Mechanical Properties of Carbonation-Enhanced Alkali-Activated Slag-Solidified Shield Muck: Temperature–Humidity Coupling Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Shield Muck
2.1.2. Ground Granulated Blast Furnace Slag
2.1.3. Alkali Activator
2.2. Test Schemes
2.3. Experiment Test Method
2.3.1. Compressive Strength Testing
2.3.2. Porosity of the Carbonated Zone
2.3.3. Pore Solution PH Analysis
2.3.4. Qualitative Analysis of Carbonation Products
2.3.5. Quantitative Analysis of Carbonation Products
2.3.6. Thermogravimetric
2.3.7. Microstructural Morphology of Carbonation Zones
3. Results and Discussion
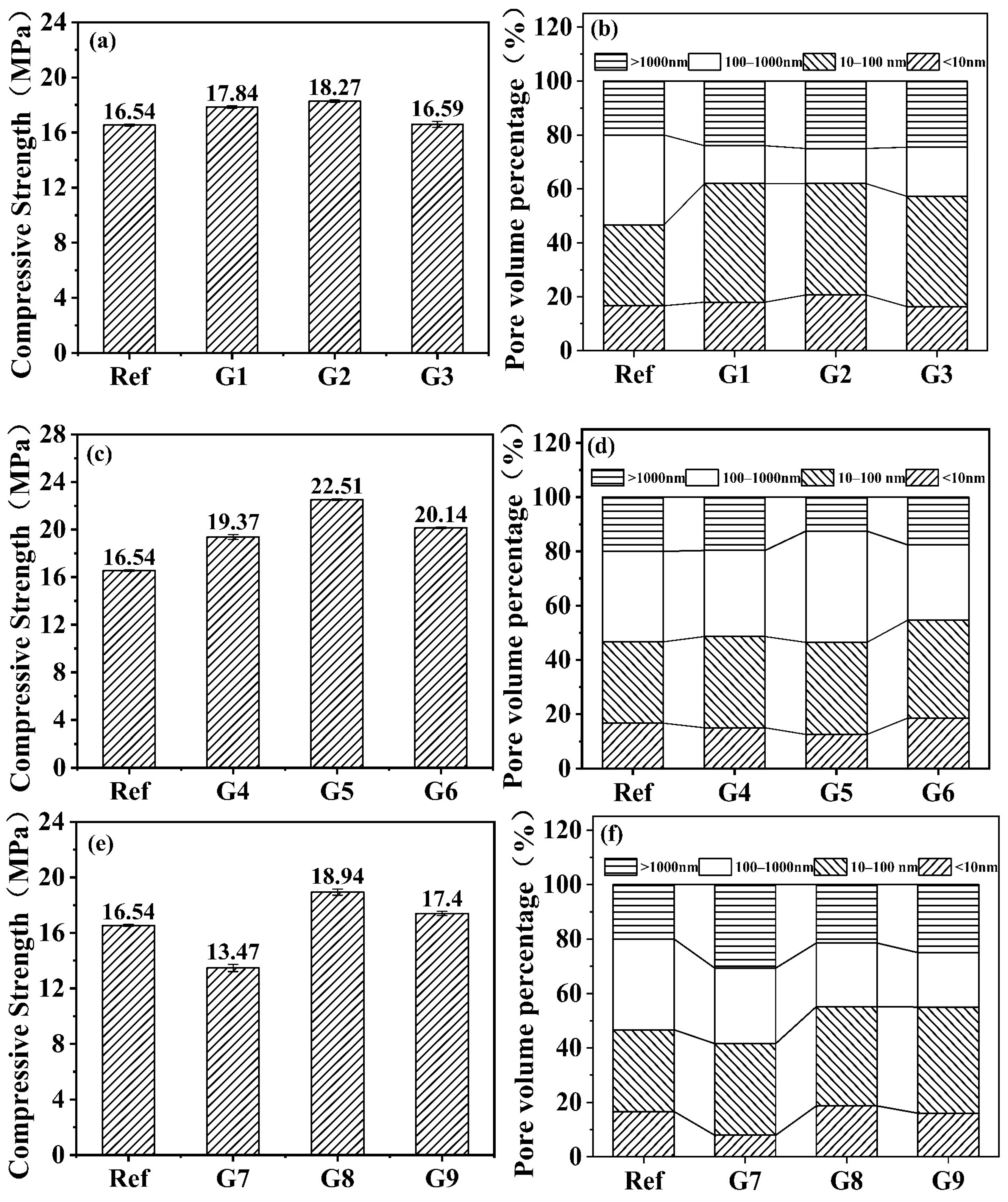
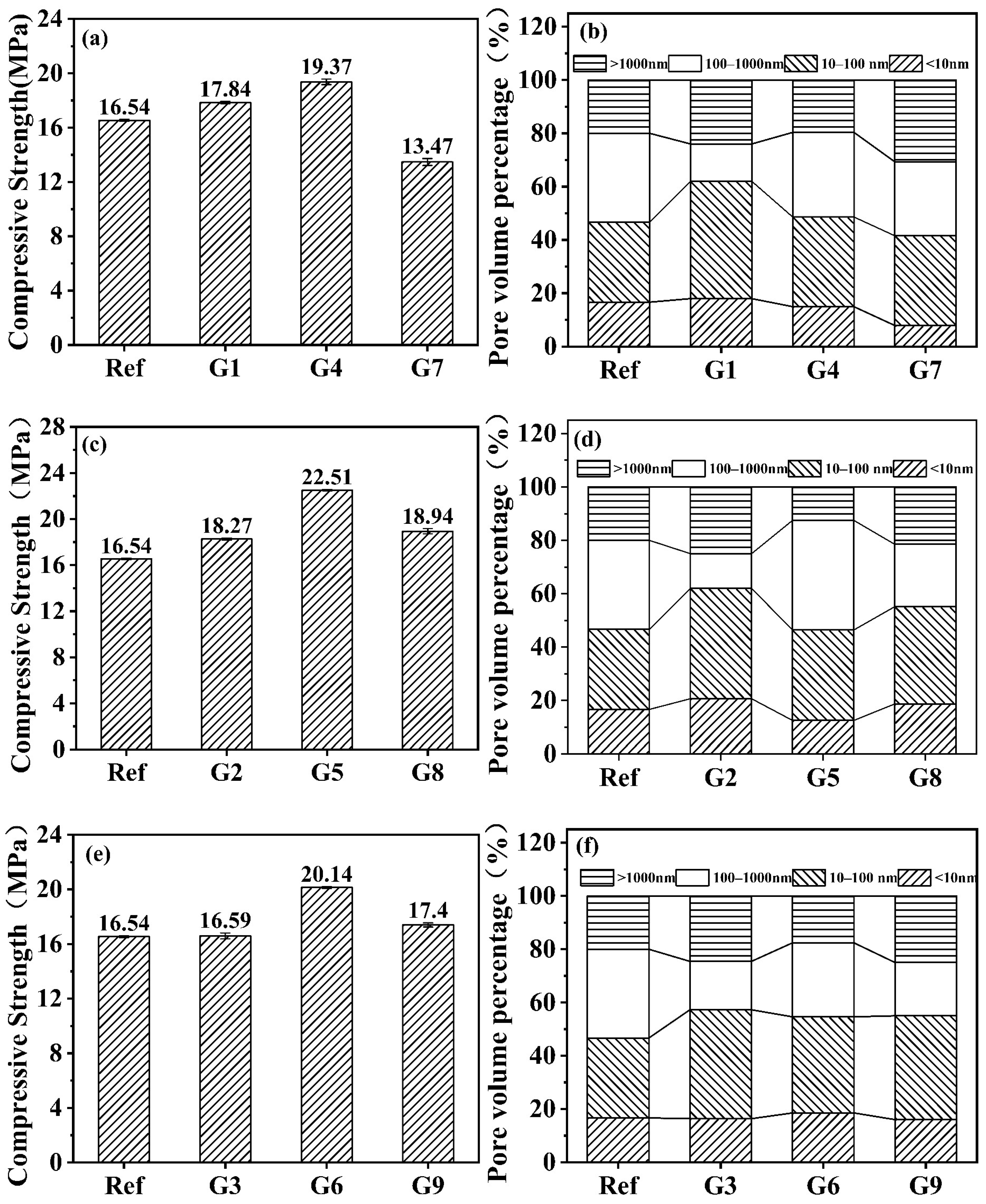
3.1. Compressive Strength and Pore Distribution
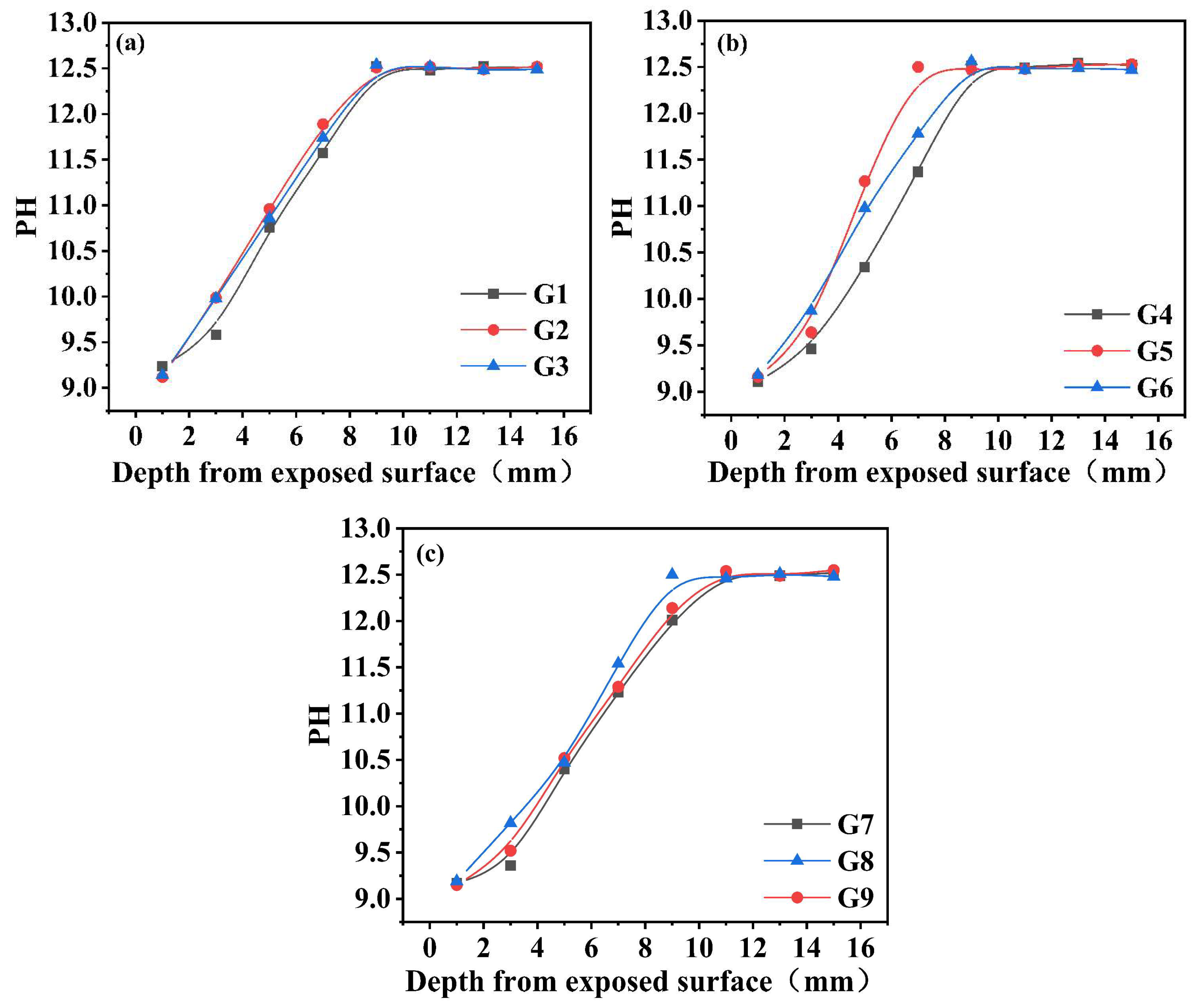
3.2. Alkaline Environment Evolution
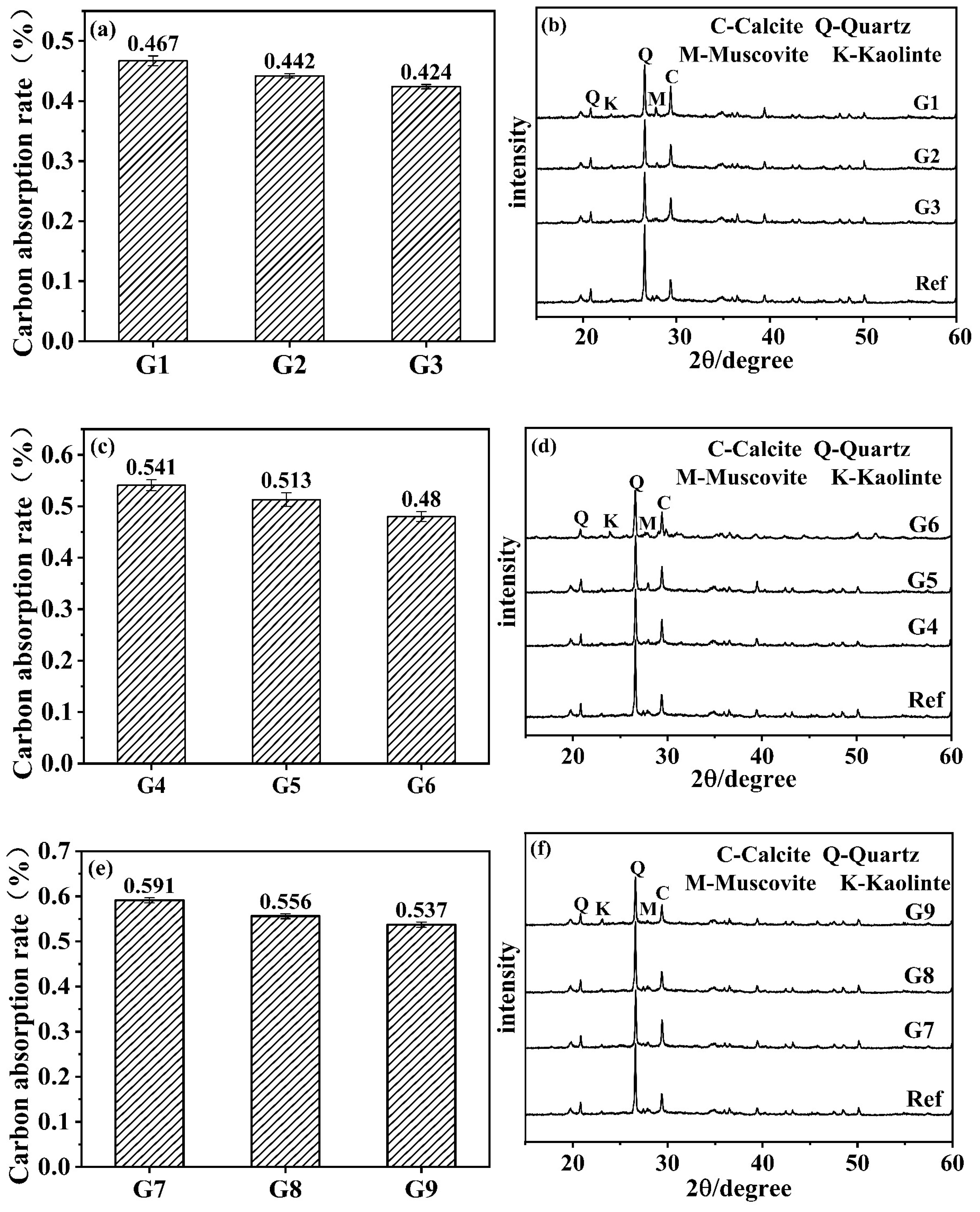
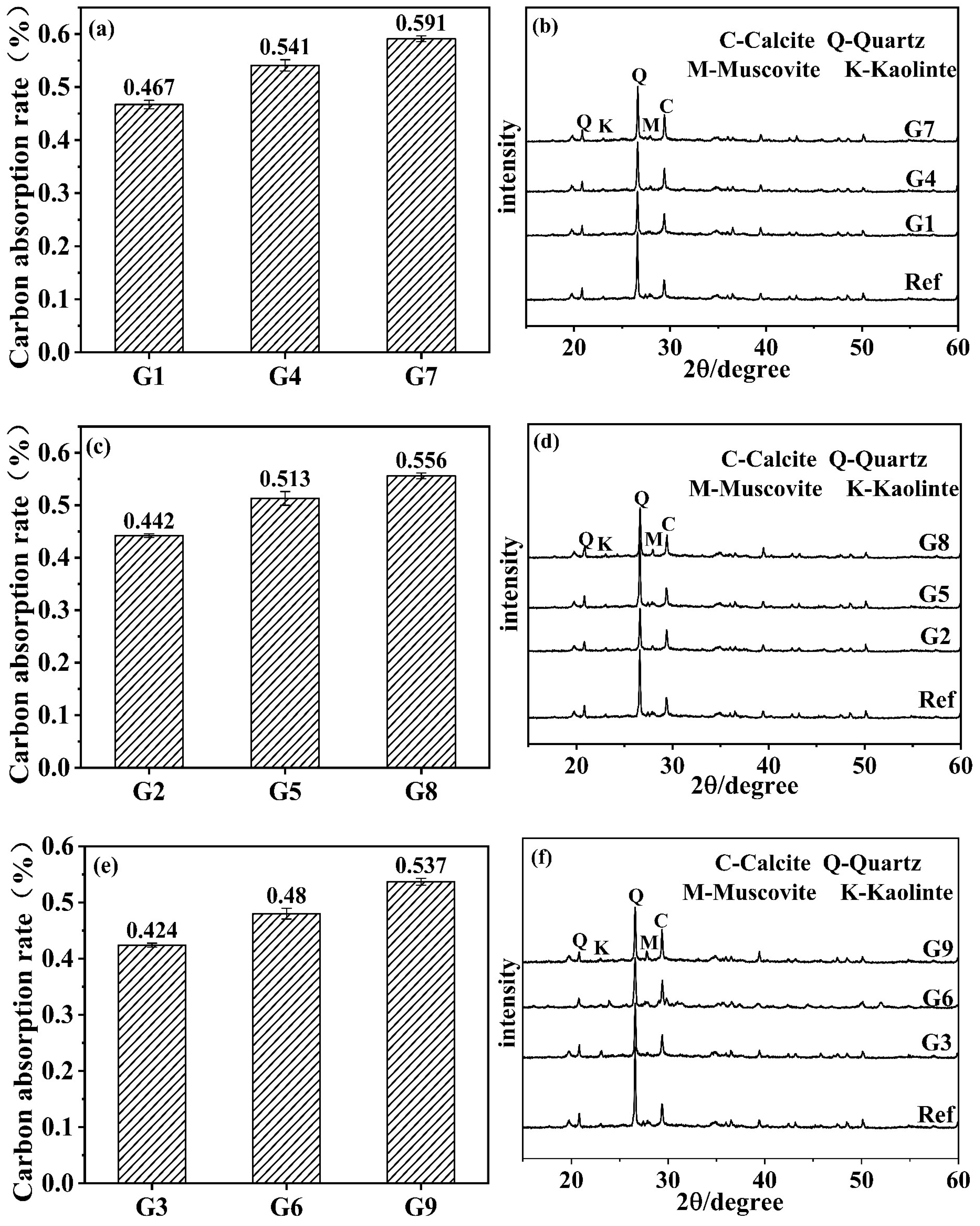
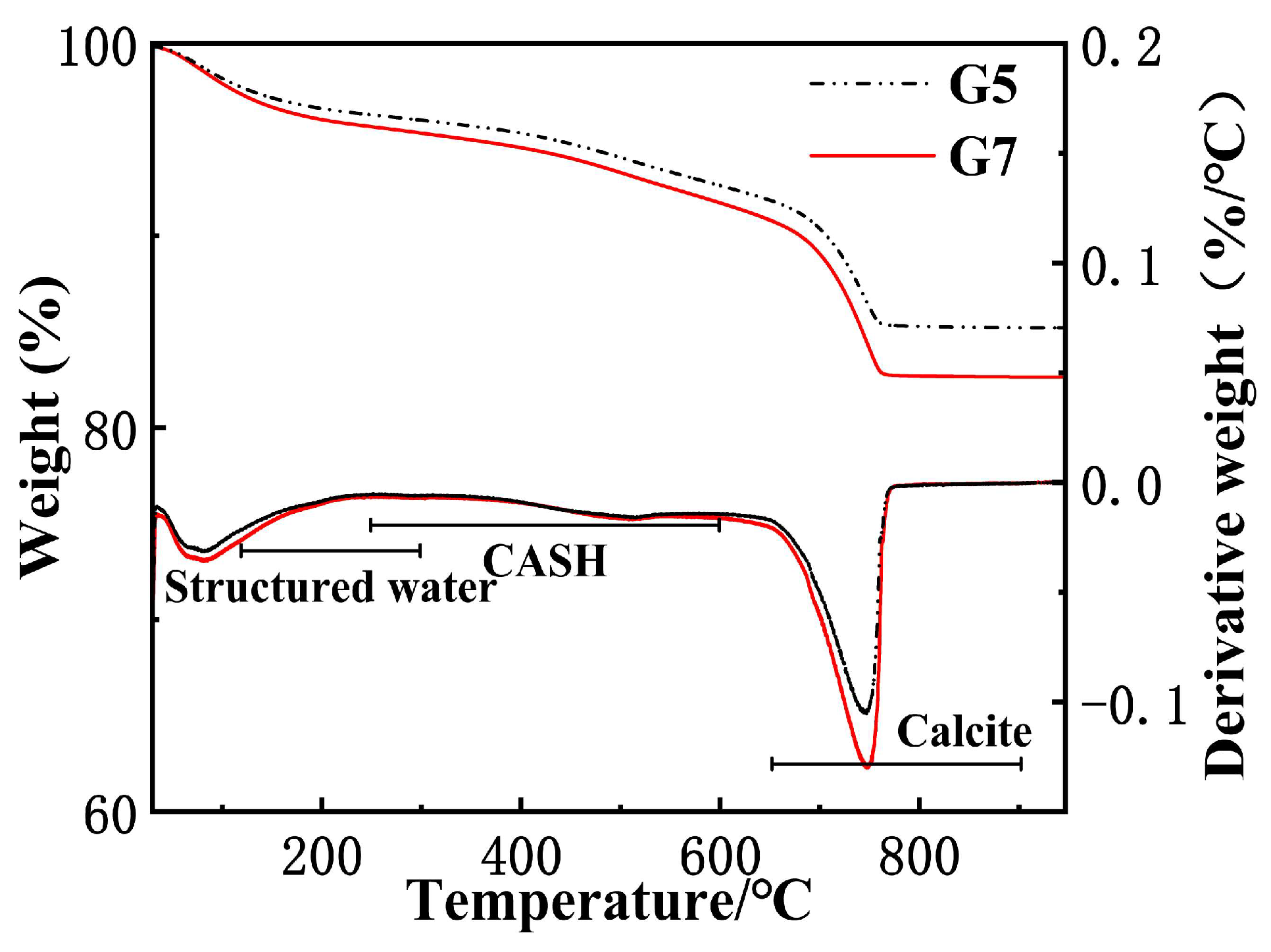
3.3. Carbonation Product Characterization
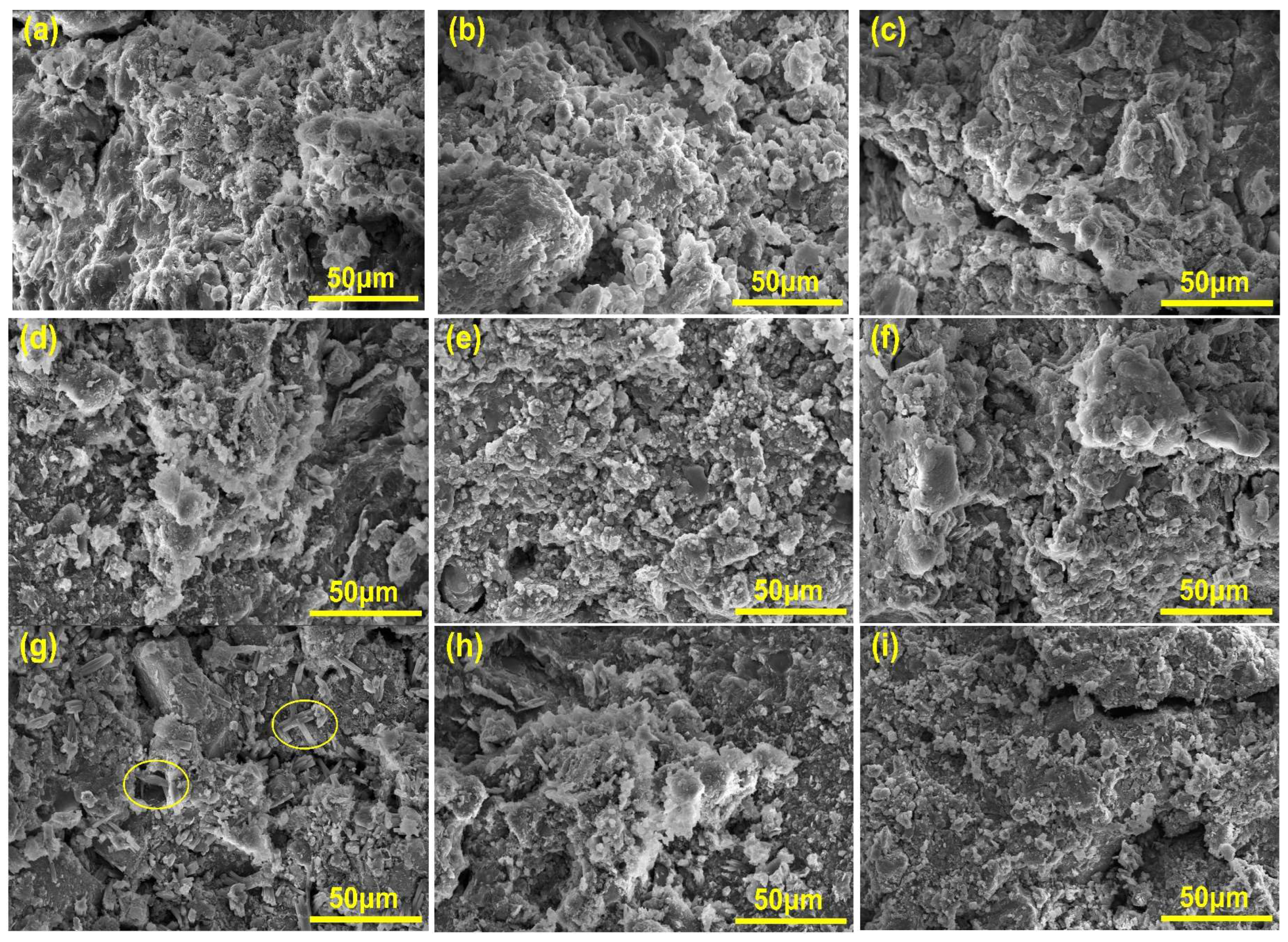
3.4. Microscopic Test Analysis
4. Conclusions
- (1)
- The proportion of harmful pores (>1000 nm) exhibited a highly significant negative correlation with compressive strength, while pores <1000 nm showed a weaker positive correlation. Reducing harmful pore content constitutes the key to improving mechanical performance in alkali-activated slag shield machine muck solidified bodies;
- (2)
- The 28-day compressive strength demonstrated a distinct initial increase followed by a decrease across the tested temperature–humidity gradient range. The maximum strength occurred at 20 °C/70% RH, showing a 36.1% enhancement when compared with standard curing, fully confirming that controlled temperature–humidity conditions during carbonation curing can effectively improve mechanical properties of alkali-activated slag shield machine muck solidified bodies;
- (3)
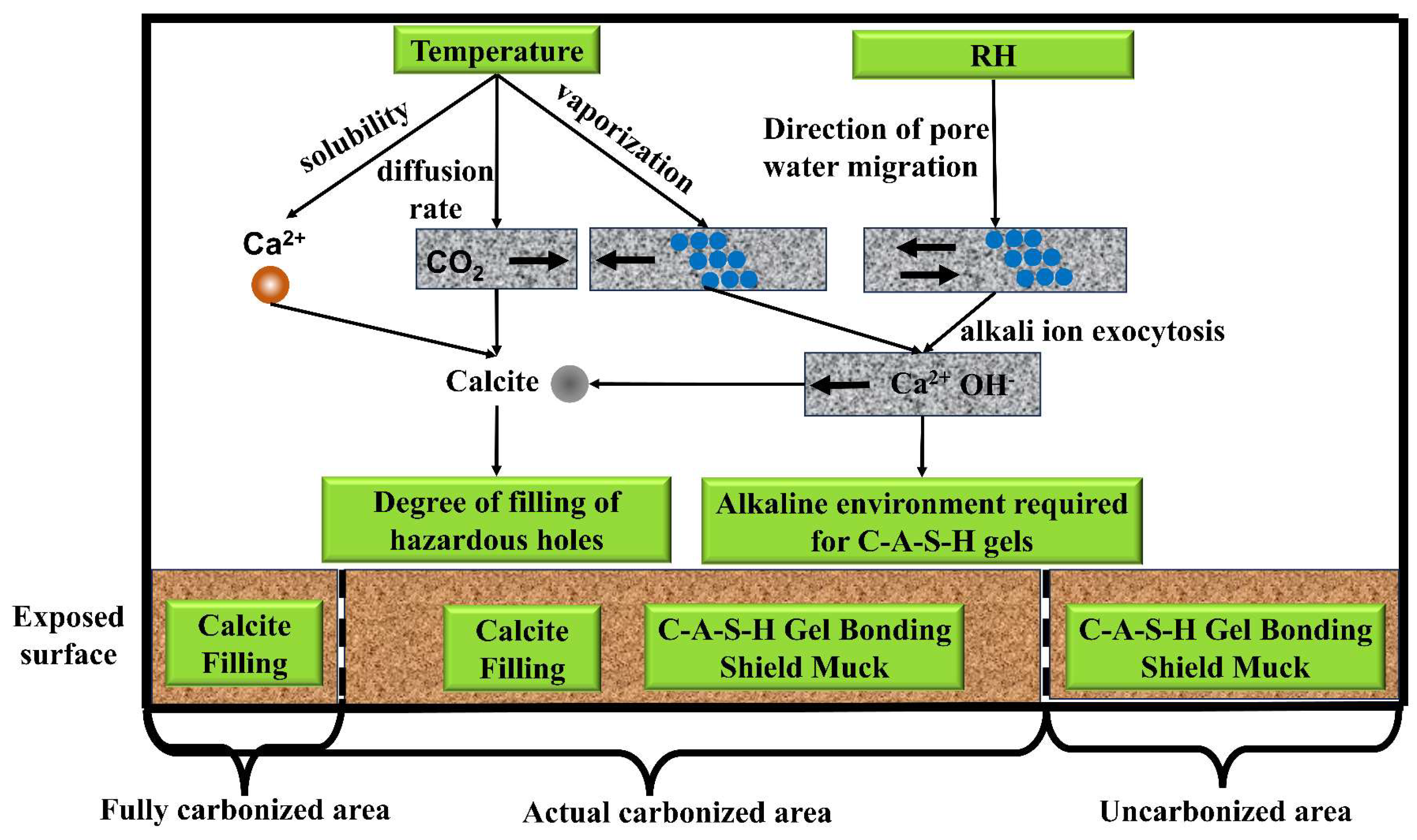
