Abstract
This study evaluates various strains of soil bacterial for use in the development of new biopreparations. Mesophilic spore-forming bacteria were isolated from cultivated soil and analysed for their enzymatic activity, ability to decompose crop residues, and antagonistic properties towards selected phytopathogens. Notably, this is the first cytotoxicity assessment of soil bacterial metabolites on Spodoptera frugiperda Sf-9 (fall armyworm). Bacillus subtilis, Bacillus licheniformis, Bacillus velezensis, Paenibacillus amylolyticus, and Prestia megaterium demonstrated the highest hydrolytic potential for the degradation of post-harvest residues from maize, winter barley, and triticale. They exhibited antimicrobial activity against at least three of the tested phytopathogens and demonstrated the ability to solubilize phosphorus. Metabolites of B. licheniformis (IC50 = 8.3 mg/mL) and B. subtilis (IC50 = 144.9 mg/mL) were the most cytotoxic against Sf-9. We recommend the use of the tested strains in industrial practice as biocontrol agents, plant growth biostimulants, crop residue decomposition stimulants, and bioinsecticides. Future studies should focus on assessing the efficacy of using these strains under conditions simulating the target use, such as plant microcosms and greenhouses and the impact of these strains on the abundance and biodiversity of native soil microbiota. This research can serve as a model procedure for screening other strains of bacteria for agricultural purposes.
1. Introduction
Biopreparations are becoming increasingly important, due to the growing demand for food and the simultaneous generation of increasing amounts of agrifood waste. The strategic policy of the European Union (the European Green Deal including the “farm to fork” strategy) is to guarantee food security while achieving climate neutrality. In accordance with these regulations, it is planned to radically reduce the use of pesticides and artificial fertilizers and to significantly increase the area of organic farms in the coming years [1,2,3]. Soil cultivation methods are also being shifted from arable practices to no-till farming, with the aim of preserving soil biodiversity. Therefore, both scientists and farmers are looking for effective and environmentally friendly products to increase agricultural productivity, in particular new biopreparations for decomposing plant waste biomass.
Biopreparations are increasingly used in agriculture and horticulture products, due to their ecological safety, wide range of possible applications, and compliance with legal guidelines [4,5]. Biopreparations are products derived from living organisms or their metabolites [6]. They include fungal, bacterial, bacterial/fungal-enzymatic, bacterio-fungal, and enzymatic preparations [7]. They include microorganisms such as Bacillus thuringiensis, Verticillium lecanii, Metarhizium anisopliae, and Trichoderma viride, as well as plant extracts, including neem, citronella oils, and water extract of garlic [4,8,9,10].
Biopreparations can be used as biopesticides, biofertilizers, biostimulants, and biodegradation stimulating agents [4,6,7,8,10]. Biopesticides are natural substances used to control pests and diseases in plants. They can contain beneficial microorganisms that help control plant pathogens. Examples include fungi like Trichoderma and bacteria such as Bacillus sp., which can inhibit the growth of harmful fungi like Botrytis cinerea, plant extracts that have antimicrobial properties, and can help in controlling plant diseases or substances like chitosan, which enhance plant resistance to pathogens and improve overall plant health [6]. Biofertilizers include Azospirillum Azotobacter, Rhizobium, and Pseudomonas fluorescens [11,12,13], as well as various plant extracts (fruits, leaves, and microalgal seaweeds) [14,15]. They can improve soil quality and support plant growth through the synthesis of growth regulators, biocontrol of phytopathogens, and induction of resistance to plant stress conditions. They also improve the absorption of elements that are difficult to access. This is due to their ability to dissolve phosphorus, potassium, and zinc [16,17]. Biofertilizers are one of the most promising solutions for maintaining or increasing current food production rates while at the same time ensuring environmental stability. Biostimulants have the ability to stimulate plant growth. The most popular biostimulants are humic substances, which are produced as a result of the chemical and biological decomposition of organic matter. Biostimulants include Trichoderma sp., algae extract, and chitosan protein hydrolysates [18,19,20,21]. Decomposition accelerators are designed to intensify the decomposition of organic matter contained in agricultural waste, such as crop residues (roots, stems, leaves, seed pods), which consist mainly of cellulose, hemicellulose, and lignin [22,23,24,25]. The decomposition of such type of waste involves a series of physical, chemical, and biological processes, during which nutrients are released and made available to plants [25,26]. During microbial degradation, the enzymatic activity of the strain and the production of extracellular hydrolases are important factors [22,23]. Bacteria including Bacillus sp., Paenibacillus sp., as well as fungi have been shown to be the main decomposers in soil ecosystems, with bacteria being responsible for the initial decomposition of organic matter and fungi playing a key role in later stages of decomposition [27].
The aim of the current work was to evaluate various strains of soil bacterial for use in the development of new biopreparations for agriculture. Spore-forming bacteria were selected for this study, due to their low sensitivity to environmental conditions and ability to withstand prolonged storage in the form of spores compared to vegetative bacteria, making them suitable for industrial applications [28]. Mesophilic spore-forming bacteria were isolated from cultivated soil, and their optimal growth conditions were determined. We conducted quantitative and qualitative analyses of their enzymatic activity, evaluated their potential to decompose crop residues, and examined their antagonistic properties towards selected phytopathogens. The results allowed us to select the most biotechnologically useful strains and to identify their potential agricultural applications. The literature presents the benefits of biopreparations and the positive characteristics of microorganisms. However, there are not many studies that present methods of obtaining and screening strains in order to construct new biopreparations [5,6,7]. This research can serve as a model procedure for screening other strains of bacteria for agricultural purposes.
2. Materials and Methods
The bacteria were isolated from soil samples after maize cultivation. After preliminary identification, the biotechnological potential of the bacteria was assessed. The research process is presented in Figure 1.
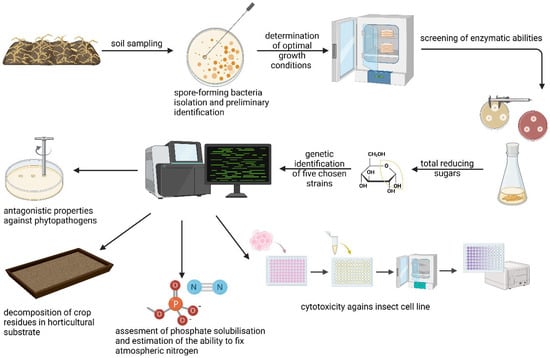
Figure 1.
Research process overview.
2.1. Soil and Harvest Residue Samples
Soil samples were collected from 18 plots on a farm located in central Poland (Lodz voivodeship), where six different maize crops were cultivated for grain and silage. The soil samples are described in Table S1 (Supplementary Materials). All samples were collected in the autumn (October 2022) season. The samples were tested for pH (pHmeter FiveEasy Plus FP20, Mettler Toledo, Zurych, Switzerland), elemental composition (elemental analyser, NE 2500, CE Instruments, Wigan, UK), and number of spore-forming mesophilic bacteria (using the culture method described previously by Szulc et al., 2024 [29]).
Crop residues of maize, winter barley, and triticale grown on the studied fields were provided by the farmer. The crop residues were characterized in terms of dry matter and dry organic matter (Table S2) and subsequently used in submerged cultures to study enzyme activity.
2.2. Isolation and Identification of the Tested Strain
Based on their macroscopic features, the spore-forming bacteria were isolated from plates with TSA medium (tryptic soy agar, Merck-Millipore, Warsaw, Poland) and spread on pure cultures. Next, bacterial identification was performed using API 50 CH and API 20 E tests (BioMérieux, Marcy-l’Étoile, France), as well as MALDI-TOF MS (Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry). For microbial identification, the direct smear method with formic acid on-plate extraction was used in combination with the MALDI-TOF MS technique, as described previously by Liszkowska et al., 2023 [30]. Direct smear method with formic acid on-plate extraction was applied for microbial identification with MALDI-TOF MS technique. A single colony of each isolate was placed onto a dedicated steel target plate using sterile inoculation loop. Then, 0.5 μL 25% formic acid (MERCK, Darmstadt, Germany) was combined with bacterial biomass, mixed, and left until almost dry. Next, 1 μL of saturated α-cyano-4-hydroxycinnamic acid (α-CHCA; MERCK, Germany) matrix solution (40 mg/mL in acetonitrile:ethanol:water 1:1:1 (v/v) with 3% trifluoroacetic acid (TFA; MERCK, Germany)) was added, stirred, and left to air-dry for 30 min. The dried samples were analyzed with an AXIMA-iD Plus Confidence MALDI-TOF mass spectrometer (Kratos Analytical Ltd., Manchester, UK and Shimadzu Corporation, Kyoto, Japan). The spectra were collected using Launchpad 2.9 software (Kratos Analytical Ltd. and Shimadzu Corporation, Japan) with the linear positive ion mode. The mass-to-charge (m/z) ratio range was 2000 to 20,000 Da, with 50 Hz laser frequency and 90% laser power. For each sample a mass spectrum of 200 profiles (five laser shots per profile) were acquired. The overnight cells of strain E. coli DH5α (TAKARA BIO Inc., Kusatsu, Japan) cultivated on LB agar at 30 °C were used as an identification control. For comparative analysis of obtained and reference spectra the SARAMIS Premium 4.11 software (Spectral Archive and Microbial Identification System; bioMérieux, Craponne, France) was utilized. Results were expressed as confidence scores values [%]. Scores ≥70% were considered sufficient for identification. Genetic identification based on the nucleotide sequence of gene 16S rRNA was performed on the strains with the greatest enzymatic activity, according to the method described in Krakova et al. (2017) [31]. All strains were deposited in the collection of the Department of Environmental Biotechnology, Lodz University of Technology (Łodź, Poland).
2.3. Determination of Optimal Growth Conditions
To determine optimal growth conditions, the tested strains were first activated on solid Lysogeny Broth (LB) at 30 °C until colony growth was macroscopically noticeable (24–48 h). Next, the obtained single colonies were streaked on solid LB, TSA, and nutrient broth agar (NA) media plates and incubated at 10 °C, 20 °C, and 30 °C. Bacterial growth on the agar plates was monitored every 24 h for 7 days. The compositions of the media are presented in Table S3.
