1. Introduction
In orthopaedic rehabilitation following lower limb surgeries, such as fracture repair, total hip replacement, or knee arthroplasty, partial weight-bearing is widely recognised as a cornerstone of effective postoperative care. The clinical rationale for prescribing partial weight-bearing is twofold. On the one hand, applying a controlled mechanical load to the healing bone stimulates osteoblastic activity and bone remodelling, essential for the recovery and strengthening of the skeletal structure [
1,
2]. Conversely, limiting the load prevents excessive stress that could lead to complications such as fracture malunion, implant failure, or undesired alterations in gait biomechanics [
3,
4]. Despite its importance, achieving the recommended weight-bearing remains challenging. Traditional methods of teaching and monitoring partial weight-bearing, such as using home scales, verbal instructions, or tactile cues (e.g., placing a hand under the foot), have been found lacking in several respects. These static modalities fail to capture the dynamic nature of weight distribution during ambulation and daily activities, leading to poor correlation between estimated and actual load application [
3]. Research has repeatedly reported that patients, regardless of repeated instruction, struggle to consistently reproduce the given weight-bearing limits over time, with notable deviations noted during dynamic activities such as walking [
5,
6]. The resulting discrepancies can shift the centre of gravity, adversely increasing muscular effort, particularly in the abductor muscles, and introduce compensatory movement patterns that further compromise rehabilitation outcomes [
7]. In recent years, biofeedback systems have injected a new dimension into postoperative rehabilitation protocols. These systems, which include digital portable insoles and wearable sensor arrays, provide continuous, real-time feedback on the load exerted on the lower limbs. Unlike traditional static assessments, these devices enable patients to adjust their gait dynamically, ensuring that the load remains within prescribed limits during functional activities [
5,
7]. Despite the demonstrated efficacy of such digital solutions, their widespread adoption is hampered by two critical limitations: high costs and evidence of benefit restricted to short-term outcomes. Consequently, an unmet need remains for accessible, economically viable tools to deliver sustained improvements in load perception and weight-bearing accuracy.
Several biofeedback solutions have been explored to help patients respect partial-weight-bearing (PWB) limits. Laboratory-based force-plate or visual/auditory treadmill systems can improve load awareness but are costly and bound to specialised gait labs [
8]. Instrumented crutches with load-triggered sound give real-time cues during overground walking, yet they require batteries and deliver feedback only when crutches contact the ground [
9]. Wireless smart-insole platforms (e.g., SmartStep™, OpenGo-Science) stream plantar-pressure data to a smartphone, but prices > EUR 600 and the need for Bluetooth pairing limit routine use [
10]. More recently, capacitive sensor insoles with on-board electronics have been proposed, but they still depend on continuous power and visual monitoring [
11]. Vibrotactile belts or smart glasses can offload visual demand, yet the wearables remain bulky, and their efficacy data are confined to balance-impaired populations rather than orthopaedic PWB [
12].
Our incentiviser costs < EUR 5 in off-the-shelf parts, is fitted in <5 min, functions without electricity, and delivers an unmistakable somatosensory cue regardless of lighting, ambient noise, or the patient’s field of view. These attributes make it attractive for low-resource settings and unsupervised home rehabilitation, where electronic devices may be impractical.
