Polyamine and Isoamylamine Levels in Peripheral Blood Cells and Plasma as Biomarkers of Structural and Functional Alterations in Acute Myocardial Infarction and Chronic Heart Failure Patients
Abstract
1. Introduction
2. Methods
2.1. Participants and Study Design
2.2. Separation of Cells and Plasma from Peripheral Blood
2.3. Determination of Polyamines via HPLC
2.4. Chemicals
2.5. Statistical Analysis
3. Results
3.1. Description of Participants
3.2. Clinical Data on the Patients
3.3. Correlation Between Polyamine Levels in Peripheral Blood Cells and Plasma
3.4. Discriminant Function Analysis of Participants in the Control Group and Patients with AMI and CHF Based on the Amine Levels Measured in Blood Cells and Plasma
3.5. Polyamine Levels in Peripheral Blood Cells and Plasma for Patients and the Control Group
3.6. Correlation Between Polyamines in Peripheral Blood Cells and Plasma and the Clinical and Analytical Parameters of the Patients
3.7. Lineal Regression Analysis of Clinical Parameters of Patients with AMI and CHF as Dependent Variables and Polyamines as Predictor Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schibalski, R.S.; Shulha, A.S.; Tsao, B.P.; Palygin, O.; Ilatovskaya, D.V. The role of polyamine metabolism in cellular function and physiology. Am. J. Physiol. Cell Physiol. 2024, 327, C341–C356. [Google Scholar] [CrossRef]
- Bordallo, C.; Cantabrana, B.; Velasco, L.; Secades, L.; Meana, C.; Méndez, M.; Bordallo, J.; Sánchez, M. Putrescine modulation of acute activation of the β-adrenergic system in the left atrium of rat. Eur. J. Pharmacol. 2008, 598, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Meana, C.; Bordallo, J.; Bordallo, C.; Suarez, L.; Cantabrana, B.; Sanchez, M. Functional effects of polyamines via activation of human beta1- and beta2-adrenoceptors stably expressed in CHO cells. Pharmacol. Rep. 2010, 62, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.; Secades, L.; Bordallo, C.; Bordallo, J.; de Boto, M.J.; Rubin, J.M.; Hidalgo, A.; Cantabrana, B.; Sanchez, M. Role of putrescine on androgen-elicited positive inotropism in the left atrium of rats. J. Cardiovasc. Pharmacol. 2008, 52, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Bordallo, C.; Rubin, J.M.; Varona, A.B.; Cantabrana, B.; Hidalgo, A.; Sanchez, M. Increases in ornithine decarboxylase activity in the positive inotropism induced by androgens in isolated left atrium of the rat. Eur. J. Pharmacol. 2001, 422, 101–107. [Google Scholar] [CrossRef]