2.4. Screening of Enzymatic Activities
Screening of hydrolytic activity using the plate method was performed according to the method described by Gerlicz et al. (2024) [32]. Briefly, 2 μL of bacterial inocula (OD660 = 1) was spotted in triplicate on agar screening media containing 1:4 (v/v; milk:medium) skimmed milk for proteolytic activity, 2% (w/v) starch for amylolytic activity, 2% (w/v) carboxymethylcellulose (CMC) for cellulolytic activity, 1% (w/v) apple pectin for pectinolytic activity, 2% (w/v) xylan for xylanolytic activity, or 0.1% (w/v) of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) for ligninolytic activity. The plates were incubated at the optimal growth temperature for 5 days. Then, the plates were flooded and incubated for 15 min (with staining solutions, if needed) to observe clear zones around the bacterial colonies. The compositions of the solid screening media and staining solutions are presented in Table S3. The clear or coloured zones around the microbial colonies were then measured, and the Enzymatic Activity Index (EAI) was calculated using the formula:
where:
- —average diameter of clear/coloured zones around microbial colonies [mm].
- —average diameter of microbial colonies [mm].
2.5. Establishment of Post-Culture Filtrate
Submerged cultivation of the selected 10 strains was performed in 250 mL flasks with 50 mL of minimal mineral medium (0.5 g/L NaCl, 0.5 g/L NH4Cl, 0.3 g/L K2HPO4, 0.4 g/L KH2PO4, 0.1 g/L MgSO4, 0.1 g/L yeast extract) supplemented with 2% (w/v) agricultural waste residues. The sterile media were inoculated with 1 mL of sterile MiliQ-water-washed bacterial cell-suspension of OD660 = 1 (cells harvested from LB medium activation culture) and incubated at 20 °C or at 30 °C for 5 days on a rotatory shaker (INFORS HT, Bottmingen, Switzerland) at 140 rpm. The post-culture liquids were separated from the non-degraded residues by vacuum filtration using a Büchnel funnel and 70-mm Munktell filter paper (Chem-Land, Stargard, Poland). The filtrates were stored at −20 °C prior to further analyse protein content, the concentration of reducing sugars, and enzymatic activities. The non-degraded residues were air-dried for at least 24 h. The negative control was uninoculated, sterile medium with each crop residue incubated under the same cultivation conditions.
2.6. Hydrolytic Activities in Submerged Cultures
For amylolytic, xylanolytic, cellulolytic, and pectinolytic activity assays, 200 µL of buffer (0.1 M acetate of pH 5.5 or 0.1M phosphate buffer pH 7.0) and 200 µL of 0.5% (w/v) substrate solution (soluble starch, birchwood xylan, carboxymethylcellulose, or apple pectin) were combined with 25 µL of the post-culture filtrate (or buffer for the negative control), then incubated for 30 min at 30 °C and 300 rpm. Subsequently, 650 µL of 1% DNS solution was added to each sample. The samples were then incubated for 5 min at 95 °C, cooled on ice, and centrifuged (5 min, 10,000 rpm). Finally, 300 µL of the obtained supernatant (in triplicate) was pipetted into microplate wells. The absorbance was measured at 540 nm. The enzymatic activity (U/mL) in the post-culture filtrate was determined as the release of 1 µmol of reducing sugars from the substrate per minute and calculated using standard curves for D-glucose (Chempur, Katowice, Poland) prepared under the same conditions. To determine if the enzymatic activities were substrate-dependent, the ANOVA test was performed in R programming (ver 4.4.2).
2.7. Total Reducing Sugars
The post-culture filtrates were centrifuged (10 min; 3500 rpm). Then, 100 μL of each supernatant was mixed with 100 μL of 1% 3,5-dinitro-2-hydroxybenzoic acid (DNS; Sigma-Aldrich, St. Louis, MI, USA) solution (30% sodium potassium tartrate, 1.6% NaOH) (Chempur, Poland) and incubated for 5 min at 95 °C in triplicate. Next, 800 μL of distilled water was added to the sample and thoroughly mixed. After mixing, 300 µL of the sample was transferred to three wells of a microplate. The absorbance was measured at 540 nm. The amounts of total reducing sugars [mg/g] released from the tested crop residues were calculated using the standard curve for D-glucose (Chempur, Poland) under the same conditions, using the formula
where:
- —absorbance of sample [-];
- —absorbance of control [-];
- a—standard curve slope index [-];
- V—volume of the liquid culture medium [mL], 50 mL;
- m—mass of the crop residue [g], 1 g.
The negative control was the filtrate from uninoculated, post-culture medium with each crop residue incubated under the same cultivation conditions.
2.8. Decomposition of Crop Residues in Horticultural Substrates
On the basis of their enzymatic activity, five strains were tested for their ability to decompose crop residues. For this purpose, unsterilized dried maize harvest residue was cut into 3-cm lengths. Then, 17 g of the residue was weighed and placed in plastic cuvettes (32 × 23 × 7 cm) with a volume of 1 L. Suspensions of the tested strains (100 mL/cuvette) were applied to the crop residues.
The substrates with crop residues in cuvettes were moistened in distilled water to 70–80% water content (measured using a Moisture Meter HH2, Cambridge, UK) and incubated in the dark in a BINDER KB 720 E.6 AS IS incubator, at a constant temperature of 20 °C (±1°C) and 80 ± 5% relative air humidity (RH). The humidity of the substrates was kept constant by adding appropriate amounts of distilled water when necessary. The quality and decomposition dynamics of the harvest residues were assessed on the basis of weekly visual assessments on a five-point scale (5—undecomposed crop residues, 1—fully decomposed crop residues). The experiment was performed in triplicate for each variant.
2.9. Antagonistic Properties Against Phytopathogens
The antagonistic properties of five selected bacteria strains were tested against the following phytopathogens: Alternaria tenuissima DSM 63360, Rhizoctonia solani DSM 22843, Colletotrichum coccodes DSM 62126, Phoma exigua DSM 62040 (German Collection of Microorganisms and Cell Cultures GmbH, DSMZ, Braunschweig, Germany); Alternaria solani Z184 (Plant Breeding and Acclimatization Institute (IHAR), National Research Institute, Radzików, Poland); Fusarium avenaceum IOR 1571; Fusarium proliferatum IOR 2377; Fusarium graminearum IOR 1540 (Institute Of Plant Protection, National Research Institute, Poznań, Poland). The tests were conducted using the agar well diffusion method [33] with three independent repetitions.
2.10. Assessment of Phosphate Solubilization
The ability of selected bacteria to solubilize phosphates was assessed on Pikovskaya medium (pH 6.8–7.0). The composition of the medium (in g/L) was as follows: glucose—10.0; Ca3(PO4)2—5.0; (NH4)2SO4—0.5; NaCl—0.2; MgSO4 × 2H2O—0.1; KCl—0.2; yeast extract—0.5; MnSO4—0.001; FeSO4—0.001; agar—20.0 [34]. The medium was inoculated pointwise and incubated for 7 days at 30 °C. The experiment was carried out in triplicate. After the incubation period, clear zones around the bacterial colonies indicated the ability of the bacteria to solubilize phosphates [35].
2.11. Ability to Fix Atmospheric Nitrogen
The ability to fix atmospheric nitrogen was tested on a mineral medium without a nitrogen source (pH 6.8–7.0). The composition of the medium (in g/L) was as follows: glucose—10.0; MgSO4 × 7H2O—5.0; KH2PO4—1.0; agar—20.0. The medium was inoculated pointwise and incubated for 7 days at 30 °C. The experiment was carried out in triplicate. After incubation, the appearance of the colony indicated the ability of the strain to fix nitrogen from the air.
2.12. Cytotoxicity Against Insect Cell Line—Cell Culture and 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
The cytotoxicity of the post-culture Tryptic soy broth (TSB) medium filtrates from the five bacterial strains with the highest enzymatic activity was evaluated. A normal cell line of ovarian pupa from the insect Spodoptera frugiperda Sf-9 (fall armyworm) was used. The Sf-9 cell line was selected for this research because it is the most established insect cell model for the study of agricultural cytotoxicity. The cell line was cultured as described in our previous studies [36]. The cytotoxicity of the post-culture filtrates was determined after 24 h of exposure to Sf-9 cells using the MTT assay. Test samples were added to each well (in four replicates) in the following final concentrations (mg/mL): 1.6, 3.13, 6.25, 12.5, 25, 50, 100, and 200. For the negative control, cells were incubated in culture medium only. The MTT test and IC50 calculations were conducted according to the methods described in our previous studies [37].
3. Results
3.1. Isolation and Identification of Soil Isolates
The bacteria were isolated from soil under a no-till farming system, where all crop residues were left in the field in the form of mulch after harvesting the main crop. The pH of the soil samples was slightly acidic or acidic (pH 3.9–5.7). The samples varied in elemental composition and contained 28.15–57.68% carbon, 0.09–0.30% nitrogen, 0.02–0.14% phosphorus, 4.56–8.55% hydrogen, and 0.02–0.69% sulphur. The average carbon to nitrogen (C:N) ratio was 259. The number of spore-forming bacteria ranged from 106 to 107 CFU/g (Table S1), there were not any statistically important connections between bacterial count and soil constitution. Based on morphological differences between the colonies on agar plates, 24 isolates were selected for identification and further study based on their macroscopic features: growth intensity, colony size, colony edges, color and pigment production, consistency, and texture. The spore-forming bacteria were isolated from plates with TSA medium (tryptic soy agar, Merck-Millipore, Poland) and spread on pure cultures.
Different approaches were used to identify the bacteria isolated from soil. After basic diagnostic activities, API biochemical tests and the MALDI TOF-MS procedure were performed. However, these did not always give consistent or acceptable results. Therefore, 16S rDNA gene sequencing was performed on the strains with the greatest biotechnological potential.
The vast majority of the isolated bacteria belonged to the genus Bacillus (Table 1). Bacteria of the genera Lysinibacillus, Paenibacillus, and Prestia were also identified. These bacteria constitute a large heterogeneous group within the phylum Firmicutes and order Bacillales.

Table 1.
Taxonomic affiliations of the tested soil bacteria.
The strains WK-16 and WK-21, identified as strains of the food-borne pathogen B. cereus, were excluded from further studies. However, we also recognize that until the whole-genome sequencing of given B. cereus isolates of interest and bioinformatical analysis for the toxin-encoding genes (nheABC, hblCDA, entFM, cytK) presence will be performed, one cannot be sure if the isolated B. cereus representative is in fact a pathogenic strain. For that reason, we excluded the potentially dangerous strains from further study.