2. Materials and Methods
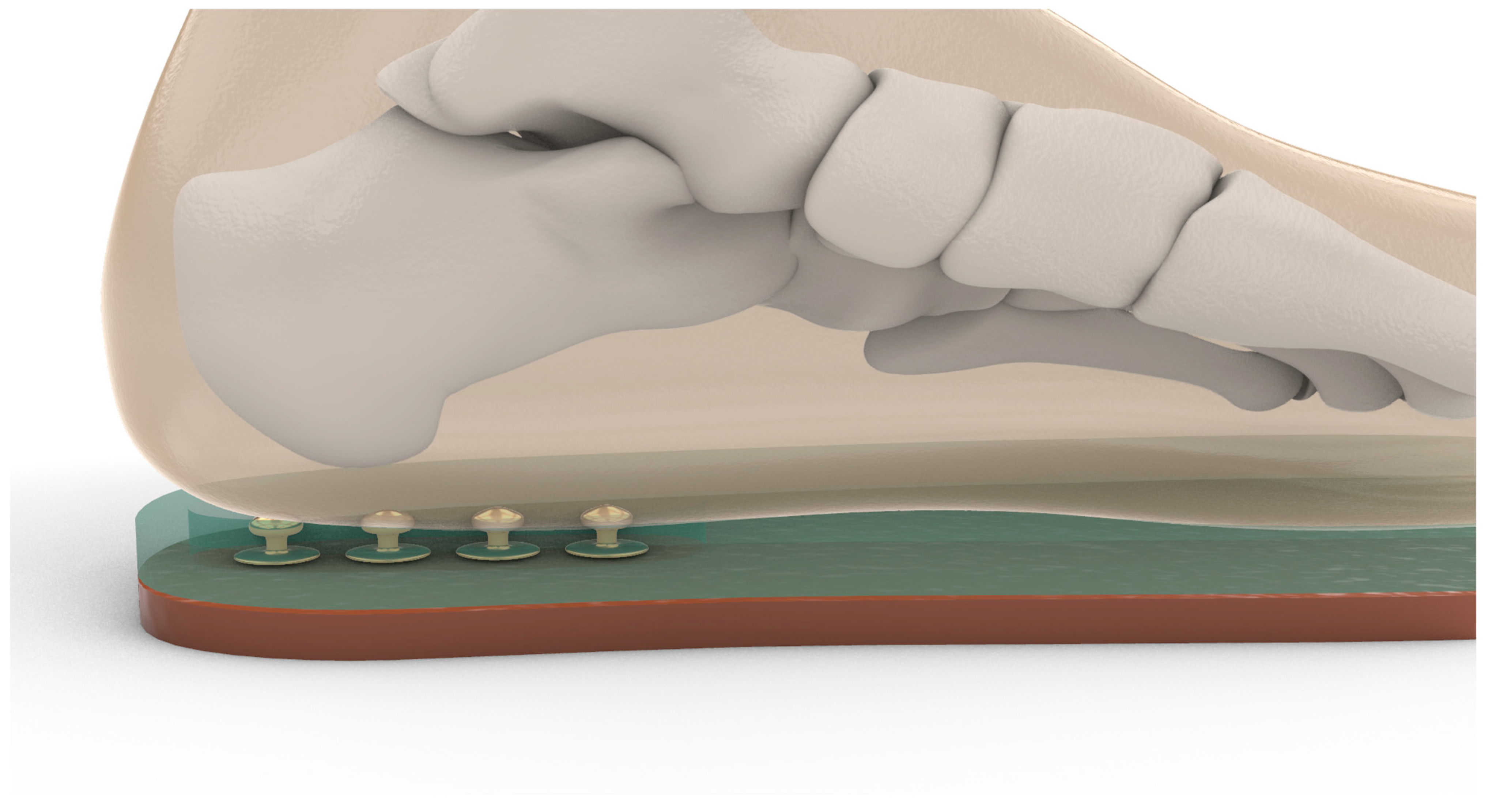
The tactile incentive insole used in this study was manually constructed using preformed rubber insoles, onto which four metallic snap buttons were affixed in a vertical line along the midline of the heel. The first button was positioned 2.5 cm from the posterior end of the insole, with the remaining three placed at 1.5 cm intervals. The principle behind the device is proprioceptive stimulation through tactile feedback. When the load applied to the operated limb reaches a specific threshold, compression of the overlying material allows the plantar surface of the foot to contact the buttons, generating a distinct sensory signal. Different layers of compressible materials—foam rubber and neoprene of varying densities and thicknesses (6 mm and 8 mm)—were added on top of the base insole to modulate the activation threshold. The material height and resistance combination was individually calibrated to ensure the patient perceived the feedback only when the prescribed partial weight-bearing target (e.g., 20% of body weight) was reached (
Figure 1 and
Figure 2).