- Sanchez, M.; Secades, L.; Bordallo, C.; Meana, C.; Rubin, J.M.; Cantabrana, B.; Bordallo, J. Role of polyamines and cAMP-dependent mechanisms on 5alpha-dihydrotestosterone-elicited functional effects in isolated right atria of rat. J. Cardiovasc. Pharmacol. 2009, 54, 310–318. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Xue, G.; Zhang, L.; Zhang, W.; Wang, L.; Lu, F.; Li, H.; Bai, S.; Lin, Y.; et al. Exercise training preserves ischemic preconditioning in aged rat hearts by restoring the myocardial polyamine pool. Oxid. Med. Cell Longev. 2014, 2014, 457429. [Google Scholar] [CrossRef]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef]
- Wei, C.; Li, H.; Wang, Y.; Peng, X.; Shao, H.; Li, H.; Bai, S.; Xu, C. Exogenous spermine inhibits hypoxia/ischemia-induced myocardial apoptosis via regulation of mitochondrial permeability transition pore and associated pathways. Exp. Biol. Med. 2016, 241, 1505–1515. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Shantz, L.M.; Feith, D.J.; Pegg, A.E. Targeted overexpression of ornithine decarboxylase enhances beta-adrenergic agonist-induced cardiac hypertrophy. Biochem. J. 2001, 358, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Flamigni, F.; Stefanelli, C.; Guarnieri, C.; Caldarera, C.M. Modulation of ornithine decarboxylase activity and ornithine decarboxylase-antizyme complex in rat heart by hormone and putrescine treatment. Biochim. Biophys. Acta 1986, 882, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Tipnis, U.R.; He, G.Y.; Li, S.; Campbell, G.; Boor, P.J. Attenuation of isoproterenol-mediated myocardial injury in rat by an inhibitor of polyamine synthesis. Cardiovasc. Pathol. 2000, 9, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.J.; Russell, D.H. Polyamine biogenesis in left ventricle of the rat heart after aortic constriction. Am. J. Physiol. 1972, 222, 1199–1203. [Google Scholar] [CrossRef][Green Version]
- Cubria, J.C.; Reguera, R.; Balana-Fouce, R.; Ordonez, C.; Ordonez, D. Polyamine-mediated heart hypertrophy induced by clenbuterol in the mouse. J. Pharm. Pharmacol. 1998, 50, 91–96. [Google Scholar] [CrossRef]