3.2. Biochemical Characterization of Soil Bacteria
3.2.1. Determination of Optimal Growth Conditions
The optimal growth conditions for selected isolated strains (n = 22) were characterized based on macroscopic observation of the growth plates with three different solid media (LB, TSA, and NA) incubated at three temperatures (10 °C, 20 °C, and 30 °C). The optimal growth temperature (OGT) varied between strains. The OGT for 19 of the 22 strains was 30 °C. However, WK-3, WK-9, and WK-17 showed an OGT of 20 °C. None of the tested bacterial isolates exhibited an OGT of 10 °C (Table S4). The OGTs for each strain were applied in the next step of the research.
3.2.2. Preliminary Assessment of the Enzymatic Activity of Soil Bacteria in Plate Assays
The 22 selected strains were examined for the production of extracellular hydrolytic enzymes, using the on-plate method with commercial substrates (Figure 2).
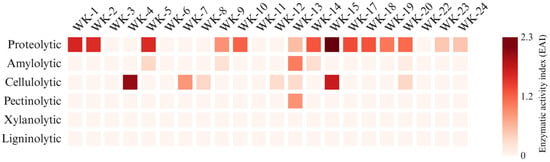
Figure 2.
On-plate hydrolytic activities (as enzymatic activity index, EAI) of WK strains after 7 days at respective optimal growth temperatures (Table S4). None of the isolates showed xylanolytic or ligninolytic activities.
Most of the studied strains (63.6%) exhibited proteolytic activity for the hydrolysis of casein. Approximately 32% were able to degrade carboxymethyl cellulose (CMC). Few strains showed the ability to degrade starch (22.7%). Only one strain, WK-13, showed pectinolytic activity (Figure S1). None of the isolates showed xylanolytic or ligninolytic activity under the screening conditions.
On-plate biochemical tests revealed that some of the strains possess rich enzymatic potential that might be useful in the biodegradation of plant residues. Particularly interesting in this regard are the strains WK-13, which showed the ability to hydrolyse casein, starch, CMC, and pectin, and WK-20, which is able to hydrolyse casein, starch, and CMC. The 10 strains with the highest and most diverse activities desirable for the degradation of crop residues were selected for further studies aimed at assessing the biotechnological potential of the bacteria (WK-4, WK-5, WK-7, WK-8, WK-9, WK-12, WK-13, WK-14, WK-15, and WK-20).
3.2.3. Extracellular Enzyme Activities of Isolated Bacteria Strains in Submerged Cultures
To assess the biodegradation potential of the 10 selected isolates that exhibited the highest and most diverse range of enzymatic activities in on-plate biochemical assays, submerged cultures were established in media containing maize, winter barley, and triticale residues as the primary carbon sources. After a 5-day incubation period, the supernatants were analysed for cellulolytic, amylolytic, pectinolytic, and xylanolytic activities. Given the pH-dependent nature of enzyme activity, assays were conducted at pH 5.5 and 7.0 (Figure 3). We have selected a model acidic pH of 5.5 and neutral pH 7.0 as a standard in comparative studies.
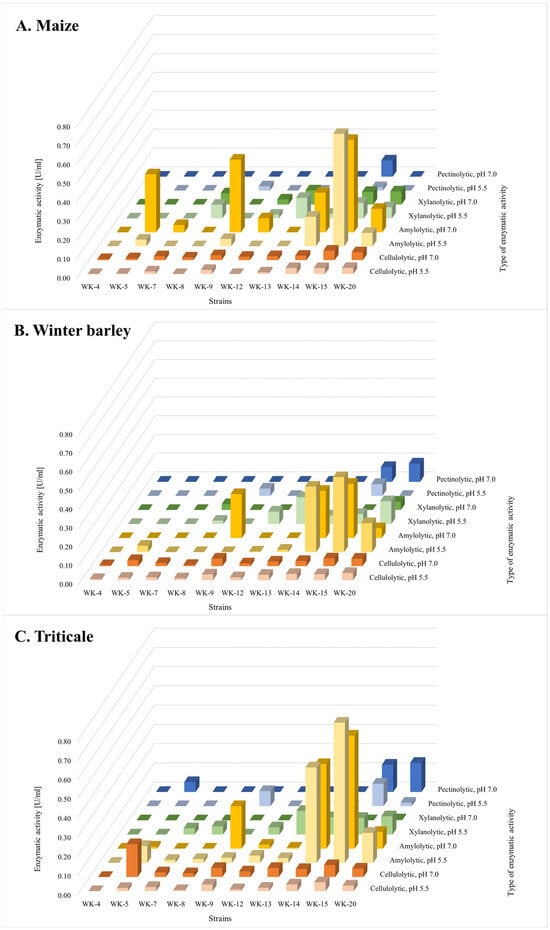
Figure 3.
Enzymatic activities in post-culture filtrates of selected WK strains after 5-day cultivation with (A) maize, (B) winter barley, and (C) triticale.
The experimental results indicate that strains WK-5, WK-9, WK-14, WK-15, and WK-20 have the highest hydrolytic potential for the degradation of all three types of post-harvest residues. These strains exhibited the most significant amylolytic, cellulolytic, and xylanolytic activities in their supernatants. Interestingly, despite exhibiting broad-spectrum hydrolytic activities in on-plate tests, strain WK-13 showed relatively low potential for the degradation of post-harvest residues in submerged cultures in comparison to strains WK-14 and WK-15.
Statistical analysis of the results revealed that the hydrolytic activities of most of the tested strains, if exhibited, were significantly substrate-dependent (p-value < 0.05). Differences were found to be dominant for isolate WK-5 with cellulolytic (pH 5.5; p-value = 0.023; pH 7.0 p-value = 3.33 × 10−7) and amylolytic (pH 5.5; p-value = 1.32 × 10−4; pH 7.0 p-value = 1.7 × 10−5) activities, as well as WK-14 and WK-15 for all measured activities (p-value from 6.18 × 10−7 to 0.043) indicating statistically significant difference between samples. Exceptions were observed in the case of cellulolytic activity for WK-14 in pH 5.5 (p-value = 0.244) and WK-15 in pH 7.0 (p-value = 0.089). Interestingly, the cellulolytic activity of isolate WK-20 did not seem to depend on the type of post-harvest residue used as a primary source of carbon (pH 5.5, p-value = 0.087; pH 7.0, p-value = 0.666), despite other activities varying significantly (p-value from 4.59 × 10−5 to 5.83 × 10−3). This effect may be due to the specific biodegradation potential of Bacillaceae strains [38,39]. It could also be related to the complex structures of the natural maize, winter barley, and triticale residues used in this study, which have diverse carbohydrate compositions and availabilities, inducing various enzymatic responses [40,41,42].
3.2.4. Total Sugar Produced by Soil Bacteria in Batch Cultures
Total reducing sugars (TRS) (Figure 4) were determined in post-culture liquids after 5-day submerged cultivation with maize, winter barley, or triticale residues.
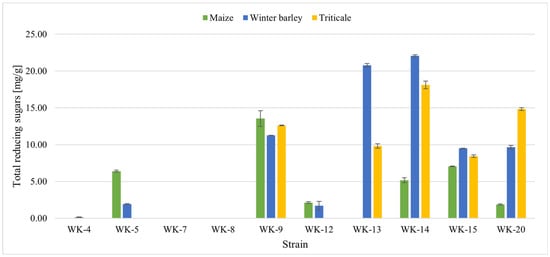
Figure 4.
Concentrations of total reducing sugars in post-culture filtrates of WK strains cultivated with various crop residues for 5 days.
The highest amounts of TRS released from the polysaccharide structure of the post-harvest residues after 5 days of incubation were observed for strains WK-9, WK-13, WK-14, WK-15, and WK-20. However, only four strains (WK-9, WK-14, WK-15, and WK-20) increased TRS for all three types of crop residues, indicating synthesis of more efficient or more versatile enzymes or enzyme combinations. Reducing sugars were not detected in any samples inoculated with either WK-7 or WK-8. The absence of TRS in those liquids could be a result of the low enzymatic activity of those isolates, when cultivated in the presented conditions (results in Figure 3). The low enzymatic activity of those bacteria (in comparison to other strains) and therefore low content of TRS in post-culture liquids could be due to the non-optimal conditions for WK-7 and WK-8 cultivation chosen for this study (composition minimal mineral medium, temperature, or time of the incubation) or due to the lack of enzymes able to catalyze degradation of complex substrates, such as maize, triticale and winter barely residues.
The five most promising strains for the biodegradation of post-harvest residues were selected for further research: P. megaterium WK-5, P. amylolyticus WK-9, B. velezensis WK-14, B. subtilis WK-15, and B. licheniformis WK-20.
Among them, B. subtilis and B. licheniformis are producers of enzymes with the GRAS (Generally Recognized as Safe) status granted by the FDA (Food and Drug Administration) [43]. In addition, the strain belonging to B. velezensis has been assessed by the EFSA (European Food Safety Authority—EFSA) as safe for use as a feed additive for pigs and has been granted the QPS (Qualified Presumption of Safety) status [44]. Paenibacillus amylolyticus and Prestia megaterium do not have GRAS or QPS status, but we have found that they do not pose a risk to human health under Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work [45]. These strains are not listed in groups 2–4 of bacteria for workers’ health hazards and therefore are unlikely to cause adverse health effects on humans under the terms of the Directive.
3.3. Decomposition of Crop Residues
Application of the tested microorganisms to crop residues mixed with non-sterilized horticultural substrate resulted in significantly accelerated decomposition compared to residues treated with water. However, the activity of a mixture of the microorganisms was not significantly different from the activity of the individual microorganisms. This indicates the beneficial effect of all tested microorganisms on accelerating the decomposition of crop residues, with almost complete decomposition within 81 days in a substrate containing 80% water at a temperature of 20 °C. Decomposition in the control sample was less advanced (Figure 5).