Before recruitment, we performed an a priori power calculation to determine the minimum number of participants required to detect a clinically meaningful improvement in partial weight-bearing (PWB) accuracy. The literature reports a minimal clinically significant difference (MCID) of ±2 kg for 20% body weight prescriptions in post-operative gait training; we set the target effect (Δ) at 3 kg, i.e., a change large enough to bring the average patient inside the MCID window [
13,
14]. Based on previous smart-insole studies [
8,
10], we expected a pooled standard deviation (SD) of 5 kg. Assuming a two-sided α = 0.05 and power (1 − β) = 0.80, a simple two-sample
t-test yields.
Rehabilitation was conducted in an inpatient setting at the Novaggio Rehabilitation Clinic (Ticino, Switzerland), with daily physiotherapy sessions delivered by licenced therapists and the observation period extended from T0 (postoperative day 1) to T1 (hospital discharge, typically postoperative day 14 ± 2).
To compensate for a potential 20% attrition rate typical of early post-operative rehabilitation, we increased the target sample to 34 participants (17 per group). The study recruited 34 random patients who had undergone lower limb orthopaedic surgery, meeting the inclusion criteria of being aged between 18 and 85 years and having undergone surgery for femoral fracture, hip arthroplasty, trimalleolar ankle fracture, pelvic fracture, or knee arthroplasty.
At the beginning of every calendar week, all new admissions who met the inclusion criteria were listed in order of entry and assigned an incremental study ID. An independent research assistant, who had no clinical contact, uploaded that week’s ID list to Random.org (
https://www.random.org/ (accessed on 18 May 2025)) and drew the same numbers for treatment and three for the control. The resulting allocation sheet was released to the ward staff only after baseline measurements were completed. Because the tactile incentiviser is visible and palpable, neither patients nor treating physiotherapists could be blinded; the randomisation assistant was the only person otherwise masked to group identity.
Exclusion criteria included pre-existing walking difficulties, neurological conditions, and foot size outside the measurement range of the insole. Before recruitment, all patients were informed about the study and provided signed informed consent.
- -
Age between 18 and 85 years
- -
Underwent lower limb orthopaedic surgery, including the following:
- ○
Femoral fracture fixation
- ○
Total hip arthroplasty (THA)
- ○
Hip hemiarthroplasty
- ○
Trimalleolar ankle fracture
- ○
Pelvic fracture
- ○
Total knee arthroplasty (TKA)
- -
Ability to walk with or without assistance
- -
Provision of informed consent
- -
Severe cognitive impairment
- -
Neurological conditions affecting gait
- -
Pre-existing walking disability
- -
Foot size incompatible with the pressure insole
- -
Refusal to participate
The recruitment was conducted at the Rehabilitation Clinic in Novaggio and the Physiatry Department of San Carlo Hospital in Bordighera. The recruiting physicians and physiotherapists were aware of the study protocol. Participants were randomly assigned to the intervention or control group. The digital insole used for measuring plantar pressure was GeBiom [
15], GP MobilData–Go-tec (v.7, 48149 Münster, Germany), and the pressure measurement system was GP MobilData wireless. The software divided the plantar surface into several regions to evaluate the foot’s load. The sensory feedback incentivator was designed and created from preformed rubber insoles with protruding automatic buttons, which provided feedback and encouraged sensitivity to the load. Four buttons were placed per insole in a vertical line along the heel, with the first button 2.5 cm from the end of the insole and the other three buttons spaced 1.5 cm apart along the midline. Depending on the load, the starting insole or three materials of variable density (foam rubber, 6 mm neoprene, or 8 mm neoprene) were used to increase the patient’s sensitivity to proprioceptive stimuli and impose increased load on the limb (
Figure 2). Before clinical use, each insole was statically calibrated on a custom hydraulic press equipped with a 0–500 N load cell (accuracy ±0.5 N). Three polyurethane-foam inserts of increasing hardness (35, 45, and 60 Shore A; thickness 6 mm) were available and selected according to the participant’s body mass. The insole was pre-loaded in 10 N steps; the tactile dome was adjusted until it collapsed at 20% ± 1% of the individual’s body weight, representing our protocol’s prescribed partial weight-bearing limit. The procedure was repeated thrice per insole, yielding a coefficient of variation < 4%.