- Caldarera, C.M.; Casti, A.; Rossoni, C.; Visioli, O. Polyamines and noradrenaline following myocardial hypertrophy. J. Mol. Cell Cardiol. 1971, 3, 121–126. [Google Scholar] [CrossRef]
- Meana, C.; Rubin, J.M.; Bordallo, C.; Suarez, L.; Bordallo, J.; Sanchez, M. Correlation between endogenous polyamines in human cardiac tissues and clinical parameters in patients with heart failure. J. Cell Mol. Med. 2016, 20, 302–312. [Google Scholar] [CrossRef]
- Kim, J.; Baek, Y.; Lee, S. Dietary polyamine intake lowers the risk of all-cause and cardiovascular disease-related mortality: Follow-up of the Korean National health and nutrition Examination survey 2007–2015. J. Funct. Foods 2024, 119, 106268. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Chiba, F. Food polyamine and cardiovascular disease--an epidemiological study. Glob. J. Health Sci. 2012, 4, 170–178. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Harris, S.P.; Patel, J.R.; Marton, L.J.; Moss, R.L. Polyamines decrease Ca(2+) sensitivity of tension and increase rates of activation in skinned cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1383–H1391. [Google Scholar] [CrossRef] [PubMed]
- Chao de la Barca, J.M.; Bakhta, O.; Kalakech, H.; Simard, G.; Tamareille, S.; Catros, V.; Callebert, J.; Gadras, C.; Tessier, L.; Reynier, P.; et al. Metabolic Signature of Remote Ischemic Preconditioning Involving a Cocktail of Amino Acids and Biogenic Amines. J. Am. Heart Assoc. 2016, 5, e003891. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Liu, C.; Mehta, A.; Ko, Y.A.; Tahhan, A.S.; Dhindsa, D.S.; Uppal, K.; Jones, D.P.; Butler, J.; Morris, A.A.; et al. N8-Acetylspermidine: A Polyamine Biomarker in Ischemic Cardiomyopathy With Reduced Ejection Fraction. J. Am. Heart Assoc. 2020, 9, e016055. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Liu, L.; Yin, L.; Xu, X.; Xiong, J.; Zhao, J. Association of Serum Polyamines with Cardiovascular Events and All-Cause Mortality in Chronic Kidney Disease. Cardiorenal Med. 2025, 15, 238–248. [Google Scholar] [CrossRef]
- Lambertos, A.; Ramos-Molina, B.; Cerezo, D.; López-Contreras, A.J.; Peñafiel, R. The mouse Gm853 gene encodes a novel enzyme: Leucine decarboxylase. Biochim. Et Biophys. Acta Gen. Subj. 2018, 1862, 365–376. [Google Scholar] [CrossRef] [PubMed]