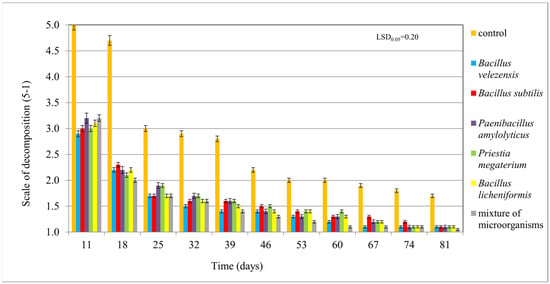
Figure 5.
Decomposition of maize crop residues at 20 °C in non-sterilized horticultural substrate moistened with distilled water (control), with the addition individual microorganisms and a mixture of microorganisms, on a five-point quality scale (5—non-decomposed residues, 1—completely decomposed residues). NIR was calculated at a significance level of p = 0.05.
3.4. Antagonistic Properties Against Phytopathogens
The antimicrobial activities of the five selected strains (B. subtilis, B. licheniformis, B. velezensis, P. amylolyticus, and P. megaterium) were evaluated against phytopathogens. All the strains exhibited antimicrobial activity against at least three of the tested phytopathogens (Figure 6).
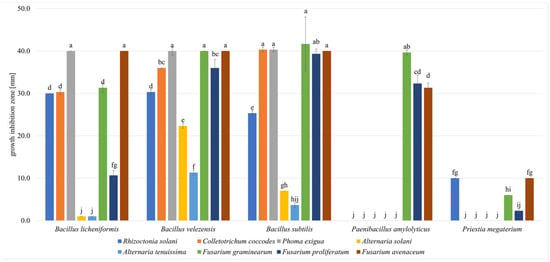
Figure 6.
Antimicrobial activity of bacteria strains against phytopathogens measured using the agar-plug diffusion method as growth inhibition zones. a–j—results with different letters within the sample are significantly different (Tukey’s test, α = 0.05).
The strains B. licheniformis, B. velexensis, and B. subtilis exhibited antimicrobial activity against all the tested phytopathogens. B. velezensis and B. subtilis exhibited the largest zones of inhibition. B. velezensis inhibited P. exigua with a mean zone of 40.0 ± 1.0 mm, as well as F. graminearum and F. avenaceum with a mean zone of 40.0 ± 0.0 mm. B. subtilis inhibited F. graminearum with a mean zone of 41.7 ± 6.0 mm, as well as C. coccodes and P. exigua with a mean zone of 40.3 ± 0.6 mm. P. amylolyticus showed antimicrobial activity (inhibition zones ≥ 30 mm) against the maize-related pathogens F. graminearum, F. proliferatum, and F. avenaceum, but did not exhibit antimicrobial activity against the other phytopathogens. P. megaterium exhibited the lowest level of antimicrobial activity. Although it showed activity against four of the tested phytopathogens, the growth inhibition zones were ≤10 mm.
3.5. Phosphate Solubilization and Ability to Fix Atmospheric Nitrogen
Phosphate solubilization was assessed on Pikovskaya medium. The tested strains demonstrated the ability to solubilize phosphorus (Table 2).

Table 2.
Assimilation of atmospheric nitrogen and solubilization of phosphorus.
Atmospheric nitrogen fixation was estimated on mineral medium without a nitrogen source. All tested strains except B. licheniformis exhibited growth on the nitrogen-free medium, suggesting that they may be capable of fixing nitrogen from the air (Table 2).
3.6. Cytotoxic Activity of Cell Culture Supernatants (Metabolites) of Soil Bacteria Against Insect Cell Line Sf-9
Metabolites of soil bacteria including P. megaterium, P. amylolyticus, and B. velezensis did not show any cytotoxicity in the tested range of concentrations. B. subtilis metabolites also showed no cytotoxicity up to a concentration of 100 mg/mL. However, above this concentration their cytotoxicity increased rapidly and at the highest tested concentration (200 mg/mL) reached 95.66 ± 0.36%. The strongest cytotoxicity was shown by the B. licheniformis metabolites, with a great increase in cytotoxicity observed already at a concentration of 3.13 mg/mL, reaching 90.96–99.23% in the concentration range 25–200 mg/mL (Figure 7).
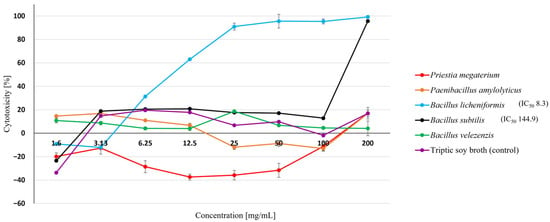
Figure 7.
Cytotoxicity of soil bacteria metabolites after 24 h exposure to insect Sf-9 cells. Each data point represents the mean absorbance value from four replicates. Results are presented as mean ± SD. IC50 (mg/mL), unless no IC50 was obtained.
The IC50 values could only be calculated for the most cytotoxic metabolites of B. licheniformis (8.3 mg/mL) and the less cytotoxic B. subtilis (144.9 mg/mL) (Figure 7). It was not possible to calculate the IC50 values or metabolites of the other soil bacteria, due to the lack of cytotoxicity.
4. Discussion
It is important to note that in Bacillales, phenotypic characters may vary within and between species, often leading to inconsistent species identification [46]. Standard MALDI-TOF MS is faster, but reliable differentiation within the B. cereus group is difficult using commercial databases [47], especially for closely related environmental isolates with metabolic profile variations in comparison to collection strains. This technique can be significantly improved with development and application of novel, genus, or group specific MALDI-TOF MS identification protocols or dedicated databases, containing reference mass spectra of well-characterized representatives of targeted microorganisms isolated from various environments. Species distinction by 16S ribosomal DNA gene sequencing also does not guarantee correct taxonomic assignment [48]. Alternative diagnostic approaches include specific PCR targeting toxins, virulence plasmids, and chromosomal markers. However, these also do not always provide completely satisfactory results [49,50]. Identification of Bacillales bacteria is therefore difficult and should be performed using different approaches to obtain the most reliable results.
Bacteria from the B. cereus group are commonly found in soil and food and are often an etiological factor causing opportunistic food poisoning [50]. Therefore, the isolates from this group (WK-16 and WK-21) should not be used in biopreparations for industrial applications.
Most of the tested bacterial strains preferred TSA medium and a growth temperature of 30 °C. However, some of the isolated soil bacteria (characterized by an OGT of 20 °C and the ability to grow at 10 °C) may possess adaptations to survive at lower temperatures. Many bacteria isolated from soil samples exhibit resistance to temperature fluctuation, which is regarded as one of the key properties for survival in seasonally changing and highly competitive environments. Such microorganisms are known to play an important role in reducing cold stress in plants, including crops, by improving the bioavailability of nutrients and minerals, reducing osmotic stress, and regulating phytohormones [51,52].
During the screening of enzymatic activity, most of the tested strains exhibited proteolytic, cellulolytic, and amylolytic activity. However, no xylanolytic or ligninolytic activity was observed. The strains WK-14 and WK-15 exhibited relatively high cellulolytic and amylolytic activities in all types of submerged cultures. Strain WK-15 also exhibited high xylanolytic and pectinolytic activity, but interestingly did not exhibit high amylolytic activity when grown on solid screening medium with starch or pectinolytic potential when grown with pectin. Similarly, no clear zones were observed around WK-14 colonies cultured on CMC- and starch-supplemented solid media during on-plate assays. This suggests that submerged cultivation with complex crop residues may support excretion of hydrolytic enzymes that were not detected by on-plate screening conducted with high-purity grade reagents.
On the other hand, the broad hydrolytic potential exhibited during on-plate tests by strain WK-13 was not confirmed by the results of enzymatic activity assays in post-culture liquids. This may be linked to various factors, such as the media composition (e.g., carbon and nitrogen sources), different oxygenation levels in each cultivation method, and the presence of other biotic or abiotic inhibitors.
During the saccharification of lignocellulosic substrates (such as wheat, triticale, and winter barley residues) by microorganisms and their various enzymes, complex carbohydrates are broken down to oligo- and monosaccharides [53]. Interestingly, none of the samples of post-harvest residues inoculated with strains WK-7 and WK-8 exhibited the release of total reducing sugars (TRS). This might be due to the low levels of enzyme secretion, low or inhibited enzyme activity, or the uptake of already released sugars for cell metabolism. Oligo- and monosaccharides, especially glucose, play crucial roles in cell development and growth, acting as energy carriers, gene expression regulators, building blocks for cell components, and moieties for the synthesis of crucial metabolites [54].
The soil bacteria Bacillus sp. P3 has been found to have biodegradation potential against several different types of lignocellulosic biomass. The maximal reducing sugar release was noticed after 20 days of incubation (55.5 mg/g), with a slight decrease between days 5 (50.2 mg/g) and 10 (41.2 mg/g). This indicates fluctuation of TRS content, probably due to microbial metabolism [55]. Strains of B. velezensis UPF-B1 and UPF-B2 have been reported to biodegrade triticale and sugarcane bagasse. The highest-reducing sugar concentrations were reached after 5 days (120 h), with around 18 mg/mL, for both strains grown on mixed substrate at pH 5.5 and pH 7.0 [41]. The marine strains Bacillus safensis NIORKK2 (cellulase) and Bacillus sp. NIORKK7 (xylanase) have been shown to have biodegradation potential against various biomasses, including rice bran, wheat bran, rice, wheat hay, and maize cobs, as well as mango and bamboo leaves. The maximal reducing sugar concentrations ranged from 56.94 mg/g to 206.21 mg/g for cellulolytic enzymes and 53.91 mg/g to 178.87 mg/g for xylanolytic enzymes with untreated substrates [38]. Prior pretreatment of the substrates led to increases in the release of reducing sugars. Applying a cocktail of both enzymes resulted in further increases in saccharification effectiveness, suggesting that cellulolytic and xylanolytic enzymes have a synergistic effect on lignocellulosic biomass degradation [38]. The amounts of TRS released from the substrates after 5-day incubation differed significantly. The highest concentrations of TRS were observed for strain WK-14 with winter barley (22.07 mg/mL) and triticale (18.10 mg/mL). However, mulching is considered the only feasible agrotechnical pretreatment method for in situ biodegradation of post-harvest residues. On-field conditions are also far from optimal for strain growth and biodegradation, due to fluctuating conditions of temperature, oxygenation, and humidity, as well as the presence of natural competitors.