The insoles were calibrated to apply a specific load on the heel before the material reached zero height, and the buttons provided distinct tactile feedback. The complete insole was placed inside the patient’s shoe to provide sensory feedback on the correct load. The sensory feedback incentivator was used in the treatment group’s evaluation and treatment phases. The evaluation protocol included collecting demographic data, medical history, and conducting physical examinations such as the Numeric Rating Scale (NRS) and the 6-Minute Walking Test (6MWT) [
16,
17,
18,
19,
20]. The study design was quasi-experimental, where the treated group received treatment with an incentivator while the control group did not receive any treatment. The primary outcome measure of the study was the change in loaded weight (Δ loaded weight), defined as the difference between the theoretical weight and the actual weight measured at two time points: T0 (before the start of treatment) and T1 (at the end of hospitalisation). Statistical analysis compared the treatment and control groups regarding their Δ loaded weight at T0 and T1. Specifically, values at T0 and T1 were compared using parametric tests when normality was met, or non-parametric alternatives: the
t-test (or Student’s
t-test). A non-parametric test, the Wilcoxon-Mann–Whitney test for paired data, was also performed for confirmation. The significance level for both tests was set at
p < 0.05, meaning that results at the 95th percentile were considered valid, resulting in 95% confidence that the differences between the groups were significant. SPSS
® v19.0 and Excel 2019 were used for data analysis.
Outcome assessments were performed at three fixed time points: baseline (post-operative day 1, T0), mid-rehabilitation (post-operative day 7 ± 1, Tmid) and at hospital discharge (post-operative day 14 ± 2, T1).
A post hoc power analysis (two-tailed independent-samples t test, α = 0.05) was performed in GPower 3.1 using the effect size subsequently observed for the primary outcome to quantify the achieved statistical power.
The normality of each continuous variable was assessed using the Shapiro–Wilk test. When the distribution did not deviate significantly from normality (p > 0.05), intra-group changes were analysed with the paired Student’s t test; otherwise, the non-parametric Wilcoxon signed-rank test was applied. The choice of inter-group test (independent-samples t or Mann–Whitney) followed the same criterion.
This study was conducted by the ethical standards outlined by the Declaration of Helsinki and approved by the University of Insubria Ethics Committee (protocol number 0026262).
3. Results
A total of 34 patients were included in the study and evenly allocated to intervention and control groups (
n = 17 each).
Table 1 presents the main demographic and clinical characteristics at baseline.
Results from the study were objectively quantified by digitally recording the load expressed in Kg maintained by patients during ambulation, using measurements at the beginning, middle, and end of treatment (
Figure 3).
The experimental treatment’s effectiveness was calculated by considering the difference between the load granted during the orthopaedic visit and the load used by the patients (Δ weight loaded), comparing the average values and standard deviations between the experimental and control groups. In the experimental group, a Δ load was observed at the beginning of treatment without using the load incentiviser, equal to 12.9 kg ± 10.9. At the beginning of therapy utilising the incentiviser, the Δ weight loaded decreased to 5.6 kg ± 8.5. In the middle of treatment, following the integration of the load incentiviser in the treatment plan, the Δ weight loaded was 4.4 kg ± 6.3, and at the end of treatment, it was 2.0 kg ± 3.0. The change in load perception was confirmed by the
T-test between T0 and T1, with a
p-value = 0.021251 and therefore <0.05, confirming the alternative hypothesis of a difference between the beginning and end of treatment at 95% (
Table 2).