- The temperature–humidity conditions during carbonation curing showed negligible but measurable effects on the alkaline environment of pore solutions. These effects primarily manifested through humidity-controlled pore solution dilution and ion migration processes, as well as temperature-regulated Ca2+ dissolution and pore solution evaporation;
- (4)
- The carbon absorption rates gradually increased with a rising temperature and decreasing humidity, indicating intensified carbonation reactions. The crystalline carbonation product was identified as calcite, while the hydration product remained the C-A-S-H gel phase. Under high-temperature/low-humidity conditions, an accelerated decomposition of C-A-S-H gel through carbonation promoted continuous growth and a coarsening of calcite crystals, leading to non-uniform deposition. This ineffective filling of harmful pores ultimately degraded mechanical performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.; Li, W.; Yang, F.; Cao, T.; Wu, Z.; Lu, Y.; Wu, C. Study on Resourceful Treatment and Carbon Reduction Intensity of Metro Shield Slag: An Example of a Tunnel Interval of Shenzhen Line 13. Buildings 2023, 13, 2816. [Google Scholar] [CrossRef]
- Wu, S.; Shi, J.; Wu, J.; Li, L.; Jin, H. Risk Factor Recognition of Shield Tunnel Crossing Underneath the Existing Subway Tunnel. IOP Conf. Ser. Earth Environ. Sci. 2021, 676, 012134. [Google Scholar] [CrossRef]
- Wu, Z.; Ye, C.; Cao, F. Performance and Microstructure of Grouting Materials Made from Shield Muck. Materials 2024, 17, 4074. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, M.; Zhou, Q.; Wang, B.; Wei, W.; Chen, J. On recycling earth pressure balance shield muck with residual foaming agent: Defoaming and antifoaming investigations. Environ. Sci. Pollut. Res. 2024, 31, 8046–8060. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wu, S.; Qin, J.; Li, X.; Liu, X.; Zhang, Y.; Mao, J.; Nie, W. Multiscale mechanical characterizations of ultrafine tailings mixed with incineration slag. Front. Earth Sci. 2023, 11, 1123529. [Google Scholar] [CrossRef]