- ŞahutoĞlu, A.S. Comparative modelling of a novel enzyme: Mus musculus leucine decarboxylase. Turk. J. Chem. 2020, 44, 817–832. [Google Scholar] [CrossRef]
- Delbès-Paus, C.; Pochet, S.; Helinck, S.; Veisseire, P.; Bord, C.; Lebecque, A.; Coton, M.; Desmasures, N.; Coton, E.; Irlinger, F.; et al. Impact of Gram-negative bacteria in interaction with a complex microbial consortium on biogenic amine content and sensory characteristics of an uncooked pressed cheese. Food Microbiol. 2012, 30, 74–82. [Google Scholar] [CrossRef]
- Coton, M.; Delbés-Paus, C.; Irlinger, F.; Desmasures, N.; Le Fleche, A.; Stahl, V.; Montel, M.C.; Coton, E. Diversity and assessment of potential risk factors of Gram-negative isolates associated with French cheeses. Food Microbiol. 2012, 29, 88–98. [Google Scholar] [CrossRef]
- Sánchez, M.; Suárez, L.; Banda, G.; Barreiro-Alonso, E.; Rodríguez-Uña, I.; Rubín, J.M.; Cantabrana, B. Age-associated polyamines in peripheral blood cells and plasma in 20 to 70 years of age subjects. Amino Acids 2023, 55, 789–798. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Beghini, A.; Sammartino, A.M.; Papp, Z.; von Haehling, S.; Biegus, J.; Ponikowski, P.; Adamo, M.; Falco, L.; Lombardi, C.M.; Pagnesi, M.; et al. 2024 update in heart failure. ESC Heart Fail. 2025, 12, 8–42. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef] [PubMed]
- Escribano, M.I.; Legaz, M.E. High performance liquid chromatography of the dansyl derivatives of putrescine, spermidine, and spermine. Plant Physiol. 1988, 87, 519–522. [Google Scholar] [CrossRef]
- Saygin, D.; Wanner, N.; Rose, J.A.; Naga Prasad, S.V.; Tang, W.H.W.; Erzurum, S.; Asosingh, K. Relative quantification of beta-adrenergic receptor in peripheral blood cells using flow cytometry. Cytom. Part A J. Int. Soc. Anal. Cytol. 2018, 93, 563–570. [Google Scholar] [CrossRef]
- Bree, F.; Gault, I.; d’Athis, P.; Tillement, J.P. Beta adrenoceptors of human red blood cells, determination of their subtypes. Biochem. Pharmacol. 1984, 33, 4045–4050. [Google Scholar] [CrossRef]
- Horga, J.F.; Gisbert, J.; De Agustín, J.C.; Hernández, M.; Zapater, P. A beta-2-adrenergic receptor activates adenylate cyclase in human erythrocyte membranes at physiological calcium plasma concentrations. Blood Cells Mol. Dis. 2000, 26, 223–228. [Google Scholar] [CrossRef]