Enzymes play a crucial role in the decomposition of plant residues [23,56]. The addition of the tested microorganisms is expected to enhance the microbial decomposition of residues. Other researchers have demonstrated the ability of certain microbial strains to decompose various crop residues. For instance, Avdeeva et al. (2016) found that B. subtilis IMB B-7516 and B. licheniformis IMB B-7515 strains exhibited the ability to decompose a range of crop residues, achieving a decomposition rate of 40–60% under laboratory conditions [57]. In a recent study by Chhetri et al. (2022) [58], the use of multi-enzyme producing Bacillus sp. was shown to be an effective and eco-friendly method for the biodegradation of organic waste, with synergistic effects. This approach has potential benefits for commercial enzyme production and biotechnological applications. The synergistic effects of strains may also explain why the mixture of microorganisms used in this study resulted in the fastest decomposition of residues.
The availability of phosphorus, a crucial nutrient for plant growth and development, is often limited by its occurrence in insoluble forms in soil. Phosphate-solubilizing microorganisms (PSMs) have been shown to convert these insoluble phosphates into plant-available forms, enhancing plant growth and reducing dependence on inorganic fertilizers [59,60]. Various PSMs, including bacteria and fungi, have been identified as being capable of effectively solubilizing phosphorus [61,62,63].
Extensive studies have investigated the ability of various Bacillus sp. strains to solubilize phosphorus under laboratory conditions, revealing significant potential for agricultural applications. For instance, B. subtilis has shown promising results in solubilizing inorganic phosphorus. In a study involving Bacillus strains isolated from San Luis, Argentina, B. subtilis SL-7 demonstrated a high solubilization index on Pikovskaya’s agar, outperforming the recognized plant growth-promoting bacterium B. velezensis FZB42 [63]. Additionally, B. velezensis strain Ag75 has been identified as a potent phosphate solubilizer, significantly increasing biomass and shoot phosphorus content in maize, and enhancing yields in both maize and soybean by 17.8% and 26.5%, respectively, in field experiments [64]. P. megaterium has also been highlighted for its phosphorus solubilization capabilities, particularly through the production of organic acids such as succinic acid, which is strongly correlated with increased soluble phosphorus concentration [65]. This species has been shown to be effective at solubilizing phosphorus from various sources, including poultry bones and fish bones [66,67,68]. Although P. amylolyticus, part of the Paenibacillus genus, may utilize mechanisms including organic acid production to solubilize phosphorus from insoluble compounds, enhancing availability to plants, the literature on its specific phosphorus solubilization capabilities remains limited [69,70]. The P. amyloliticus strains tested in this study possess the ability to solubilize phosphorus.
Various methodologies have been employed to study nitrogen fixation, including culturing of diazotrophic bacteria in nitrogen-free media and measuring nitrogenase activity using spectrophotometry and reagents such as Nessler’s reagent [71] or the acetylene reduction assay [72]. The use of 16S rRNA PCR and sequencing enables the identification and confirmation of nitrogen-fixing bacteria [73]. The fixation of atmospheric nitrogen by bacteria is a crucial component of sustainable agriculture, as it reduces reliance on synthetic fertilizers [74]. Four of the five strains tested in our study are capable of fixing atmospheric nitrogen. Bacillus species are known for their plant growth-promoting traits. According to the literature, they are able to fix nitrogen, making it available to plants, produce growth hormones, and solubilize phosphate [75]. Studies have also shown that certain Bacillus strains, including B. subtilis and B. licheniformis, as well as P. megaterium, exhibit high nitrogenase activity in the acetylene reduction assay [76]. B. subtilis and P. megaterium isolated from soils in Vietnam cultivated with cassava have been found to have nitrogen-fixing abilities. The authors highlighted the importance of optimizing cultivation conditions for the growth and metabolic activity of nitrogen-fixing organisms. One of the most critical parameters affecting their growth and activity is pH [77]. The B. licheniformis tested in the present study did not exhibit growth on nitrogen-free medium. It is possible that the isolated strain does not possess the ability to fix nitrogen, or the growth conditions may have affected its activity. The nitrogen-fixation abilities of P. amylolyticus have not been studied extensively, although other Peanibacillus species have been shown to be capable of fixing nitrogen [78,79]. P. amylolitycus exhibited growth on the nitrogen-free medium, which suggest that it may be capable of nitrogen fixation.
The results obtained in this study regarding the antimicrobial activity of the selected bacterial strains are consistent with findings reported in the literature. B. subtilis is particularly well known for its strong antimicrobial properties against various phytopathogens. This is primarily attributed to its production of secondary metabolites, including nonribosomal peptides and other antimicrobial compounds, which endow it with robust biocontrol capabilities. In other studies, B. subtilis has demonstrated antimicrobial activity against a broad range of pathogens, including Saccharicola, Cochliobolus, Alternaria, Fusarium spp., and Ralstonia solanacearum [80,81,82]. The findings of the present study, in which B. subtilis exhibited the largest growth-inhibition zones against several pathogens, align well with those reported previously. B. licheniformis also exhibited significant antimicrobial activity, which is supported by its ability to produce a diverse array of antimicrobial substances, such as bacteriocins, peptides, proteins, and cyclic lipopeptides. Studies have shown that this strain effectively inhibits pathogens such as R. solani, Sclerotium rolfsii, Fusarium culmorum, Pythium spp., Alternaria alternata, and Phoma medicaginis [83,84]. Fermentation supernatants of B. licheniformis TG116 have demonstrated efficacy against a variety of phytopathogens [85]. In this study, B. licheniformis displayed significant antifungal activity, particularly against R. solani and F. graminearum. The antimicrobial properties of B. velezensis are also well-documented, with strains exhibiting substantial activity against phytopathogens such as F. graminearum [86,87] and F. oxysporum [88]. Additionally, B. velezensis has demonstrated activity against Phytophthora sojae and R. solanacearum [89]. Genomic analyses have underscored the biocontrol potential of B. velezensis, revealing the presence of antimicrobial biosynthetic gene clusters [90]. In this study, B. velezensis exhibited consistent and robust inhibitory effects, particularly against Fusarium sp. and P. exigua. P. amylolyticus has also been reported to possess antimicrobial activity, producing non-ribosomal peptides and polyketides that target a range of phytopathogens [91]. Previous studies have shown Peanibacillus sp. efficacy in inhibiting notable pathogens such as F. oxysporum and R. solani [92]. The results of the present study confirm these findings, as P. amylolyticus displayed substantial antimicrobial activity against three tested phytopathogens (Fusarium sp.) but not against R. solani. P. megaterium exhibited comparatively lower antimicrobial activity than the other strains. However, previous reports have noted its effectiveness against pathogens such as F. culmorum and F. graminearum [93], as well as against Agrobacterium tumefaciens, Erwinia carotovora, and R. solanacearum [94]. While its activity in this study was limited, P. megaterium may still hold potential for specific biocontrol applications under different conditions or against different phytopathogens. The alignment of these findings with existing literature underscores the antimicrobial potential of the selected bacterial strains against diverse phytopathogens. Particularly high broad-spectrum activity was demonstrated by B. licheniformis, B. subtilis, and B. velezensis. These results highlight their potential applicability as biocontrol agents, contributing to sustainable agricultural practices and the management of plant diseases.
Our study is in the first to investigate the cytotoxicity of soil bacterial metabolites on an insect cell model, Sf-9. B. licheniformis and B. subtilis metabolites have been previously shown to have cytotoxic effects towards various human and mammalian cell lines, including embryonic kidney (HEK293), ovary Chinese hamster (CHO-K1), colon cancer Caco-2, breast cancer MDA-MB-231, prostate cancer PC-3, lung cancer NCI-H23, stomach cancer NUGC-3, and renal cancer ACH [95,96,97,98]. The cytotoxic metabolites of B. licheniformis and B. subtilis include small cyclic lipopeptide lichenysin A, linear lipopeptide gageostatin, catecholate siderophore bacillibactin, macrolactin A, and enediyne [97,98,99,100,101]. The B. subtilis and B. licheniformis strains used in the present study could probably be applied in biopreparations for the control of insect pathogens. However, more research is needed into their metabolites and biological properties. Our study is in the first to investigate the cytotoxicity of soil bacterial metabolites towards the Sf-9 insect cell model.
Based on the obtained results, we recommend the following uses of the isolated bacterial strains: B. licheniformis, B. subtilis, and B. velezensis as biocontrol agents; B. subtilis, B. licheniformis, B. velezensis, P. amylolyticus, and P. megaterium as plant growth biostimulants; B. subtilis, B. licheniformis, B. velezensis, P. amylolyticus, and P. megaterium as crop residue decomposition stimulants; B. licheniformis and B. subtilis as bioinsecticides. These microorganisms can be used individually or in a mixture of several strains (to produce a biopreparation with a broad spectrum of action), provided that the cultivation conditions are optimized to obtain the highest possible yield of spores.
In the current study, samples were collected exclusively from fields after maize cultivation in central Poland (warm temperate zone of the transitional type). It should be noted that they may not be microorganisms characteristic of other types of crops and climatic conditions in the world, which may affect the adaptability of the tested bacteria in the conditions of target use as soil biopreparations. Future studies incorporating diverse soil types, climate regions, and more refined experimental designs are necessary to generalize the findings and fully evaluate bacterial adaptability for broader agricultural applications. Additionally, the experimental conditions, such as temperature range and nutritional assessments, are currently under further investigation to provide a more comprehensive understanding of bacterial growth characteristics. Future work should also focus on assessing the efficacy of using the isolated strains under conditions simulating the target use—e.g., in plant microcosms and greenhouse crops and assessment of the impact of applying these strains on the abundance and biodiversity of native soil microbiota.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15126400/s1. Suplementary materials: Table S1. Description of the tested soil samples, Table S2. Characteristic of harvest residues samples, Table S3. Media and staining solutions used in research, Table S4. The optimal growth conditions of isolated bacterial strains after 7 days, Figure S1. Hydrolytic activities of selected WK strains on screening media supplemented with (1) skimmed milk: (a) strain WK-2 after 5 days at 30°C (EAI = 1.59); (b) strain WK-20 after 5 days at 30 °C (EAI = 1.14) and (c) strain WK-7 after 5 days at 30 °C (no proteolytic activity); (2) soluble starch: (d) strain WK-7 after 5 days at 30 °C (without iodine staining); (e) strain WK-7 after 5 days at 30 °C (with iodine staining; no amylolytic activity) and (f) strain WK-6 after 5 days at 30 °C (EAI = 0.33); (3) carboxymethylcellulose: (g) strain WK-7 after 4 days at 30 °C (without Congo Red staining); (h) strain WK-13 after 5 days at 30°C (EAI = 0.30) and (i) strain WK-14 after 5 days at 30 °C (no cellulolytic activity).