- Mustafa Al Bakri, A.M.; Muhammad Faheem, M.T.; Sandhu, A.V.; Alida, A.; Mohd Salleh, M.A.A.; Ruzaidi, C.M. Microstructure Studies on Different Types of Geopolymer Materials. Appl. Mech. Mater. 2013, 421, 384–389. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Pham, V.T.H.Q.; Dang, T.P.; Dao, T.K. Leachability of heavy metals in geopolymer-based materials synthesized from red mud and rice husk ash. AIP Conf. Proc. 2018, 1954, 040014. [Google Scholar]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Majidi, B. Geopolymer technology, from fundamentals to advanced applications: A review. Mater. Technol. 2009, 24, 79–87. [Google Scholar] [CrossRef]
- Beltrame, N.A.M.; Dias, R.L.; Witzke, F.B.; Medeiros-Junior, R.A. Effect of carbonation curing on the physical, mechanical, and microstructural properties of metakaolin-based geopolymer concrete. Constr. Build. Mater. 2023, 406, 133403. [Google Scholar] [CrossRef]
- Ye, K.; Yang, L.; Zeng, J.; Tian, J.; Zeng, F.; Ye, G.; Mu, Y. Enhancing the strength of carbonated γ-dicalcium silicate by internal carbonation curing. Constr. Build. Mater. 2025, 469, 140512. [Google Scholar] [CrossRef]
- Shang, X.; Chen, Y.; Qi, Y.; Chang, J.; Yang, J.; Qu, N. Comparative life cycle environmental assessment of recycled aggregates concrete blocks using accelerated carbonation curing and traditional methods. Constr. Build. Mater. 2023, 404, 133207. [Google Scholar] [CrossRef]
- Duan, S.; Liao, H.; Cheng, F.; Tao, M. Effect of curing condition and carbonization enhancement on mechanical properties of fly ash -desulfurization gypsum—Steel slag blocks. J. CO2 Util. 2020, 38, 282–290. [Google Scholar] [CrossRef]
- Oke, J.A.; Abuel-Naga, H. Ultrasonic Non-Destructive Testing of Accelerated Carbonation Cured-Eco-Bricks. Appl. Sci. 2024, 14, 8954. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Song, L.; Qu, Z.; Wang, H. Influence of Carbon Dioxide Curing on the Corrosion Resistance of Reinforced Cement Mortar under the External Erosion of NaCl Freeze–Thaw Cycle. Appl. Sci. 2022, 12, 5061. [Google Scholar] [CrossRef]