- Nagai, M.; Terao, S.; Vital, S.A.; Rodrigues, S.F.; Yilmaz, G.; Granger, D.N. Role of blood cell-associated angiotensin II type 1 receptors in the cerebral microvascular response to ischemic stroke during angiotensin-induced hypertension. Exp. Transl. Stroke Med. 2011, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Suzuki, H.; Maruyama, T.; Saruta, T. Gene expression of angiotensin II receptor in blood cells of Cushing’s syndrome. Hypertension 1995, 26, 1003–1010. [Google Scholar] [CrossRef]
- Tipnis, U.R.; Frasier-Scott, K.; Skiera, C. Isoprenaline induced changes in ornithine decarboxylase activity and polyamine content in regions of the rat heart. Cardiovasc. Res. 1989, 23, 611–619. [Google Scholar] [CrossRef]
- Shimizu, M.; Irimajiri, O.; Nakano, T.; Mizokami, T.; Ogawa, K.; Sanjo, J.; Yamada, H.; Sasaki, H.; Isogai, Y. Effect of captopril on isoproterenol-induced myocardial ornithine decarboxylase activity. J. Mol. Cell Cardiol. 1991, 23, 665–670. [Google Scholar] [CrossRef]
- White, D.C.; Hata, J.A.; Shah, A.S.; Glower, D.D.; Lefkowitz, R.J.; Koch, W.J. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc. Natl. Acad. Sci. USA 2000, 97, 5428–5433. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Yang, W.; Jiang, D.; Tao, K.; Dong, A.; Cheng, H. Spermine ameliorates ischemia/reperfusion injury in cardiomyocytes via regulation of autophagy. Am. J. Transl. Res. 2016, 8, 3976–3985. [Google Scholar] [PubMed]
- Hu, J.; Lu, X.; Zhang, X.; Shao, X.; Wang, Y.; Chen, J.; Zhao, B.; Li, S.; Xu, C.; Wei, C. Exogenous spermine attenuates myocardial fibrosis in diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress and the canonical Wnt signaling pathway. Cell Biol. Int. 2020, 44, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Li, L.; Chai, N.; Chen, Y.; Wu, F.; Zhang, W.; Wang, L.; Shi, S.; Zhang, L.; et al. Spermine and spermidine reversed age-related cardiac deterioration in rats. Oncotarget 2017, 8, 64793–64808. [Google Scholar] [CrossRef]