Other parameters were also analysed, such as the standard deviations of individual subjects at different measurement times (columns), indicating the intra-step variability of the subject (how much the patient varies the load in different steps within the same measurement). Between T0 and T1, the mean value of the standard deviations goes from 2.5 kg ± 1.0 at T0, to 3.4 kg ± 1.6 at T0 with incentivator, to 2.0 kg ± 1.2 at T1. In the control group, the Δ weight loaded at the beginning of the treatment was 16.3 kg ± 14.7, while at the end of treatment, where the incentiviser was never used, the Δ weight loaded decreased to 12.8 kg ± 8.4. In this case, the statistical test with a
p-value = 0.218672 and therefore >0.05 confirms the null hypothesis of equality and the absence of improvement between the beginning and end of treatment (
Table 3).
Intra-step variability was also analysed in this group, with 3.1 kg ± 1.9 at T0 and 3.5 kg ± 3.1 at T1. Non-parametric statistics were also performed in this study. In the T0 and T1 comparison, the test group showed a greater improvement in weight load than the control group. The difference between T0 and T1 is confirmed at 99% in the test group, with a
p-value < 0.01. The comparison between the test T0 and control T0 and the absence of a
p-value < 0.01 confirms that the two groups start from homogeneous baseline conditions and can be compared. The comparison between control T0 and control T1 and the absence of a
p-value < 0.01 confirms the equality between the beginning and end of treatment and the lack of improvement in the control group. The intergroup analysis at T1 between the test and control groups, with a
p-value < 0.01, shows that the two groups behave differently at discharge. The statistical significance is confirmed. As for the NRS and 6MWT, significant improvements were observed in both the experimental and control groups (
Figure 4) (
Table 4).
The mean length of stay was 13.9 ± 1.7 days for the intervention group and 14.1 ± 1.6 days for controls.
Using the observed between-group effect size for the primary outcome (Cohen’s d = 1.71), a two-tailed independent-samples t-test with α = 0.05 and n = 17 participants per arm (total N = 34) yielded a non-centrality parameter of δ = 4.99 and an achieved statistical power of 0.998 (99.8%), as computed in G*Power 3.1.
When the primary outcome was analysed within each surgical category, the tactile incentiviser consistently lowered the load error relative to standard care. The largest absolute benefit was observed after total hip arthroplasty (–11.0 kg; Cohen’s d = 3.97), followed by femoral-shaft fracture fixation (–15.2 kg; d = 1.75) and hip hemi-arthroplasty (–12.7 kg; d = 1.46). Pelvic-fracture patients also improved (–10.1 kg), but the subgroup was too small for a reliable effect-size estimate, and only a single trimalleolar-fracture patient received the device, precluding comparison. A treatment-by-diagnosis interaction test was not significant (
p = 0.21), indicating that the incentiviser’s efficacy did not differ meaningfully between surgery types within the limits of the pilot sample (
Table 5).
Secondary outcomes showed no statistically significant differences between groups. NRS scores decreased from 4.2 ± 1.7 to 2.0 ± 1.9 in the control group and from 4.2 ± 2.5 to 1.6 ± 1.4 in the intervention group (p = 0.48). Similarly, 6MWT distance improved from 45.9 ± 74.4 m to 181.1 ± 92.3 m in controls and from 35.4 ± 66.1 m to 206.2 ± 90.2 m in the intervention group (p = 0.51).
4. Discussion
As described in the Results, the observed effect for the primary outcome (Cohen’s d = 1.71) provided an achieved power of 99.8%, confirming that the study was adequately powered despite its pilot nature.
The current study addressed the longstanding controversy regarding the standardised implementation of partial weight-bearing protocols—a topic that has generated both patient and therapist apprehension. Our findings demonstrate that using a load incentiviser, as a biofeedback device, significantly improves patients’ perception and application of partial load during rehabilitation. Specifically, the study shows an immediate enhancement in load perception, as revealed by statistically significant differences between baseline (T0) and post-stimulation (T0 stimulus) assessments. This immediate improvement appears to be linked to an enhanced anticipatory system that better prepares the neuromuscular system to regulate weight distribution during gait.
