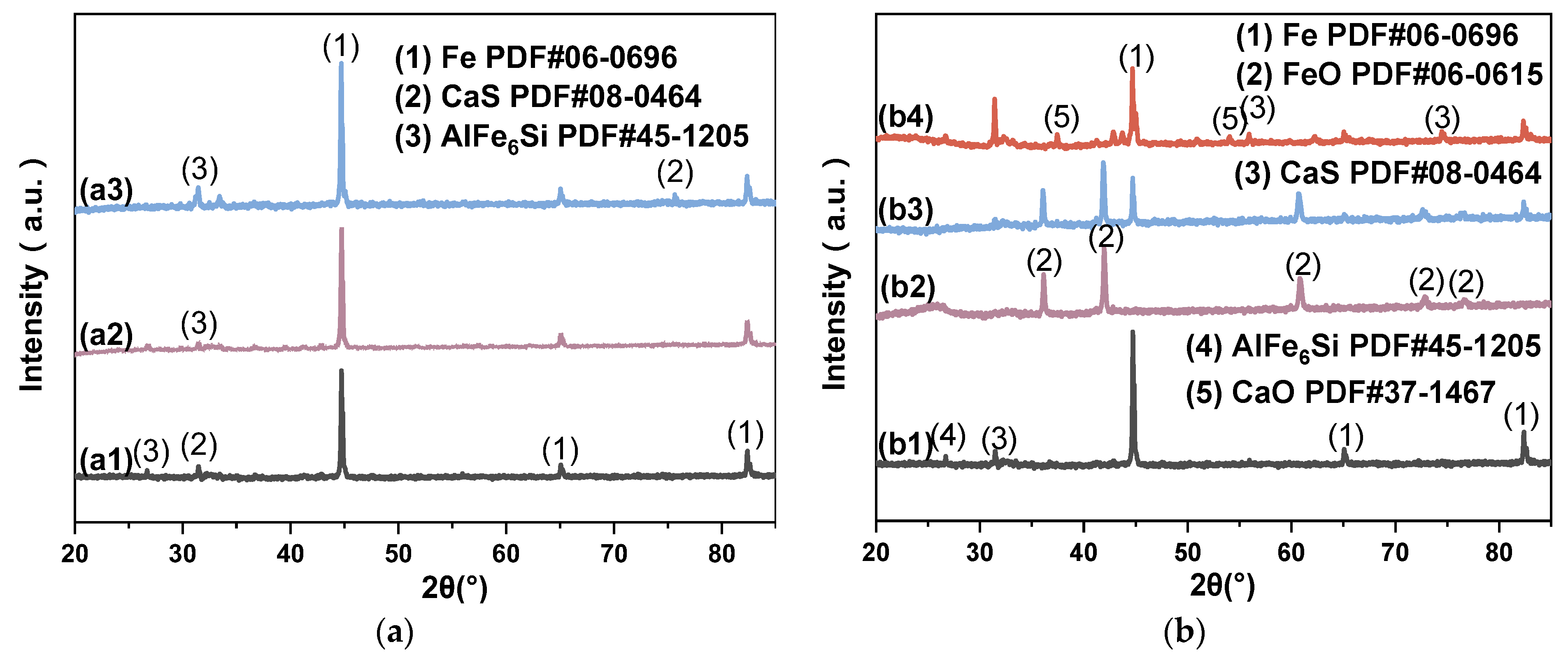

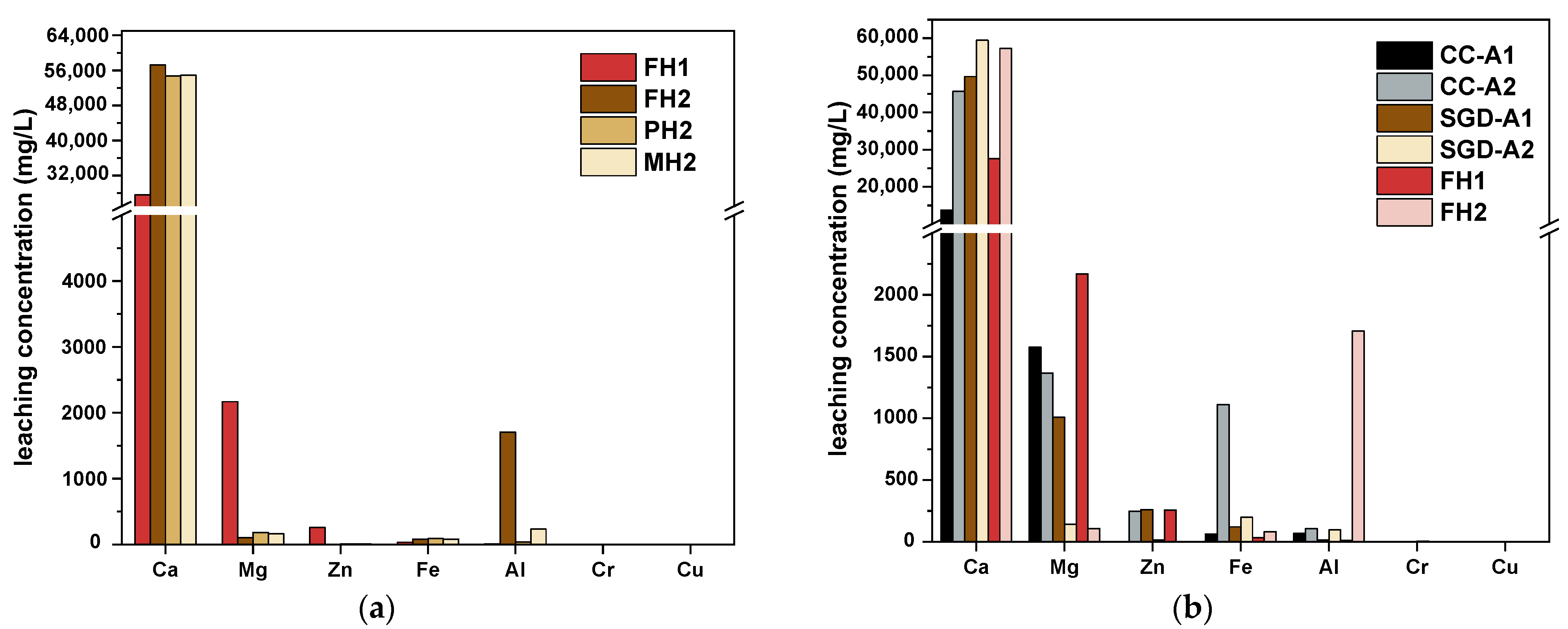
| Material | C | H | N | S |
|---|---|---|---|---|
| WT | 75.01 | 6.37 | 0.66 | 2.75 |
| WTC | 67.87 | 1.62 | 0.60 | 3.94 |
| CC | 88.53 | 1.12 | 0.88 | 0.84 |
| SMTD | 1.15 | 0.05 | 0.02 | 0.72 |
| SGD | 1.09 | 0.64 | 0.14 | 0.00 |
| Material | Ca | Al | Mg | Cu | Cr | Zn | O | Si |
|---|---|---|---|---|---|---|---|---|
| SMTD | 11.75 | 1.37 | 2.19 | - | - | 0.01 | 30.11 | 2.87 |
| SGD | 11.68 | 0.16 | 3.86 | - | - | 0.18 | 19.58 | 0.72 |
| Material | Cl | P | TFe | Fe2+ | MFe | |||
| SMTD | 0.14 | 0.08 | 49.32 | 2.27 | 0.34 | |||
| SGD | 0.12 | 0.09 | 61.74 | 27.88 | 30.91 |
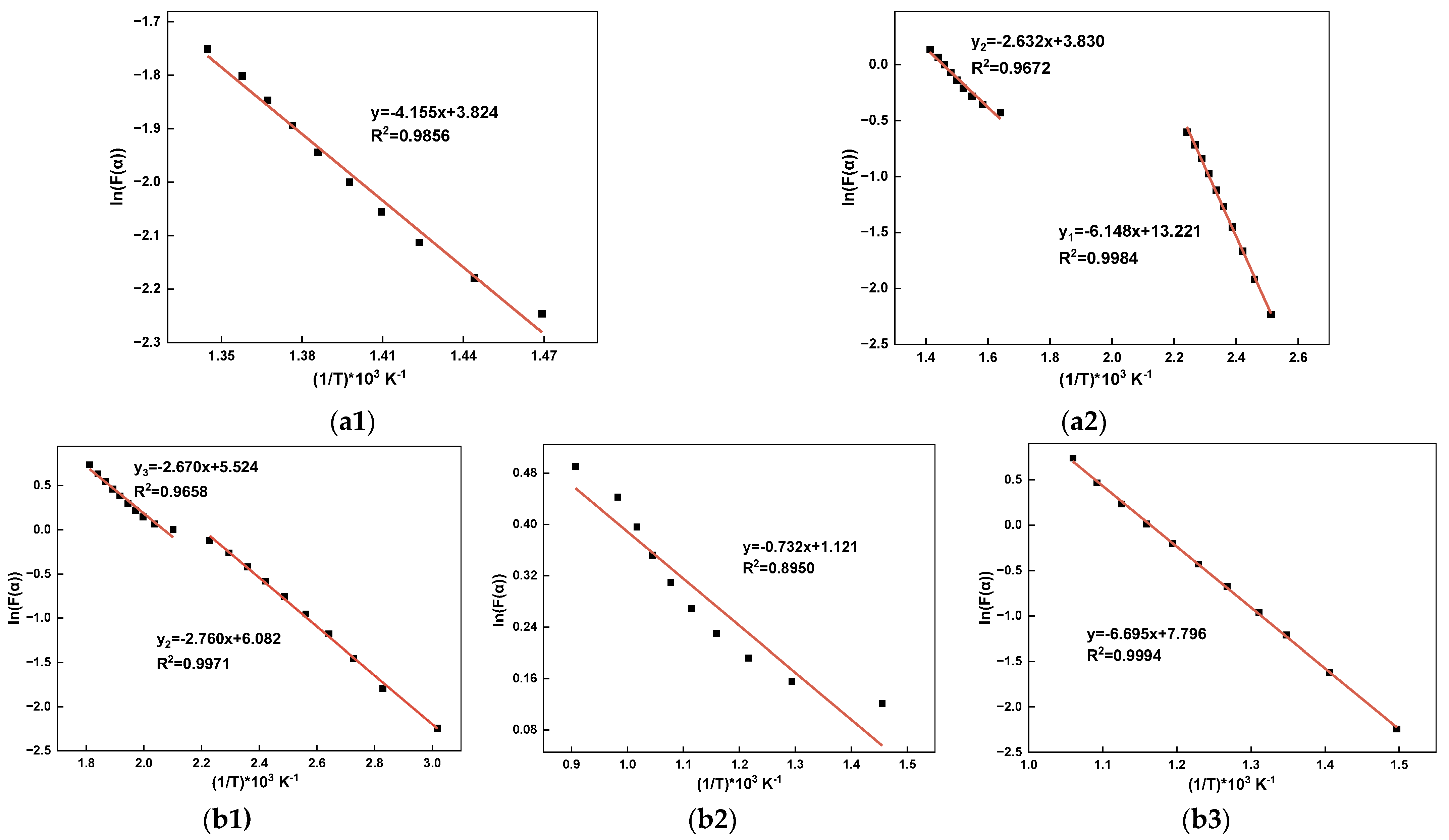
| Material | Stage | Fitted Curve | Ea/(kJ/mol) | A/(min−1) | R2 |
|---|---|---|---|---|---|