- Wei, C.; Sun, M.; Liang, X.; Che, B.; Wang, N.; Shi, L.; Fan, Y. Spermine Regulates Immune and Signal Transduction Dysfunction in Diabetic Cardiomyopathy. Front. Endocrinol. 2021, 12, 740493. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, K.; Chen, S.; Su, Q.; Zhang, W.; Zeng, L.; Lin, X.; Peng, F.; Lin, J.; Chai, D. High serum lactate dehydrogenase as a predictor of cardiac insufficiency at follow-up in elderly patients with acute myocardial infarction. Arch. Gerontol. Geriatr. 2024, 117, 105253. [Google Scholar] [CrossRef]
- Soda, K.; Uemura, T.; Sanayama, H.; Igarashi, K.; Fukui, T. Polyamine-Rich Diet Elevates Blood Spermine Levels and Inhibits Pro-Inflammatory Status: An Interventional Study. Med. Sci. 2021, 9, 22. [Google Scholar] [CrossRef]
- Senekowitsch, S.; Wietkamp, E.; Grimm, M.; Schmelter, F.; Schick, P.; Kordowski, A.; Sina, C.; Otzen, H.; Weitschies, W.; Smollich, M. High-Dose Spermidine Supplementation Does Not Increase Spermidine Levels in Blood Plasma and Saliva of Healthy Adults: A Randomized Placebo-Controlled Pharmacokinetic and Metabolomic Study. Nutrients 2023, 15, 1852. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef]
- Liberles, S.D.; Buck, L.B. A second class of chemosensory receptors in the olfactory epithelium. Nature 2006, 442, 645–650. [Google Scholar] [CrossRef]
- Moiseenko, V.I.; Apryatina, V.A.; Gainetdinov, R.R.; Apryatin, S.A. Trace Amine-Associated Receptors’ Role in Immune System Functions. Biomedicines 2024, 12, 893. [Google Scholar] [CrossRef] [PubMed]

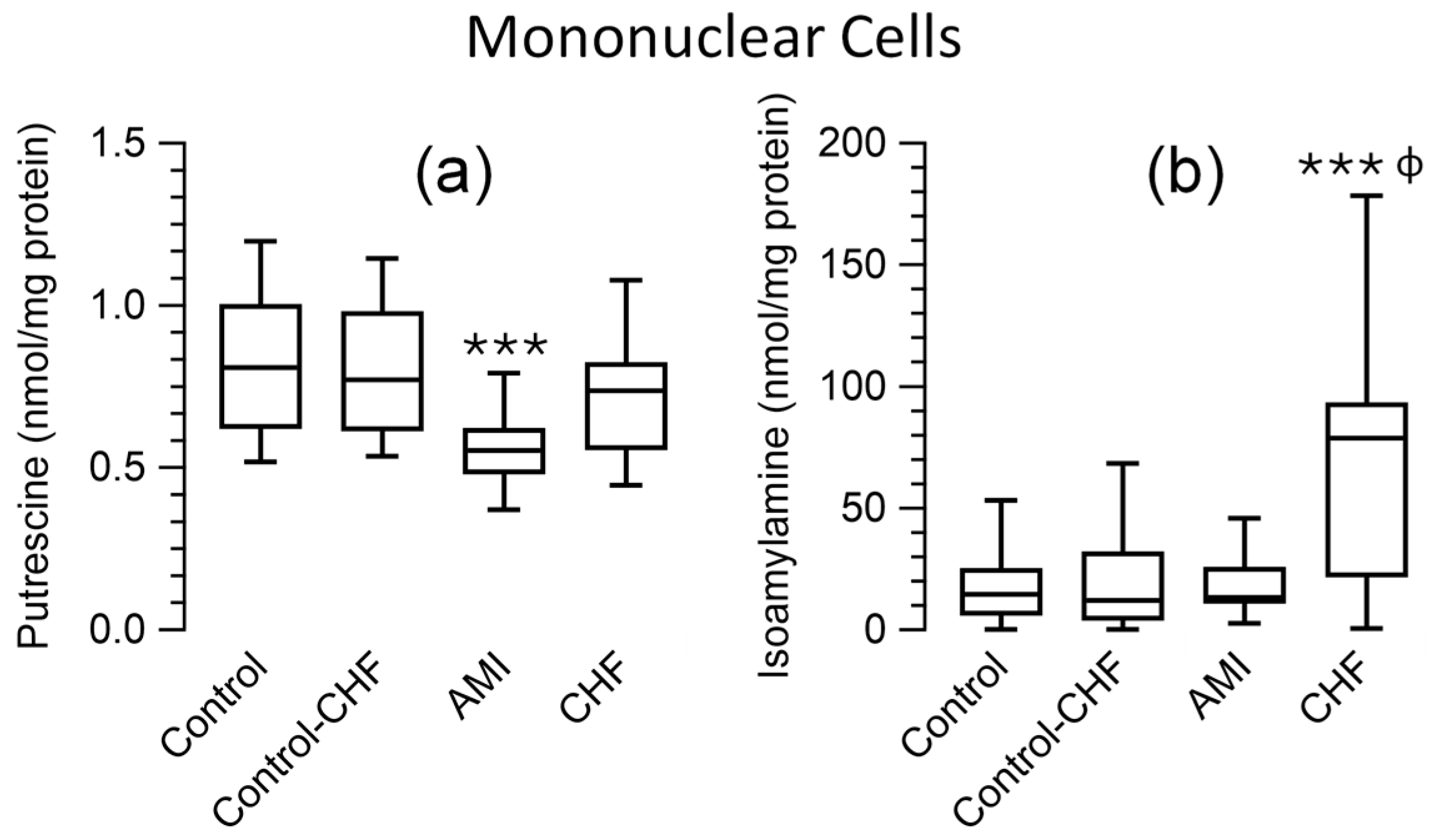
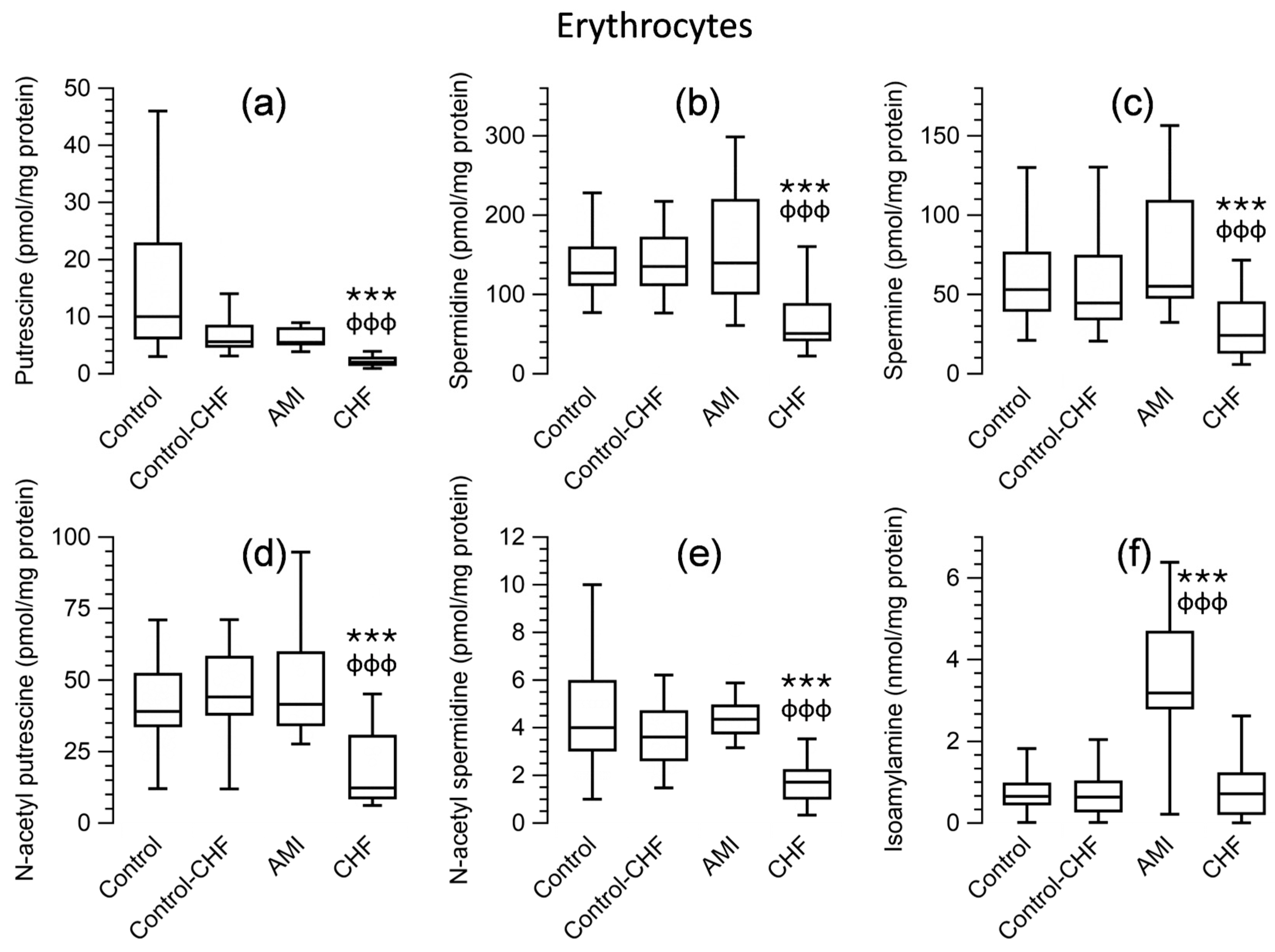
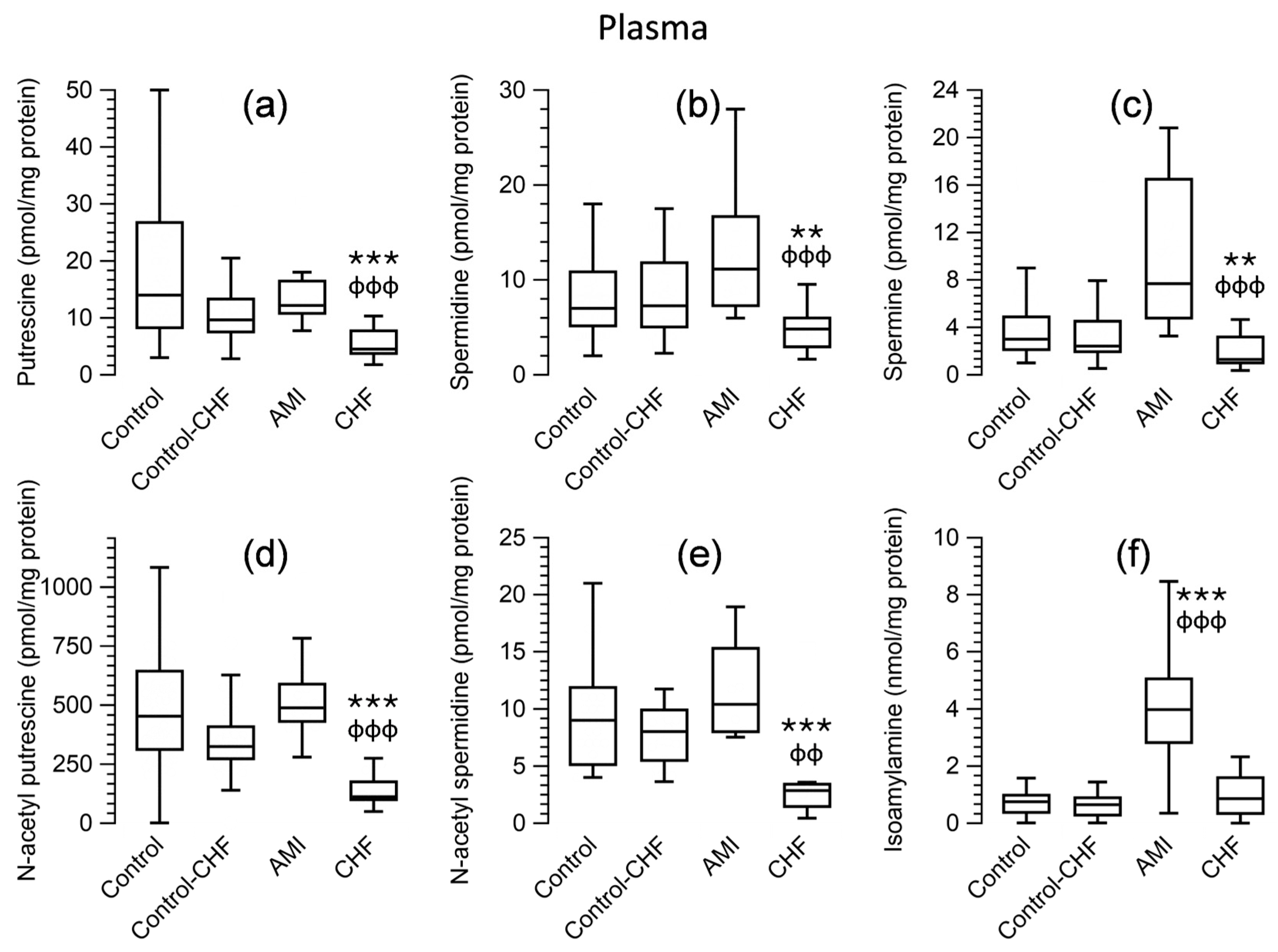
| Control | Control (≥58 years) | AMI | CHF | |
|---|---|---|---|---|
| Study participants (n) | 86 | 46 | 25 | 18 |
| Gender (male/female) | 44/42 | 20/26 | 20/5 | 10/8 |
| Age (median [percentile 25–75]) | 59 (47–65.3) | 63.5 (61–69.4) | 58 (49–71.5) | 69.5 (63.7–77.2) *** |
| NSTEMI/STEMI (n) | 5/15 | |||
| HFrEF/HFmrEF/HFpEF (%) | 16.7/4.2/79.2 | 88.9/5.6/5.6 | ||
| Heart rate (beats per minute) | 70 (65–80) | 85 (84.5–91) | ||
| Sistolic blood pressure (mm Hg) | 120 (100–128.75) | 130 (125–137.5) | ||
| Diastolic blood pressure (mm Hg) | 70 (60–70) | 75 (70–77.5) | ||
| LVEF (%) | 53.5 (50–60) | 29 (23.75–32.25) | ||
| Left atria size (mm) | 38.85 (34.7–41.6) | 45 (42–50) | ||
| Septum (mm) | 11.5 (10–13) | |||
| NT-proBNP (pg/mL) | 2049.5 (1094.75–3203.25) | |||
| Creatine Kinase (U/L) | 840 (352.5–1752.75) | |||
| Troponin (ng/mL) | 1903.5 (537.25–5240) | |||