Author Contributions
Conceptualization, J.S. and P.R.; Methodology, J.S., P.R., M.S. (Marcin Sypka), R.J., A.M.B., B.G., and A.N.; Formal analysis, P.R., M.S. (Marcin Sypka), and A.N.; Investigation, J.S., P.R., M.S. (Marcin Sypka), M.S. (Maria Stryjek), R.J., and A.N.; Resources, J.S., A.M.B., R.J., and B.G.; Data curation, J.S.; Writing—original draft preparation, J.S., P.R., M.S. (Marcin Sypka), A.N., A.M.B., B.G., and M.S. (Maria Stryjek); Writing—review and editing, J.S., P.R., and M.S. (Marcin Sypka); Visualization, J.S., P.R., M.S. (Marcin Sypka), R.J., and A.N.; Supervision, J.S.; Project administration, J.S.; Funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by The Agency for Restructuring and Modernisation of Agriculture, Poland [grant number 00077.DDD.6509.000167.2022.05].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings of this study are available within the paper and its Supplementary Information. Nucleotide sequences determined during the study are available under the genome assembly accession numbers: ASM4051364v1, ASM4051367v1, ASM4051368v1, ASM4051371v1, ASM4051399v1.
Acknowledgments
Graphical abstract, Figure 1 and Figure 2 were prepared using BioRender software (https://www.biorender.com/). This article was completed while the first and second authors were a Doctoral Candidate of the Interdisciplinary Doctoral School at Lodz University of Technology, Poland.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TSA | Tryptic Soy Agar |
| MALDI-TOF MS | Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry |
| LB | Lysogeny Broth |
| NA | Nutrient Broth Agar |
| EAI | Enzymatic Activity Index |
| TRS | Total reducing sugars |
| TSB | Tryptic Soy Broth |
| OGT | optimal growth temperature |
| CMC | carboxymethyl cellulose |
| SD | standard deviation |
| PSMs | phosphate-solubilizing microorganisms |
References
- European Commission. European Commission COM(2019)640. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vl4cnhyp1ort (accessed on 14 July 2024).
- European Commission. European Commission COM(2020)380. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vl8tqb8jwtyy (accessed on 14 July 2024).
- European Commission. European Commission COM(2020)381. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vl8tofp7dtuc (accessed on 14 July 2024).
- Narwade, J.D.; Odaneth, A.A.; Lele, S.S. Solid-state fermentation in an earthen vessel: Trichoderma viride spore-based biopesticide production using maize cobs. Fungal Biol. 2023, 127, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Rowińska, P.; Gutarowska, B.; Janas, R.; Szulc, J. Biopreparations for the decomposition of crop residues. Microb. Biotechnol. 2024, 17, e14534. [Google Scholar] [CrossRef] [PubMed]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants, and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Toader, G.; Chiurciu, V.; Filip, V.; Burnichi, F.; Toader, E.V.; Enea, C.; Trifan, D.; Rîșnoveanu, L. Bacterial biopreparations-a ‘green revolution’ for agriculture. Res. J. Agric. Sci. 2020, 52, 198–205. [Google Scholar]
- Marwal, A.; Srivastava, A.K.; Gaur, R.K. Plant viruses as biopesticides. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 181–194. [Google Scholar] [CrossRef]
- Steglińska, A.; Bekhter, A.; Wawrzyniak, P.; Kunicka-Styczyńska, A.; Jastrząbek, K.; Fidler, M.; Śmigielski, K.; Gutarowska, B. Antimicrobial activities of plant extracts against Solanum tuberosum L. phytopathogens. Molecules 2022, 27, 1579. [Google Scholar] [CrossRef]
- Chakraborty, N.; Mitra, R.; Pal, S.; Ganguly, R.; Acharya, K.; Minkina, T.; Sarkar, A.; Keswani, C. Biopesticide consumption in India: Insights into the current trends. Agriculture 2023, 13, 557. [Google Scholar] [CrossRef]
- Divya, K.; Singh, R.; Thakur, I. Response of biofertilizers and foliar application of zinc on yield and economics of lentil (Lens culinaris, Fabaceae). Int. J. Environ. Clim. Change 2023, 13, 1040–1045. [Google Scholar] [CrossRef]
- Upadhyay, H.; Banik, D.; Aslam, M.; Singh, J. Beneficial microbiomes for sustainable agriculture: An ecofriendly approach. In Current Trends in Microbial Biotechnology for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2021; pp. 227–244. [Google Scholar] [CrossRef]
- Vishal, R.S.; Singh, A.P.; Pradhan, A. Influence of biofertilizers and nitrogen on yield and economics of barley (Hordeum vulgare L.). Int. J. Plant Soil Sci. 2023, 35, 196–202. [Google Scholar] [CrossRef]
- Anli, M.; Ait-El-Mokhtar, M.; Akensous, F.-Z.; Boutasknit, A.; Ben-Laouane, R.; Fakhech, A.; Ouhaddou, R.; Raho, O.; Meddich, A. Biofertilizers in date palm cultivation. In Date Palm; CABI: Wallingford, UK, 2023; pp. 266–296. [Google Scholar] [CrossRef]
- Bairwa, P.; Kumar, N.; Devra, V.; Abd-Elsalam, K.A. Nano-biofertilizers synthesis and applications in agroecosystems. Agrochemicals 2023, 2, 118–134. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microbiol. 2022, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, A.; De Luca, L.; Graziani, G.; Cepparulo, M.; El-Nakhel, C.; Giordano, M.; Rouphael, Y.; Ritieni, A.; Romano, R.; Di Vaio, C. Biostimulants application on Olea europaea L. in Mediterranean conditions increases the production and bioactive compounds of drupes and oil. Agriculture 2022, 12, 2173. [Google Scholar] [CrossRef]
- Halshoy, H.; Mahmood, A.; Tofiq, G. Effect of plant biostimulants on growth, yield, and some mineral composition of broccoli plants (Brassica oleracea var. italica). Tikrit J. Agric. Sci. 2023, 23, 130–140. [Google Scholar] [CrossRef]
- Malécange, M.; Sergheraert, R.; Teulat, B.; Mounier, E.; Lothier, J.; Sakr, S. Biostimulant properties of protein hydrolysates: Recent advances and future challenges. Int. J. Mol. Sci. 2023, 24, 9714. [Google Scholar] [CrossRef]
- Pérez-Aguilar, H.; Lacruz-Asaro, M.; Arán-Ais, F. Evaluation of biostimulants based on recovered protein hydrolysates from animal by-products as plant growth enhancers. J. Plant Sci. Phytopathol. 2023, 7, 42–47. [Google Scholar] [CrossRef]
- Madhavan, T.; Neethu, K.G.; Patel, T.K.; Vyas, T.K.; Ganesh, S. Exploring microbes for their cellulolytic and lignolytic enzyme activity for manure preparation. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3808–3816. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental factors affecting the mineralization of crop residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Maharjan, K.K.; Noppradit, P.; Techato, K. Suitability of vermicomposting for different varieties of organic waste: A systematic literature review (2012–2021). Org. Agric. 2022, 12, 581–602. [Google Scholar] [CrossRef]
- Pržulj, N.; Tunguz, V. Significance of harvest residues in sustainable management of arable land I. Decomposition of harvest residues. Arch. Tech. Sci. 2022, 1, 61–70. [Google Scholar] [CrossRef]
- Sánchez, G.; del Pino, A.; Hernández, J. Decomposition of Eucalyptus sp. and Pinus taeda harvest residues under controlled temperature and moisture conditions. Open J. For. 2018, 8, 87–104. [Google Scholar] [CrossRef]
- Hellequin, E.; Monard, C.; Quaiser, A.; Henriot, M.; Klarzynski, O.; Binet, F. Specific recruitment of soil bacteria and fungi decomposers following a biostimulant application increased crop residues mineralization. PLoS ONE 2018, 13, e0209089. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P.; Christie, G. New thoughts on an old topic: Secrets of bacterial spore resistance slowly being revealed. Microbiol. Mol. Biol. Rev. 2023, 87, e0008022. [Google Scholar] [CrossRef] [PubMed]
- Szulc, J.; Okrasa, M.; Nowak, A.; Ryngajłło, M.; Nizioł, J.; Kuźniar, A.; Ruman, T.; Gutarowska, B. Uncontrolled Post-Industrial Land-Source of Metals, Potential Toxic Compounds, Dust, and Pathogens in Environment-A Case Study. Molecules 2024, 29, 1496. [Google Scholar] [CrossRef] [PubMed]
- Liszkowska, W.; Motyl, I.; Pielech-Przybylska, K.; Szulc, J.; Sypka, M.; Dziugan, P.; Berlowska, J. Plant biomass as a source of low-temperature yeasts. BioResources 2023, 18, 599–612. [Google Scholar] [CrossRef]
- Krakova, L.; Soltys, K.; Otlewska, A.; Pietrzak, K.; Purktrova, S.; Savicka, A.; Puskarova, A.; Buckova, M.; Szemes, T.; Budis, J.; et al. Comparison of methods for identification of microbial communities in book collections: Culture-dependent (sequencing and MALDI-TOF MS) and culture-independent (Illumina MiSeq). Int. Biodeterior. Biodegrad. 2017, 120, 32–38. [Google Scholar] [CrossRef]
- Gerlicz, W.; Sypka, M.; Jodłowska, I.; Białkowska, A.M. Isolation, Selection, and Identification of Keratinolytic Bacteria for Green Management of Keratin Waste. Molecules 2024, 29, 3380. [Google Scholar] [CrossRef]
- Steglińska, A.; Kołtuniak, A.; Motyl, I.; Berłowska, J.; Czyżowska, A.; Cieciura-Włoch, W.; Okrasa, M.; Kręgiel, D.; Gutarowska, B. Lactic acid bacteria as biocontrol agents against potato (Solanum tuberosum L.) pathogens. Appl. Sci. 2022, 12, 7763. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiology 1984, 17, 362–370. [Google Scholar]
- Pande, A.; Pandey, P.; Mehra, S.; Singh, M.; Kaushik, S. Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. J. Genet. Eng. Biotechnol. 2017, 15, 379–391. [Google Scholar] [CrossRef]
- Steglińska, A.; Nowak, A.; Janas, R.; Grzesik, M.; Śmigielski, K.; Kręgiel, D.; Gutarowska, B. Chitosan as an antimicrobial, anti-insect, and growth-promoting agent for potato (Solanum tuberosum L.) plants. Molecules 2024, 29, 3313. [Google Scholar] [CrossRef]
- Szulc, J.; Okrasa, M.; Majchrzycka, K.; Sulyok, M.; Nowak, A.; Szponar, B.; Górczyńska, A.; Ryngajłło, M.; Gutarowska, B. Microbiological and toxicological hazard assessment in a waste sorting plant and proper respiratory protection. J. Environ. Manag. 2022, 303, 114257. [Google Scholar] [CrossRef] [PubMed]
- Palekar, K.; Khandeparker, R.D.S. Saccharification of lignocellulosic biomass using an enzymatic cocktail from marine bacteria. Biofuels 2024, 16, 155–166. [Google Scholar] [CrossRef]
- Huang, Z.; Ni, G.; Zhao, X.; Wang, F.; Qu, M. Characterization of a GH8 β-1,4-Glucanase from Bacillus subtilis B111 and Its Saccharification Potential for Agricultural Straws. J. Microbiol. Biotechnol. 2021, 31, 1446–1454. [Google Scholar] [CrossRef]
- Woźniak, M.; Ratajczak, I.; Wojcieszak, D.; Waśkiewicz, A.; Szentner, K.; Przybył, J.; Borysiak, S.; Goliński, P. Chemical and Structural Characterization of Maize Stover Fractions in Aspect of Its Possible Applications. Materials 2021, 14, 1527. [Google Scholar] [CrossRef]
- Devos, R.; Bender, L.; Lopes, S.; Cavanhi, V.; Colvero, G.; Rempel, A.; Harakava, R.; Alves, S.; Colla, L. Multienzyme production by Bacillus velezensis strains isolated from fruit residues in submerged fermentation using triticale and sugarcane bagasse in the cultivation media. Process Biochem. 2023, 141, 90–101. [Google Scholar] [CrossRef]
- Pronyk, C.; Mazza, G. Fractionation of triticale, wheat, barley, oats, canola, and mustard straws for the production of carbohydrates and lignins. Bioresour. Technol. 2012, 106, 117–124. [Google Scholar] [CrossRef] [PubMed]
- FDA. 2025. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory (accessed on 30 May 2025).