- Muthu, M.; Sadowski, Ł. Evaluation of the Performance of Pervious Concrete Inspired by CO2-Curing Technology. Appl. Sci. 2024, 14, 4202. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Z.; Peng, S.; Liu, J.; Wu, Q.; Xu, X. State-of-the-art review of geopolymer concrete carbonation: From impact analysis to model establishment. Case Stud. Constr. Mater. 2024, 20, e03124. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Wong, L.S. Durability Performance of Geopolymer Concrete: A Review. Polymers 2022, 14, 868. [Google Scholar] [CrossRef]
- Li, Z.; Li, S. Carbonation resistance of fly ash and blast furnace slag based geopolymer concrete. Constr. Build. Mater. 2018, 163, 668–680. [Google Scholar] [CrossRef]
- Çelikten, S.; Sarıdemir, M.; Özgür Deneme, İ. Mechanical and microstructural properties of alkali-activated slag and slag + fly ash mortars exposed to high temperature. Constr. Build. Mater. 2019, 217, 50–61. [Google Scholar] [CrossRef]
- Ishida, T.; Maekawa, K.; Kishi, T. Enhanced modeling of moisture equilibrium and transport in cementitious materials under arbitrary temperature and relative humidity history. Cem. Concr. Res. 2007, 37, 565–578. [Google Scholar] [CrossRef]
- Leemann, A.; Moro, F. Carbonation of concrete: The role of CO2 concentration, relative humidity and CO2 buffer capacity. Mater. Struct. 2017, 50, 30. [Google Scholar] [CrossRef]
- Ren, P.; Wang, A.; Mo, K.H.; Ling, T.C. Fast-Hardening Production of High-Strength BOFS Aggregates by High-Temperature Carbonation Curing. ACS Sustain. Chem. Eng. 2024, 12, 13587–13597. [Google Scholar] [CrossRef]