| LDH (UI/L) | 400 (155–593) | |||
| Aspartate AT (U/L) | 149 (64–244) | |||
| Alanine AT (UI/L) | 45.5 (29–75.25) | |||
| Aspartate AT/Alanine AT | 2.61 (1.67–3.78) | |||
| eGFR (mL/min/1.73 m2) | 75 (57.5–80) | |||
| Glucose (mg/dL) | 106 (96–127) | |||
| Urea (mg/dL) | 33.5 (27.5–46.25) | |||
| Creatinine (mg/dL) | 0.82 (0.74–0.98) | |||
| Sodium (mEq/L) | 139 (136–141) | |||
| Potassium (mEq/L) | 4 (3.8–4.4) |
| Spermidine | Spermine | N-Acetyl Putrescine | N-Acetyl Spermidine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mononuclear Cells | Control | AMI | CHF | Control | AMI | CHF | Control | AMI | CHF | Control | AMI | CHF |
| Putrescine | 0.558 *** | 0.425 * | 0.926 *** | 0.467 *** | 0.3 *** | 0.467 * | 0.589 *** | |||||
| Spermidine | 0.887 *** | 0.638 *** | 0.529 *** | 0.445 *** | ||||||||
| Spermine | 0.414 *** | 0.394 *** | ||||||||||
| Erythrocytes | ||||||||||||
| Putrescine | 0.747 *** | 0.829 *** | 0.450 *** | 0.853 *** | 0.228 *** | 0.708 *** | 0.655 *** | 0.304 *** | 0.589 ** | 0.851 *** | ||
| Spermidine | 0.254 *** | 0.696 *** | 0.798 *** | 0.447 * | 0.558 ** | 0.345 ** | 0.815 *** | |||||
| Spermine | 0.68 *** | 0.846 *** | ||||||||||
| Plasma | ||||||||||||
| Putrescine | 0.445 * | 0.437 *** | 0.81 *** | 0.663 *** | 0.833 ** | 0.761 *** | ||||||
| Spermidine | 0.672 *** | 0.727 *** | ||||||||||
| Predictor Variables of LVEF | B | SE B | β | p |
|---|---|---|---|---|
| Acute Myocardial Infarction Patients (R2: 0.666; ANOVA p < 0.001) | ||||
| Constant | 43.26 | 5.04 | <0.001 | |
| Putrescine in Erythrocytes (nmol/mg protein) | −701.03 | 140.71 | −0.70 | <0.001 |
| Putrescine in Mononuclear Cells (nmol/mg protein) | 27.54 | 8.03 | 0.48 | 0.003 |
| Chronic Heart Failure Patients (R2: 0.86; ANOVA p = 0.013) | ||||
| Constant | −66.13 | 18.32 | 0.007 | |
| Isoamylamine in Mononuclear Cells (nmol/mg protein) | −0.15 | 0.03 | −1.55 | 0.002 |
| Putrescine in Mononuclear Cells (nmol/mg protein) | −55.75 | 16.85 | −1.63 | 0.011 |
| Spermidine in Mononuclear Cells (nmol/mg protein) | 25.36 | 6.01 | 2.88 | 0.003 |
| Putrescine in Erythrocytes (nmol/mg protein) | 11,841.35 | 4311.38 | 1.63 | 0.025 |
| Spermine in Erythrocytes (nmol/mg protein) | −1952.00 | 508.61 | −3.57 | 0.005 |
| Isoamylamine in Plasma (nmol/mg protein) | 12.21 | 3.55 | 1.29 | 0.009 |