- EFSA. 2025. Available online: https://www.efsa.europa.eu/en/topics/topic/qualified-presumption-safety-qps (accessed on 30 May 2025).
- European Parliament and Council of the European Union. Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work. Off. J. Eur. Communities 2000, L262, 21–45. [Google Scholar]
- Carroll, L.M.; Cheng, R.A.; Wiedmann, M.; Kovac, J. Keeping up with the Bacillus cereus group: Taxonomy through the genomics era and beyond. Crit. Rev. Food Sci. Nutr. 2022, 62, 7677–7702. [Google Scholar] [CrossRef]
- Manzulli, V.; Rondinone, V.; Buchicchio, A.; Serrecchia, L.; Cipolletta, D.; Fasanella, A.; Parisi, A.; Difato, L.; Iatarola, M.; Aceti, A.; et al. Discrimination of Bacillus cereus Group Members by MALDI-TOF Mass Spectrometry. Microorganisms 2021, 9, 1202. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus Group: Bacillus Species with Pathogenic Potential. Microbiol. Spectr. 2019, 7, 1–35. [Google Scholar] [CrossRef]
- Wielinga, P.R.; Hamidjaja, R.A.; Agren, J.; Knutsson, R.; Segerman, B.; Fricker, M.; Ehling-Schulz, M.; de Groot, A.; Burton, J.; Brooks, T.; et al. A multiplex real-time PCR for identifying and differentiating B. anthracis virulent types. Int. J. Food Microbiol. 2011, 145 (Suppl. S1), S137–S144. [Google Scholar] [CrossRef] [PubMed]
- Muigg, V.; Cuénod, A.; Purushothaman, S.; Siegemund, M.; Wittwer, M.; Pflüger, V.; Schmidt, K.M.; Weisser, M.; Ritz, N.; Widmer, A.; et al. Diagnostic challenges within the Bacillus cereus-group: Finding the beast without teeth. New Microbes New Infect. 2022, 49–50, 101040. [Google Scholar] [CrossRef] [PubMed]
- Jha, Y.; Yadav, K.A.; Mohamed, H.I. Plant growth-promoting bacteria and exogenous phytohormones alleviate the adverse effects of drought stress in pigeon pea plants. Plant Soil 2023, 505, 163–183. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Enhancement of disease resistance, growth potential, and biochemical markers in maize plants by inoculation with plant growth-promoting bacteria under biotic stress. J. Plant Pathol. 2023, 105, 729–748. [Google Scholar] [CrossRef]
- Mondal, S.; Santra, S.; Rakshit, S.; Kumar, S.; Hossain, M.; Chandra Mondal, K. Saccharification of lignocellulosic biomass using an enzymatic cocktail of fungal origin and successive production of butanol by Clostridium acetobutylicum. Bioresour. Technol. 2022, 343, 126093. [Google Scholar] [CrossRef]
- Jeckelmann, J.-M.; Erni, B. Transporters of glucose and other carbohydrates in bacteria. Eur. J. Physiol. 2020, 472, 1129–1153. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, H.; Rahman, M.S.; Chen, X.; Zhang, J.; Liu, Y.; Qin, W. Biological pretreatment of corn stover for enhancing enzymatic hydrolysis using Bacillus sp. P3. Bioresour. Bioprocess. 2021, 8, 92. [Google Scholar] [CrossRef]
- Greff, B.; Szigeti, J.; Nagy, Á.; Lakatos, E.; Varga, L. Influence of microbial inoculants on co-composting of lignocellulosic crop residues with farm animal manure: A review. J. Environ. Manag. 2022, 302, 114088. [Google Scholar] [CrossRef]
- Avdeeva, L.V.; Kharkhota, M.A.; Kharkhota, A.V. The decomposition of various types of crop residues by strains Bacillus subtilis IMB B-7516 and B. licheniformis IMB B-7515. Mikrobiol. Z. 2016, 78, 52–60. [Google Scholar] [CrossRef]
- Chhetri, R.B.; Silwal, P.; Jyapu, P.; Maharjan, Y.; Lamsal, T.; Basnet, A. Biodegradation of organic waste using Bacillus species isolated from soil. Int. J. Appl. Sci. Biotechnol. 2022, 10, 104–111. [Google Scholar] [CrossRef]
- Bhodiwal, S.; Barupal, T. Phosphate solubilizing microbes: An incredible role for plant supplements. MOJ Ecol. Environ. Sci. 2022, 7, 170–172. [Google Scholar] [CrossRef]
- Pang, F.; Li, Q.; Solanki, M.K.; Wang, Z.; Xing, Y.-X.; Dong, D.-F. Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front. Microbiol. 2024, 15, 1383813. [Google Scholar] [CrossRef]
- Bedine, M.A.B.; Iacomi, B.; Nguemezi Tchameni, S.; Sameza, M.L.; Boyom Fekam, F. Harnessing the phosphate-solubilizing ability of Trichoderma strains to improve plant growth, phosphorus uptake, and photosynthetic pigment contents in common bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 2022, 45, 102510. [Google Scholar] [CrossRef]
- Ferreira, A.P.; Oliveira, J.A.d.S.; Polonio, J.C.; Pamphile, J.A.; Azevedo, J.L. Recombinants of Alternaria alternata endophytes enhance inorganic phosphate solubilization and plant growth hormone production. Biocatal. Agric. Biotechnol. 2023, 51, 102784. [Google Scholar] [CrossRef]
- Cozzolino, M.E.; Córdoba, M.E.; Ramos, P.D.; Ferrari, S.G.; Silva, P.G. Phosphate solubilization by Bacillus isolates and its influence in a cyanobacterial co-culture. Malays. J. Microbiol. 2024, 20, 289–295. [Google Scholar] [CrossRef]
- Mosela, M.; Andrade, G.; Massucato, L.R.; Almeida, S.R.d.A.; Nogueira, A.F.; Lima Filho, R.B.d.; Zeffa, D.M.; Teixeira, G.M.; Shimizu, G.D.; Gonçalves, L.S.A. Bacillus velezenis strain Ag75 as a new multifunctional agent for biocontrol, phosphate solubilization, and growth promotion in maize and soybean crops. Sci. Rep. 2022, 12, 15284. [Google Scholar] [CrossRef]
- Zheng, B.X.; Ibrahim, M.; Zhang, D.P.; Bi, Q.F.; Li, H.Z.; Zhou, G.W.; Ding, K.; Peñuelas, J.; Zhu, Y.G.; Yang, X.R. Identification and characterization of inorganic-phosphate-solubilizing bacteria from agricultural fields with a rapid isolation method. AMB Express 2018, 8, 47. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Chojnacka, K. In situ solubilization of phosphorus-bearing raw materials by Bacillus megaterium. Eng. Life Sci. 2017, 17, 749–758. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Sojka, M.; Saeid, A. Production of phosphorus biofertilizer based on the renewable materials in large laboratory scale. Open Chem. 2019, 17, 893–901. [Google Scholar] [CrossRef]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus solubilization by Bacillus species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Li, B.; Shan, C.; Ibrahim, M.; Jabeen, A.; Xie, G.; Sun, G. Phosphate solubilization of Paenibacillus polymyxa and Paenibacillus macerans from mycorrhizal and non-mycorrhizal cucumber plants. Afr. J. Microbiol. Res. 2012, 6, 4567–4573. [Google Scholar] [CrossRef]
- Aberathna, A.A.A.U.; Satharasinghe, D.A.; Jayasooriya, A.P.; Jinadasa, R.N.; Manopriya, S.; Jayaweera, B.P.A.; Fernando, C.A.N.; Weerathilake, W.A.D.V.; Prathapasinghe, G.A.; Liyanage, J.A.; et al. Increasing the bioavailability of phosphate by using microorganisms. Int. J. Agron. 2022, 2022, 4305501. [Google Scholar] [CrossRef]
- Al Saffar, F.A. Effect of temperature on the ability of Pseudomonas stutzeri bacteria isolated from different sources to fix nitrogen. Res. Appl. Sci. Biotechnol. 2022, 1, 254–258. [Google Scholar] [CrossRef]
- Haskett, T.L.; Poole, P.S. A Simple Assay for Quantification of Plant-Associative Bacterial Nitrogen Fixation; Cold Spring Harbor Laboratory: Laurel Hollow, NY, USA, 2021. [Google Scholar] [CrossRef]
- Martinez-Vaz, B.M.; Denny, R.; Young, N.D.; Sadowsky, M.J. An alternative approach to “Identification of Unknowns”: Designing a protocol to verify the identities of nitrogen-fixing bacteria. J. Microbiol. Biol. Educ. 2015, 16, 247–253. [Google Scholar] [CrossRef][Green Version]