- You, N.; Zhu, G.; He, T.; Zhang, Y. Effect of Predrying Temperature on Carbonation of Alkali-Activated Slag Pastes. J. Mater. Civ. Eng. 2022, 34, 04022091. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Guo, Z.; Hong, J.; Wang, F. Strengthening mechanism of polyvinyl alcohol fibers on mechanical properties of geopolymer concrete subjected to a wet-hot-salt environment. Polym. Test. 2023, 127, 108199. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Fernandezjimenez, A. Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S.; Student, P.D. Durability performance of precast fly ash–based geopolymer concrete under atmospheric exposure conditions. J Mater Civ Eng. 2018, 30, 04018007. [Google Scholar] [CrossRef]
- Dheilly, R.M.; Tudo, J.; Sebaïbi, Y.; Quéneudec, M. Influence of storage conditions on the carbonation of powdered Ca(OH)2. Constr. Build. Mater. 2002, 16, 155–161. [Google Scholar] [CrossRef]
- Beruto, D.T.; Botter, R. Liquid-like H2O adsorption layers to catalyze the Ca(OH)2/CO2 solid–gas reaction and to form a non-protective solid product layer at 20 °C. J. Eur. Ceram. Soc. 2000, 20, 497–503. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Daval, D.; Chiriac, R.; Renard, F. Growth of Nanosized Calcite through Gas−Solid Carbonation of Nanosized Portlandite under Anisobaric Conditions. Cryst. Growth Des. 2010, 10, 4823–4830. [Google Scholar] [CrossRef]
- Yang, S.; Gu, M.; Lin, H.; Gong, Y. Property Improvement of Recycled Coarse Aggregate by Accelerated Carbonation Treatment under Different Curing Conditions. Sustainability 2023, 15, 4908. [Google Scholar] [CrossRef]
- Morshed, A.Z.; Shao, Y. Optimized process window for fresh concrete carbonation curing. Can. J. Civ. Eng. 2014, 41, 986–994. [Google Scholar] [CrossRef]
- Nisar, N.; Bhat, J.A. Effect of Rice Husk Ash on the Carbonation Depth of Concrete under Different Curing Ages and Humidity Levels. J. Mater. Civ. Eng. 2021, 33, 04020427. [Google Scholar] [CrossRef]
- Jun, Y.; Bae, Y.H.; Yim, H.J. Setting evaluation of cement mortar using nondestructive methods under different curing conditions: Temperatures and relative humidities. Mater. Struct. 2023, 56, 43. [Google Scholar] [CrossRef]