| Spermidine in Plasma (nmol/mg protein) | 4650.32 | 932.87 | 1.27 | 0.001 |
| Spermine-to-Spermidine Ratio in Plasma | 71.69 | 22.20 | 0.97 | 0.012 |
| Spermine-to-Spermidine Ratio in Erythrocytes | 19.98 | 5.88 | 0.67 | 0.009 |
| Predictor variables of Heart Rate | ||||
| Chronic Heart Failure Patients (R2: 0.512; ANOVA p = 0.006) | ||||
| Constant | 79.33 | 2.56 | <0.001 | |
| Spermidine in Plasma (nmol/mg protein) | 1511.67 | 444.93 | 0.72 | 0.006 |
| Predictor variables of Septum Size | ||||
| Chronic Heart Failure Patients (Adjusted R2: 0.55; ANOVA p = 0.003) | ||||
| Constant | 11.41 | 0.6 | <0.001 | |
| Isoamylamine in Erythrocytes (nmol/mg protein) | 1.64 | 0.38 | 0.88 | <0.001 |
| Putrescine in Plasma (nmol/mg protein) | −4.69 | 2.05 | −0.47 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubín, J.M.; Suárez, L.; Cantabrana, B.; Barreiro-Alonso, E.; Rodríguez-Uña, I.; Sánchez, M. Polyamine and Isoamylamine Levels in Peripheral Blood Cells and Plasma as Biomarkers of Structural and Functional Alterations in Acute Myocardial Infarction and Chronic Heart Failure Patients. Appl. Sci. 2025, 15, 6456. https://doi.org/10.3390/app15126456
Rubín JM, Suárez L, Cantabrana B, Barreiro-Alonso E, Rodríguez-Uña I, Sánchez M. Polyamine and Isoamylamine Levels in Peripheral Blood Cells and Plasma as Biomarkers of Structural and Functional Alterations in Acute Myocardial Infarction and Chronic Heart Failure Patients. Applied Sciences. 2025; 15(12):6456. https://doi.org/10.3390/app15126456
Chicago/Turabian StyleRubín, José Manuel, Lorena Suárez, Begoña Cantabrana, Eva Barreiro-Alonso, Ignacio Rodríguez-Uña, and Manuel Sánchez. 2025. "Polyamine and Isoamylamine Levels in Peripheral Blood Cells and Plasma as Biomarkers of Structural and Functional Alterations in Acute Myocardial Infarction and Chronic Heart Failure Patients" Applied Sciences 15, no. 12: 6456. https://doi.org/10.3390/app15126456
APA StyleRubín, J. M., Suárez, L., Cantabrana, B., Barreiro-Alonso, E., Rodríguez-Uña, I., & Sánchez, M. (2025). Polyamine and Isoamylamine Levels in Peripheral Blood Cells and Plasma as Biomarkers of Structural and Functional Alterations in Acute Myocardial Infarction and Chronic Heart Failure Patients. Applied Sciences, 15(12), 6456. https://doi.org/10.3390/app15126456