Addressing this gap, the present study examines a “weight-bearing sensitivity enhancer” constructed from readily available, low-cost materials. This novel biofeedback device is designed to provide continuous tactile input during ambulation, effectively bridging the gap between static instruction and the dynamic realities of everyday movement. By integrating an intuitive feedback mechanism into the rehabilitation process, the device aims to facilitate more accurate reproduction of prescribed partial weight-bearing and mitigate the deleterious effects of imprecise load application [
1,
3]. Unlike many high-end commercial systems, this prototype promises an economical solution that is potentially applicable in both clinical settings and community or home-based rehabilitation programmes.
Tactile plantar feedback devices enhance partial weight-bearing accuracy following lower-limb surgery by activating cutaneous mechanoreceptors, particularly the slow-adapting type I (SA-I) and fast-adapting type I (FA-I) receptors. The SA-I mechanoreceptors, primarily associated with Merkel cells, provide sustained responses to static pressure and play a critical role in fine touch perception. At the same time, FA-I receptors respond dynamically to changes in stimulus, facilitating the perception of vibrations and transient mechanical changes underfoot [
21]. These mechanoreceptors convey sensory information via afferent pathways to the central nervous system, including the cerebellar-thalamic loop, which integrates input for proprioceptive and motor coordination [
22]. This integration is crucial for maintaining muscle stability and balance during movement, particularly following surgery when proprioceptive feedback may be compromised.
Implementing threshold-triggered tactile cues from feedback devices evokes rapid motor corrections that enhance weight distribution awareness without reliance on visual cues. Such devices deliver real-time somatosensory feedback, helping the central nervous system compensate for the visual–spatial ambiguity often experienced post-surgery [
23]. Research demonstrates that augmented somatosensory feedback, whether through vibrotactile insoles or electrotactile systems, significantly improves aspects of motor control, including balance and limb positioning, by providing immediate corrections to missteps or misalignment [
24,
25]. This enhances the brain’s capacity to process proprioceptive information swiftly, fostering better adaptation to altered sensory conditions and reducing the risk of falls [
26].
Recent studies have illustrated the efficacy of tactile feedback in clinical rehabilitation settings. For instance, tactile stimulation devices have been reported to improve gait and balance performance in older adults, reinforcing the importance of integrated sensory feedback during walking tasks [
26]. Moreover, studies indicate that tactile feedback can effectively recalibrate proprioceptive pathways, ultimately improving postural control in various populations, including those recovering from lower limb surgeries [
27,
28]. The synthesis of tactile inputs not only aids in physical rehabilitation but also helps build a more accurate internal model of body mechanics, encouraging adaptive motor strategies essential for successful recovery [
23].
The immediate impact observed in the experimental group is particularly notable when contrasted with the control group, which received conventional partial weight-bearing training without biofeedback support. The control group showed no significant improvements in the application of partial load, suggesting that traditional techniques may lack the dynamic feedback necessary to achieve precise weight-bearing. This observation aligns with previous research indicating that biofeedback-based interventions play a pivotal role in maintaining long-term compliance with weight-bearing prescriptions, as the real-time cueing allows patients to adjust their performance continuously throughout rehabilitation [
29,
30].
Furthermore, the data indicate that the beneficial effects of the load incentiviser persist over time. Such long-term retention of proper weight-bearing may be attributed to the repetitive, self-correcting nature of the biofeedback mechanism, which reinforces correct loading behaviours. In contrast, other rehabilitation modalities, which rely primarily on verbal instructions or static measures, have not demonstrated comparable sustained improvements. This sustained effect is especially relevant given research emphasising the importance of integrating wearable and real-time biofeedback devices in rehabilitation protocols [
7,
31].
An additional strength of the load incentiviser lies in its universal applicability across different age groups. Our study indicates that improvements in load perception were independent of patient age, demonstrating that both younger and older patients can benefit from such biofeedback technologies. This finding is significant since many patients requiring partial weight-bearing protocols are elderly, and previous studies have identified age-related challenges in complying with weight-bearing instructions [
30,
32]. The demonstrated efficacy of the incentiviser across age groups supports the potential of biofeedback to serve as an essential adjunct in rehabilitation for a heterogeneous patient population.