- Liu, B.; Luo, G.; Xie, Y. Effect of curing conditions on the permeability of concrete with high volume mineral admixtures. Constr. Build. Mater. 2018, 167, 359–371. [Google Scholar] [CrossRef]
- Malladi, R.C.; Selvaraj, T. Influence of relative humidity on carbon sequestration and crystal morphology of air lime: A sustainable building material. Mater. Lett. 2024, 370, 136838. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Li, Z.; Shi, H.; Ye, G. Effect of curing condition on mechanical properties and durability of alkali-activated slag mortar. Constr. Build. Mater. 2024, 439, 137376. [Google Scholar] [CrossRef]
- Liu, T.; Wang, H.; Zou, D.; Long, X.; Miah, M.J.; Li, Y. Strength recovery of thermally damaged high-performance concrete subjected to post-fire carbonation curing. Cem. Concr. Compos. 2023, 143, 105273. [Google Scholar] [CrossRef]
- Lei, X.; Yu, H.; Feng, P.; Ling, T.C. Flue gas carbonation curing of steel slag blocks: Effects of residual heat and water vapor. Constr. Build. Mater. 2023, 384, 131330. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J.; Sahu, S. Phase evolution and strength development during carbonation of low-lime calcium silicate cement (CSC). Constr. Build. Mater. 2019, 210, 473–482. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, K.; Chen, L.; Yuan, Q. Review on accelerated carbonation of calcium-bearing minerals: Carbonation behaviors, reaction kinetics, and carbonation efficiency improvement. J. Build. Eng. 2024, 86, 108826. [Google Scholar] [CrossRef]
- Cepcianska, J.; Dragomirova, J.; Kuzielova, E.; Zemlicka, M.; Palou, M.T. Impact of the curing conditions and carbon dioxide ingress on heavyweight concrete. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1205, 012007. [Google Scholar] [CrossRef]
- BS 7755-3-1-1994; Soil Quality. Chemical Methods. Determination of Dry Matter and Water Content on a Mass Basis by a Gravimetric Method. BSI Group: Kuala Lumpur, Malaysia, 1994.