| SMTD | - | y = −4.155x + 3.824 | 32.84 | 23.96 | 0.9856 |
| SGD | 1 | y1 = −6.148x + 13.221 | 48.59 | 195,125.09 | 0.9984 |
| 2 | y2 = −2.632x + 3.830 | 20.80 | 38.05 | 0.9672 | |
| WT | 2 | y2 = −2.760x + 6.082 | 21.81 | 344.91 | 0.9971 |
| 3 | y3 = −2.670x + 5.524 | 21.10 | 204.07 | 0.9658 | |
| WTC | - | y = −0.732x + 1.121 | 5.79 | 9.11 | 0.8950 |
| CC | - | y = −6.695x + 7.796 | 52.91 | 789.31 | 0.9994 |
| Limiting Value | CC-A1 | CC-A2 | SGD-A1 | SGD-A2 | FH1 | FH2 | PH2 | MH2 | |
|---|---|---|---|---|---|---|---|---|---|
| Zn | 100 | 4.05 | 248.71 | 261.77 | 16.08 | 257.32 | 3.41 | 7.57 | 6.96 |
| Cr | 1 | 1.15 | 3.45 | 2.04 | 4.22 | 1.27 | 2.53 | 2.58 | 2.25 |
| Cu | 100 | 0.27 | 1.44 | 0.50 | 1.49 | 0.30 | 0.36 | 0.62 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, M.; Luan, C.; Lin, F. Application of Waste Tire Carbon for Iron-Containing Dust Reduction in Industrial Processes. Appl. Sci. 2025, 15, 6504. https://doi.org/10.3390/app15126504
Zeng M, Luan C, Lin F. Application of Waste Tire Carbon for Iron-Containing Dust Reduction in Industrial Processes. Applied Sciences. 2025; 15(12):6504. https://doi.org/10.3390/app15126504
Chicago/Turabian StyleZeng, Menglan, Chujun Luan, and Fawei Lin. 2025. "Application of Waste Tire Carbon for Iron-Containing Dust Reduction in Industrial Processes" Applied Sciences 15, no. 12: 6504. https://doi.org/10.3390/app15126504
APA StyleZeng, M., Luan, C., & Lin, F. (2025). Application of Waste Tire Carbon for Iron-Containing Dust Reduction in Industrial Processes. Applied Sciences, 15(12), 6504. https://doi.org/10.3390/app15126504