Beyond the objective measures of load application, the subjective benefits reported by patients further underscore the value of the load incentiviser. Participants consistently reported a heightened awareness of their weight-bearing patterns and an improved ability to transfer these learned strategies from the clinical setting to daily activities. This enhanced self-efficacy will contribute to overall patient confidence and adherence to the rehabilitation plan. In practical terms, using a load incentiviser facilitates safer limb loading, which is crucial for preventing complications in the post-surgical phase. It contributes to a more positive overall rehabilitation experience.
It is important to note that while secondary outcomes such as pain reduction and overall walking quantity did not significantly differ between groups, these results indicate that the primary benefits of biofeedback are specific to the modulation of weight distribution rather than general functional activity. In other words, the device is optimised for fine-tuning partial weight-bearing’s perceptual and motor control aspects without necessarily altering broader clinical parameters such as pain or activity level. This specificity aligns with the understanding that biofeedback devices primarily act at the neuromuscular and perceptual levels, reinforcing correct load application rather than inducing direct symptomatic relief [
33].
Pain intensity (NRS) and walking capacity (6MWT) improved in both groups, with no statistically or clinically significant between-group difference (NRS Δ = –0.4,
p = 0.46; 6MWT Δ = +25 m,
p = 0.62; Cohen’s d ≤ 0.28). This neutral finding is biologically plausible: the incentiviser is designed to optimise load accuracy rather than to modulate pain pathways or cardio-respiratory endurance, and the two-week follow-up is too short to translate biomechanical changes into functional gains. Comparable neutral results have been reported for load-biofeedback insole systems when pain and walking tests were evaluated within 4 weeks of surgery [
10]. Longer follow-up will be required to determine whether the reduced mechanical overload observed in the intervention group accelerates functional milestones or lowers complication rates.
Stratifying the primary outcome by surgery type revealed a consistent benefit of the tactile incentiviser across all diagnostic subgroups, with the largest absolute reductions in load error observed after total hip arthroplasty and femoral-shaft fracture fixation. These findings imply that tactile feedback is effective in prosthetic procedures, where postoperative pain may obscure load perception, and in fracture cases requiring strict load limitation to protect healing bone. The non-significant treatment-by-diagnosis interaction (p = 0.21) suggests that the mechanism of action is unlikely to depend on the surgical procedure itself; nevertheless, the wide confidence intervals in the smaller pelvic and ankle cohorts highlight the need for larger, diagnosis-focused trials or prospectively stratified randomisation. Such studies will clarify whether different load thresholds or feedback intensities should be tailored to specific clinical pathways.
Although both groups showed improvements in pain and walking distance, these secondary outcomes did not differ significantly between groups, suggesting that the observed benefit of the incentiviser was specific to weight-bearing control rather than overall functional recovery or analgesia.
The mean improvement of −10.8 kg reduced the average deviation to 2.0 ± 3.0 kg, bringing 88% of patients within the ±2 kg minimal clinically important difference (MCID) window defined by van Lieshout et al. (2016) [
29]. Deviations over 5 kg have been associated with delayed union and hardware failure in femoral and tibial fracture fixation and inferior early functional scores after hip arthroplasty [
8]. By restoring load accuracy to within the safety margin, the incentiviser is expected to reduce mechanical stress on the implant/bone interface and facilitate earlier progression to full weight bearing, both clinically relevant endpoints that cannot be captured by
p-values alone. Longer follow-up studies are required to verify whether the observed biomechanical benefit translates into fewer complications and faster functional milestones.
In evaluating the primary outcome of our pilot trial, which demonstrated a significant reduction in load error at discharge using plantar tactile feedback in post-operative orthopaedic patients, it is essential to compare these findings with methodologies from similar studies. For instance, the work by Josipović provides insights into in-shoe biofeedback devices that improve compliance in partial weight-bearing tasks in post-operative orthopaedic patients, supporting the efficacy of biofeedback in enhancing rehabilitation outcomes [
34]. Additionally, Argent et al. conducted a clinical trial assessing wearable sensor-based biofeedback in orthopaedic rehabilitation, which reported improvements in exercise adherence and patient outcomes during the early inpatient phase post-surgery [
35]. Both studies advocate for the importance of timely and adequate biofeedback interventions in achieving rehabilitation goals within the critical early phase post-surgery [
34,
35].