- ASTM C39/C39M-24; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2024.
- Xu, Y.; Liang, X.; Wan, C.; Yang, H.; Feng, X. Carbonation and related behaviors of hardened cement pastes under different hydration degrees. Cem. Concr. Compos. 2023, 140, 105079. [Google Scholar] [CrossRef]
- Yu, J.; Qian, J.; Tang, J.; Ji, Z.; Fan, Y. Effect of ettringite seed crystals on the properties of calcium sulphoaluminate cement. Constr. Build. Mater. 2019, 207, 249–257. [Google Scholar] [CrossRef]
- Nedeljković, M.; Ghiassi, B.; Van Der Laan, S.; Li, Z.; Ye, G. Effect of curing conditions on the pore solution and carbonation resistance of alkali-activated fly ash and slag pastes. Cem. Concr. Res. 2019, 116, 146–158. [Google Scholar] [CrossRef]
- McCaslin, E.R.; White, C.E. A parametric study of accelerated carbonation in alkali-activated slag. Cem. Concr. Res. 2021, 145, 106454. [Google Scholar] [CrossRef]
- Leemann, A.; Münch, B.; Lothenbach, B.; Bernard, E.; Trottier, C.; Winnefeld, F.; Sanchez, L. Alkali-carbonate reaction in concrete—Microstructural consequences and mechanism of expansion. Cem. Concr. Res. 2025, 195, 107903. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z. Carbonation induced phase evolution in alkali-activated slag/fly ash cements: The effect of silicate modulus of activators. Constr. Build. Mater. 2019, 223, 566–582. [Google Scholar] [CrossRef]
- Georget, F.; Soja, W.; Scrivener, K.L. Characteristic lengths of the carbonation front in naturally carbonated cement pastes: Implications for reactive transport models. Cem. Concr. Res. 2020, 134, 106080. [Google Scholar] [CrossRef]
- Zhang, P.; Lewis, J.B.; Klein-BenDavid, O.; Garrabrants, A.C.; Delapp, R.; Van Der Sloot, H.A.; Kosson, D.S. The role of environmental conditions on the carbonation of an alkali-activated cementitious waste form. Cem. Concr. Res. 2022, 151, 106645. [Google Scholar] [CrossRef]
- Rudić, O.; Grengg, C.; Seyrek, Y.; Steindl, F.; Müller, B.; Zögl, I.; Wohlmuth, D.; Ukrainczyk, N.; Mittermayr, F. Drying shrinkage and carbonation of steel slag-metakaolin alkali-activated composites: Effect of vegetable oil addition and slag aggregates. Cem. Concr. Res. 2025, 189, 107764. [Google Scholar] [CrossRef]