- Javid, H.; ul Qadir, R.; Rashid, S.; Rashid, K.; Magray, J.A.; Islam, T.; Wani, B.A.; Gulzar, S.; Nawchoo, I.A. Biological nitrogen fixation. In Advances in Plant Nitrogen Metabolism; CRC Press: Boca Raton, FL, USA, 2022; pp. 129–141. [Google Scholar]
- Jain, S.; Varma, A.; Choudhary, D.K. Perspectives on nitrogen-fixing Bacillus species. In Advances in Plant Nitrogen Metabolism; CRC Press: Boca Raton, FL, USA, 2021; pp. 359–369. [Google Scholar] [CrossRef]
- Yousuf, J.; Thajudeen, J.; Rahiman, M.; Krishnankutty, S.P.; Alikunj, A.A.; Abdulla, M.H. Nitrogen fixing potential of various heterotrophic Bacillus strains from a tropical estuary and adjacent coastal regions. J. Basic Microbiol. 2017, 57, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Cuong, P.V.; Hoa, N.P. Effect of culture conditions on nitrogen-fixing activity of bacteria isolated from cassava cultivated soils of Vietnam. Acad. J. Biol. 2021, 43, 27–35. [Google Scholar] [CrossRef]
- Weid, I.; Frois, G.; Duarte, J.; van Elsas, J.D.; Seldin, L. Paenibacillus brasilensis sp. nov., a novel nitrogen-fixing species isolated from the maize rhizosphere in Brazil. Int. J. Syst. Evol. Microbiol. 2002, 52, 2147–2153. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Liu, Z.; Zhao, D.; Liu, X.; Zhang, B.; Xie, J.; Hong, Y.; Li, P.; Chen, S.; et al. Correction: A minimal nitrogen fixation gene cluster from Paenibacillus sp. WLY78 enables expression of active nitrogenase in Escherichia coli. PLoS Genet. 2013, 9, e1003865. [Google Scholar] [CrossRef]
- Hazarika, D.J.; Goswami, G.; Gautom, T.; Parveen, A.; Das, P.; Barooah, M.; Boro, R.C. Lipopeptide mediated biocontrol activity of endophytic Bacillus subtilis against fungal phytopathogens. BMC Microbiol. 2019, 19, 71. [Google Scholar] [CrossRef]
- Jinal, N.H.; Amaresan, N. Evaluation of biocontrol Bacillus species on plant growth promotion and systemic-induced resistant potential against bacterial and fungal wilt-causing pathogens. Arch. Microbiol. 2020, 202, 1785–1794. [Google Scholar] [CrossRef]
- Kiesewalter, H.T.; Lozano-Andrade, C.N.; Wibowo, M.; Strube, M.L.; Maróti, G.; Snyder, D.; Jørgensen, T.S.; Larsen, T.O.; Cooper, V.S.; Weber, T.; et al. Genomic and chemical diversity of Bacillus subtilis secondary metabolites against plant pathogenic fungi. mSystems 2021, 6, e00770-20. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.M.; Moustafa, S.A.; Kamil, Z.; Rizk, M.; Saleh, M.; Moustafa, S. Isolation and identification of rhizosphere soil chitinolytic bacteria and their potential in antifungal biocontrol. Glob. J. Mol. Sci. 2007, 2, 57–66. [Google Scholar]
- Slimene, I.B.; Tabbene, O.; Gharbi, D.; Mnasri, B.; Schmitter, J.M.; Urdaci, M.-C.; Limam, F. Isolation of a chitinolytic Bacillus licheniformis S213 strain exerting a biological control against Phoma medicaginis infection. Appl. Biochem. Biotechnol. 2015, 175, 3494–3506. [Google Scholar] [CrossRef]
- Ling, L.; Cheng, W.; Jiang, K.; Jiao, Z.; Luo, H.; Yang, C.; Pang, M.; Lu, L. The antifungal activity of a serine protease and the enzyme production characteristics of Bacillus licheniformis TG116. Arch. Microbiol. 2022, 204, 601. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, L.; Zhang, W.; Chi, F.; Hao, X.; Bian, J.; Li, Y. Bacillus velezensis BM21, a potential and efficient biocontrol agent in control of maize stalk rot caused by Fusarium graminearum. Egypt. J. Biol. Pest Control 2020, 30, 9. [Google Scholar] [CrossRef]
- Kim, J.-A.; Song, J.-S.; Kim, P.I.; Kim, D.-H.; Kim, Y. Bacillus velezensis TSA32-1 as a promising agent for biocontrol of plant pathogenic fungi. J. Fungi 2022, 8, 1053. [Google Scholar] [CrossRef]
- Agha, S.I.; Jahan, N.; Azeem, S.; Parveen, S.; Tabassum, B.; Raheem, A.; Ullah, H.; Khan, A. Characterization of broad-spectrum biocontrol efficacy of Bacillus velezenis against Fusarium oxysporum in Triticum aestivum L. Not. Bot. Horti Agrobot. 2022, 50, 12590. [Google Scholar] [CrossRef]
- Yu, C.; Chen, H.; Zhu, L.; Song, Y.; Jiang, Q.; Zhang, Y.; Ali, Q.; Gu, Q.; Gao, X.; Borriss, R.; et al. Profiling of Antimicrobial Metabolites Synthesized by the Endophytic and Genetically Amenable Biocontrol Strain Bacillus velezensis DMW1. Microbiol. Spectr. 2023, 11, e00038-23. [Google Scholar] [CrossRef]
- Diabankana, R.G.C.; Shulga, E.U.; Validov, S.Z.; Afordoanyi, D.M. Genetic characteristics and enzymatic activities of Bacillus velezenis KS04AU as a stable biocontrol agent against phytopathogens. Int. J. Plant Biol. 2022, 13, 201–222. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Radin Shafierul, R.Y.; Mohamad Malik Al-adil, B.; Yaminudin, S.M.; Karim, M.; Ahmad, S.A.; Sabri, S. A review on the biotechnological applications of the operational group Bacillus amyloliquefaciens. Microorganisms 2021, 9, 614. [Google Scholar] [CrossRef]
- Xu, S.J.; Hong, S.J.; Choi, W.; Kim, B.S. Antifungal Activity of Paenibacillus kribbensis Strain T-9 Isolated from Soils against Several Plant Pathogenic Fungi. Plant Pathol. J. 2014, 30, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Keleş Keyfoğlu, E.N.; Tufan Dülger, A.F.; Yörük, E. Investigation of the antifungal activity of Bacillus megaterium against Fusarium species. YYU J. Agric. Sci. 2023, 33, 183–191. [Google Scholar] [CrossRef]
- Xie, Y.; Qiuju, P.; Ji, Y.; Xie, A.; Yang, L.; Mu, S.; Li, Z.; He, T.; Yang, X.; Zhao, J.; et al. Isolation and Identification of Antibacterial Bioactive Compounds from Bacillus megaterium L2. Front. Microbiol. 2021, 12, 645484. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.B.; Bjørnvad, M.E.; Rasmussen, M.D.; Petersen, J.N. Cytotoxic potential of industrial strains of Bacillus sp. Regul. Toxicol. Pharmacol. 2002, 36, 155–161. [Google Scholar] [CrossRef]
- Madslien, E.H.; Rønning, H.T.; Lindbäck, T.; Hassel, B.; Andersson, M.A.; Granum, P.E. Lichenysin is produced by most Bacillus licheniformis strains. J. Appl. Microbiol. 2013, 115, 1068–1080. [Google Scholar] [CrossRef]
- Yeak, K.Y.C.; Perko, M.; Staring, G.; Fernandez-Ciruelos, B.M.; Wells, J.M.; Abee, T.; Wells-Bennik, M.H.J. Lichenysin Production by Bacillus licheniformis Food Isolates and Toxicity to Human Cells. Front. Microbiol. 2022, 13, 831033. [Google Scholar] [CrossRef]
- Iqbal, S.; Begum, F.; Rabaan, A.A.; Aljeldah, M.; Al Shammari, B.R.; Alawfi, A.; Alshengeti, A.; Sulaiman, T.; Khan, A. Classification and multifaceted potential of secondary metabolites produced by Bacillus subtilis group: A comprehensive review. Molecules 2023, 28, 927. [Google Scholar] [CrossRef]
- Mikkola, R.; Kolari, M.; Andersson, M.A.; Helin, J.; Salkinoja-Salonen, M. Toxic lactonic lipopeptide from food poisoning isolates of Bacillus licheniformis. Eur. J. Biochem. 2000, 267, 4068–4074. [Google Scholar] [CrossRef]
- Galm, U.; Hager, M.H.; Van Lanen, S.G.; Ju, J.; Thorson, J.S.; Shen, B. Antitumor antibiotics: Bleomycin, enediynes, and mitomycin. Chem. Rev. 2005, 105, 739–758. [Google Scholar] [CrossRef]
- Nieminen, T.; Rintaluoma, N.; Andersson, M.; Taimisto, A.M.; Ali-Vehmas, T.; Seppälä, A.; Priha, O.; Salkinoja-Salonen, M. Toxinogenic Bacillus pumilus and Bacillus licheniformis from mastitic milk. Vet. Microbiol. 2007, 124, 329–339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).