Our study’s outcome of −10.8 kg reflects a substantial reduction; however, it is essential to recognise that while prior studies reported enhancements in outcomes, they did not specify clear numerical reductions in load error that could be directly compared to our quantified metric, thereby underscoring our study’s contribution to the literature [
34,
35]. Our findings add to the growing body of evidence advocating for biofeedback as a crucial adjunct in rehabilitating post-operative orthopaedic patients, suggesting further exploration into optimising feedback modalities to maximise patient recovery.
Because the incentiviser is mechanical, material costs are <EUR 5 per pair, and no batteries, electronics, or maintenance contracts are required. Fabrication can be delegated to the orthotic workshop or physiotherapy aide; fitting and static calibration take <10 min once staff receive a brief (≈1 h) hands-on tutorial covering threshold adjustment and patient education. Integration into routine practice is simplest during the first assisted ambulation session, replacing the “bathroom-scale” drill that most wards already perform but rarely repeat. Anticipated barriers include limited shoe space in patients with rigid post-operative dressings, nurses’ concerns over skin irritation, and the absence of a reimbursement code; all can be mitigated by stocking three insole sizes, adding a thin felt cover for fragile skin, and incorporating the device within the standard physiotherapy tariff.
Furthermore, the multifaceted approach of the study acknowledges the limitations inherent in previous research. Past investigations have often been constrained by small, heterogeneous patient populations and short-term follow-up periods, thereby limiting the generalizability of their findings to diverse clinical contexts [
4,
6]. Our study seeks to overcome these barriers by providing a more extended evaluation of the weight-bearing sensitivity enhancer’s performance over a longer duration and across a broader spectrum of lower limb pathologies. By doing so, we hope to offer stronger evidence regarding the long-term benefits of continuous biofeedback in enhancing partial weight-bearing accuracy and accelerating postoperative recovery.
This investigation was conceived as a pilot, single-centre trial, and the sample size, although adequately powered for the primary outcome, was too small to detect diagnosis-specific effects, infrequent adverse events, or longer-term clinical sequelae. Participants were recruited from a single orthopaedic ward with relatively homogeneous postoperative protocols; therefore, the findings may not generalise to other rehabilitation settings, patients with substantial comorbidities, or those managed without continuous professional supervision. Outcomes were collected only at discharge (~14 days), so the durability of the load-accuracy improvement and its impact on gait symmetry, pain, and bone healing beyond the inpatient phase remain unknown. Because patients and therapists cannot be blinded to allocation, behavioural and placebo effects, or greater therapist attention in the intervention arm, they cannot be completely ruled out. Finally, the mix of surgical indications (arthroplasty, internal fixation, osteotomy) introduces clinical heterogeneity that may have diluted or inflated the observed treatment effect. Future multicentre trials with longer follow-up and stratified analysis are needed to confirm these results and to evaluate cost-effectiveness and adherence in home-based use.
A further limitation is the absence of placebo control conditions. Although outcome assessors were blinded, patients and therapists were aware of device allocation, and the additional tactile stimulus may have enhanced attention or motivation independently of load accuracy. Future trials should incorporate a sham insole with inert buttons to control expectation and contact time. Another limitation of the study is the lack of clinical outcome data.
In summary, integrating an accessible, low-cost biofeedback device into rehabilitation protocols represents a promising evolution in the management of partial weight-bearing. This study is positioned within an emerging body of the literature that increasingly recognises the limitations of traditional static methods and advocates for dynamic, real-time solutions that improve load perception and enhance overall functional recovery. The extended evaluation and detailed analysis presented herein aim to lay the groundwork for future research and contribute to the standardisation of innovative, economically feasible rehabilitation strategies for patients recovering from lower limb surgery.