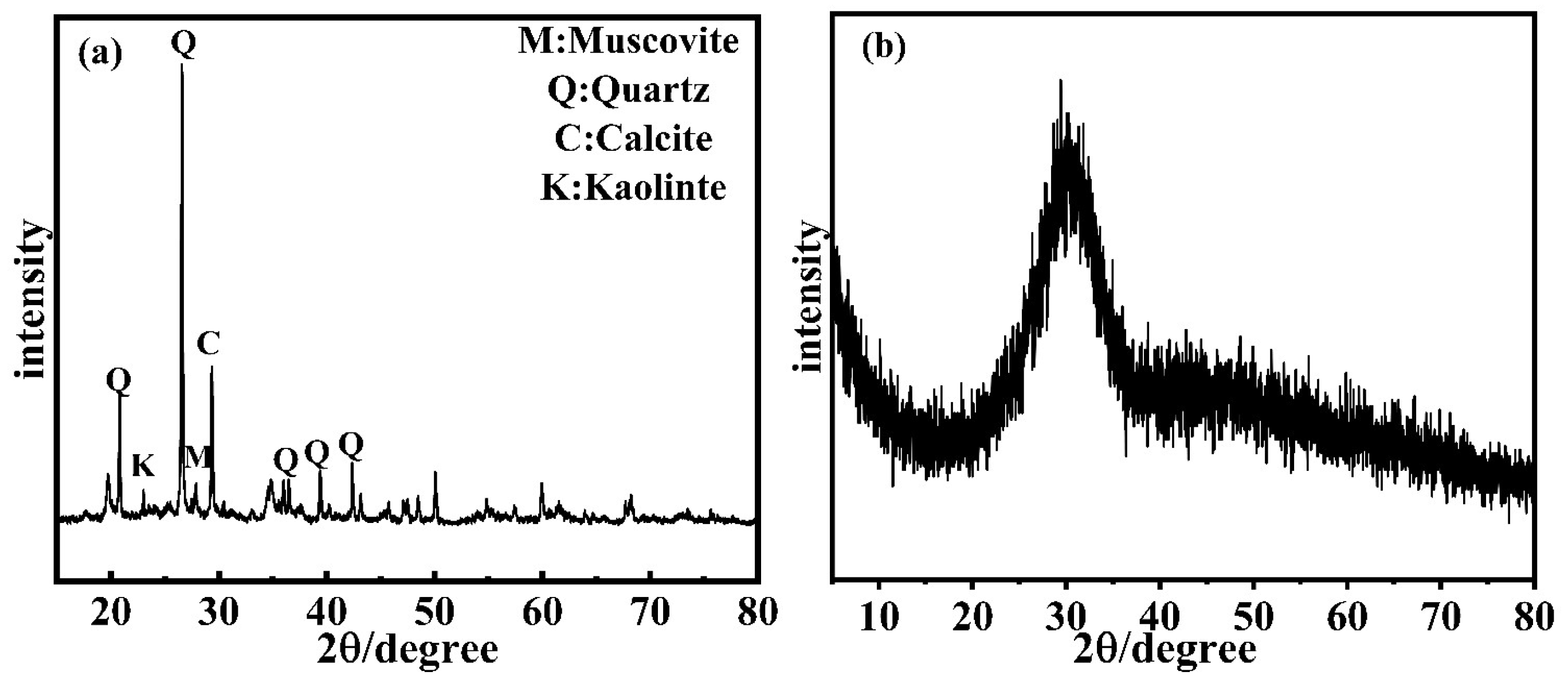
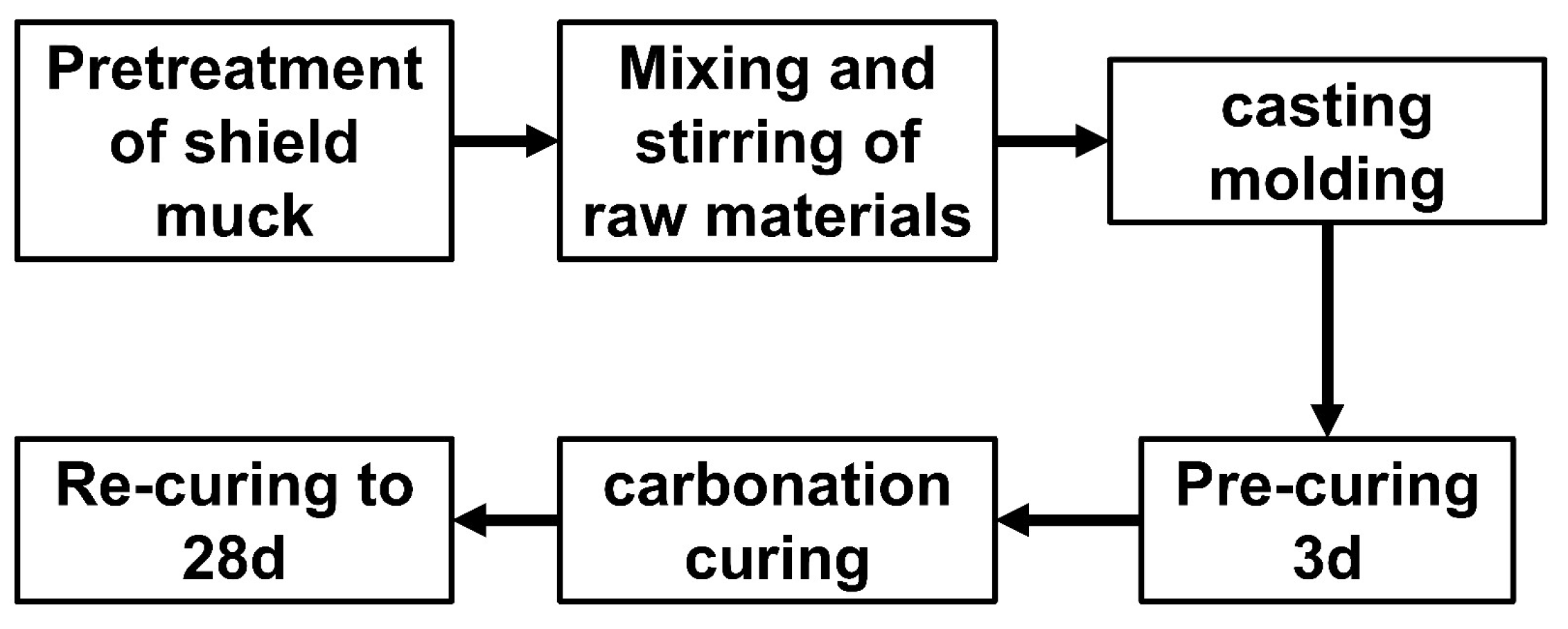


| Materials | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | TiO2 | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|---|
| Shield muck | 13.84 | 48.18 | 14.10 | 3.90 | 0.01 | 3.42 | 0.65 | 2.55 | 0.45 |
| GGBFS | 41.32 | 30.64 | 15.04 | 0.41 | 2.68 | 6.24 | 2.31 | 0.40 | 0.41 |
| Group | Temperature (℃) | Relative Humidity (%RH) | CO2 Concentration (vol%) | Carbonation Duration (h) |
|---|---|---|---|---|
| G1 | 10 | 60 | 20 | 24 |
| G2 | 70 | |||
| G3 | 80 | |||
| G4 | 20 | 60 | ||
| G5 | 70 | |||
| G6 | 80 | |||
| G7 | 30 | 60 | ||
| G8 | 70 | |||
| G9 | 80 |
| SM/g | GGBFS/g | NaOH/g | Na2SiO3/g | Water/g |
|---|---|---|---|---|
| 1300 | 409.5 | 14.94 | 32.74 | 506 |
| Group | 120–300 °C | 250–600 °C | 650–900 °C |
|---|---|---|---|
| G5 | 1.781 | 3.97 | 8.199 |
| G7 | 1.599 | 3.685 | 10.927 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Hu, S.; Li, Y.; Xi, Z.; Qian, J.; Yuan, B. Mechanical Properties of Carbonation-Enhanced Alkali-Activated Slag-Solidified Shield Muck: Temperature–Humidity Coupling Effects. Appl. Sci. 2025, 15, 5717. https://doi.org/10.3390/app15105717
Wang K, Hu S, Li Y, Xi Z, Qian J, Yuan B. Mechanical Properties of Carbonation-Enhanced Alkali-Activated Slag-Solidified Shield Muck: Temperature–Humidity Coupling Effects. Applied Sciences. 2025; 15(10):5717. https://doi.org/10.3390/app15105717
Chicago/Turabian StyleWang, Kejian, Shuangyu Hu, Ying Li, Zhiqin Xi, Jianwei Qian, and Bo Yuan. 2025. "Mechanical Properties of Carbonation-Enhanced Alkali-Activated Slag-Solidified Shield Muck: Temperature–Humidity Coupling Effects" Applied Sciences 15, no. 10: 5717. https://doi.org/10.3390/app15105717
APA StyleWang, K., Hu, S., Li, Y., Xi, Z., Qian, J., & Yuan, B. (2025). Mechanical Properties of Carbonation-Enhanced Alkali-Activated Slag-Solidified Shield Muck: Temperature–Humidity Coupling Effects. Applied Sciences, 15(10), 5717. https://doi.org/10.3390/app15105717






