Monodisperse Generation of Fragrance-Loaded Microcapsules with Hydrophilic Polymer Shells Using Microfluidic Devices
Abstract
1. Introduction
2. Results and Discussion
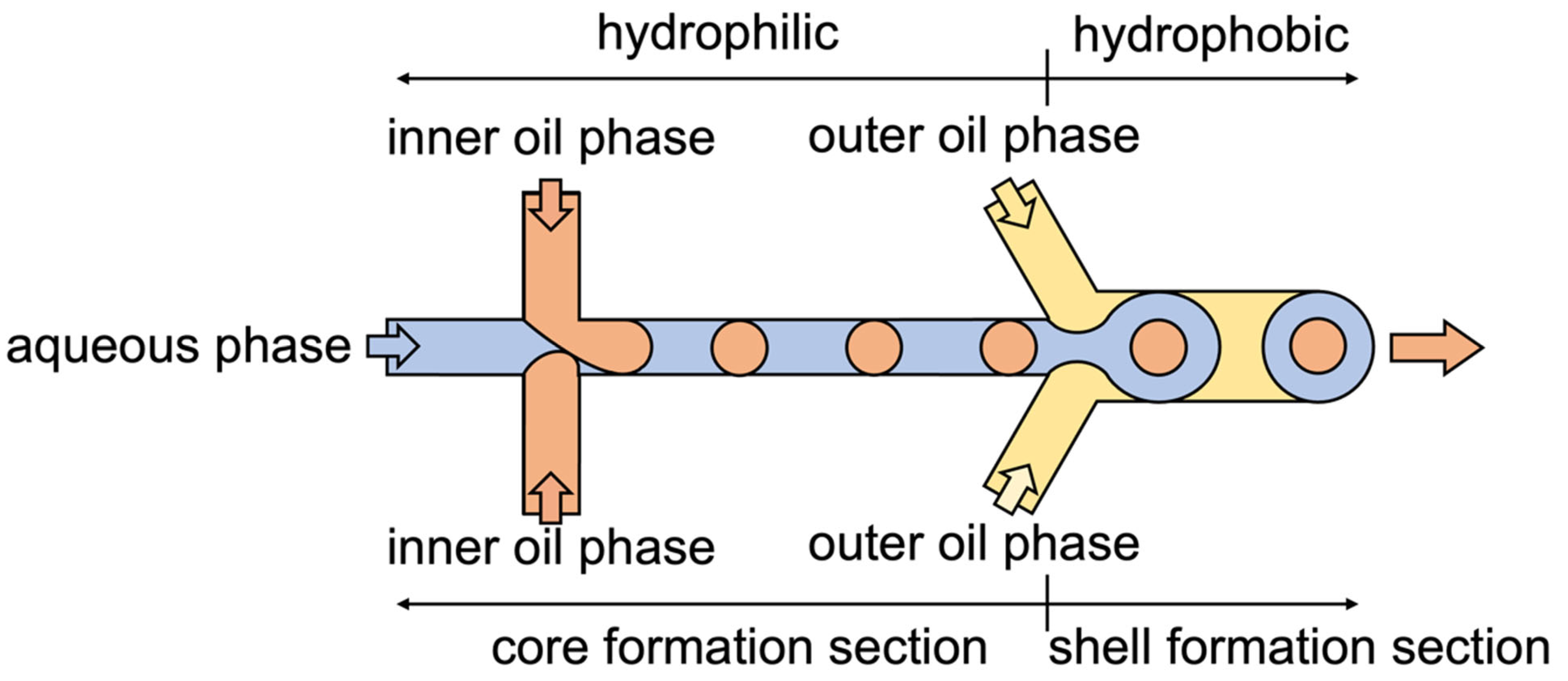
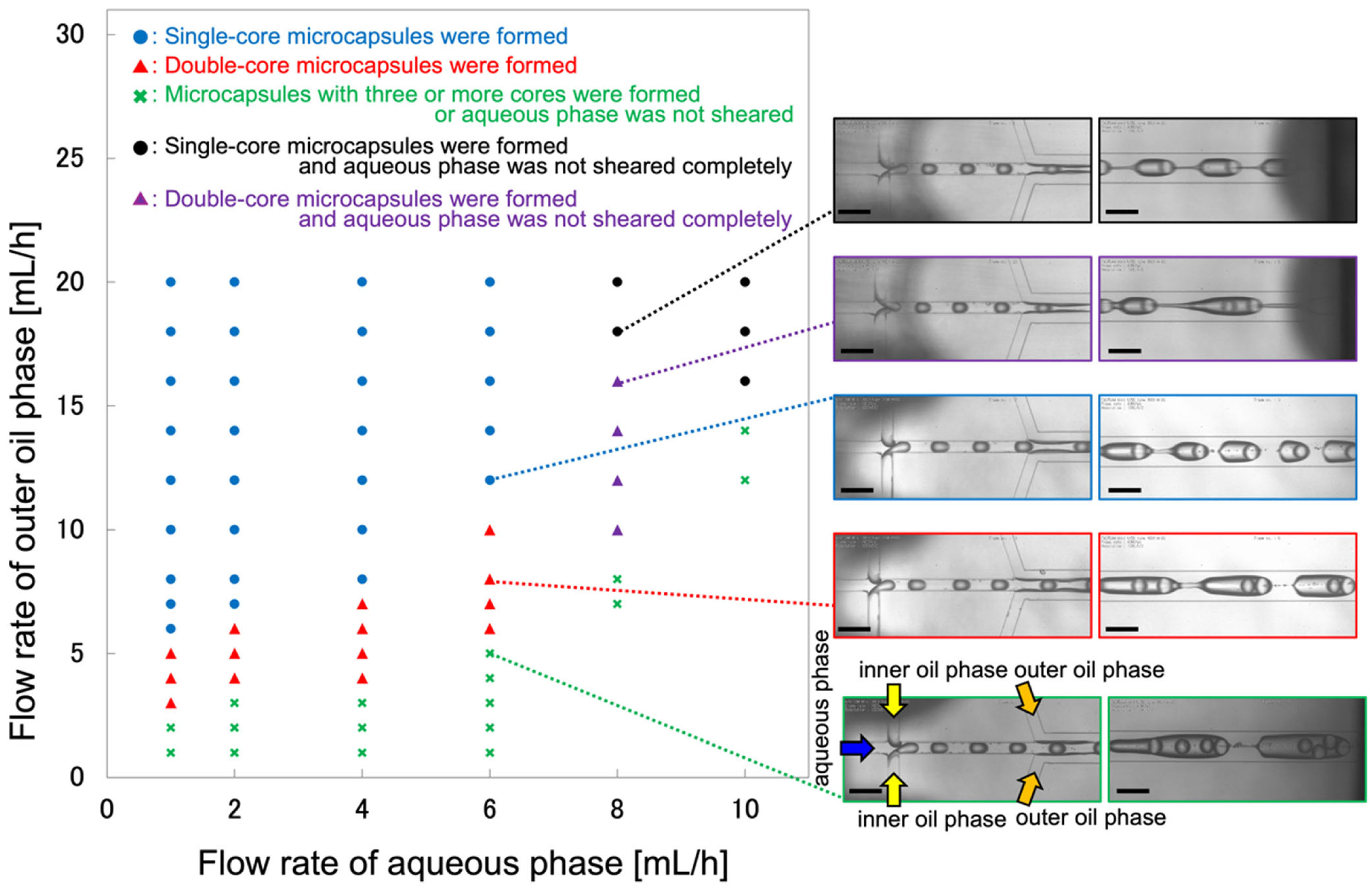
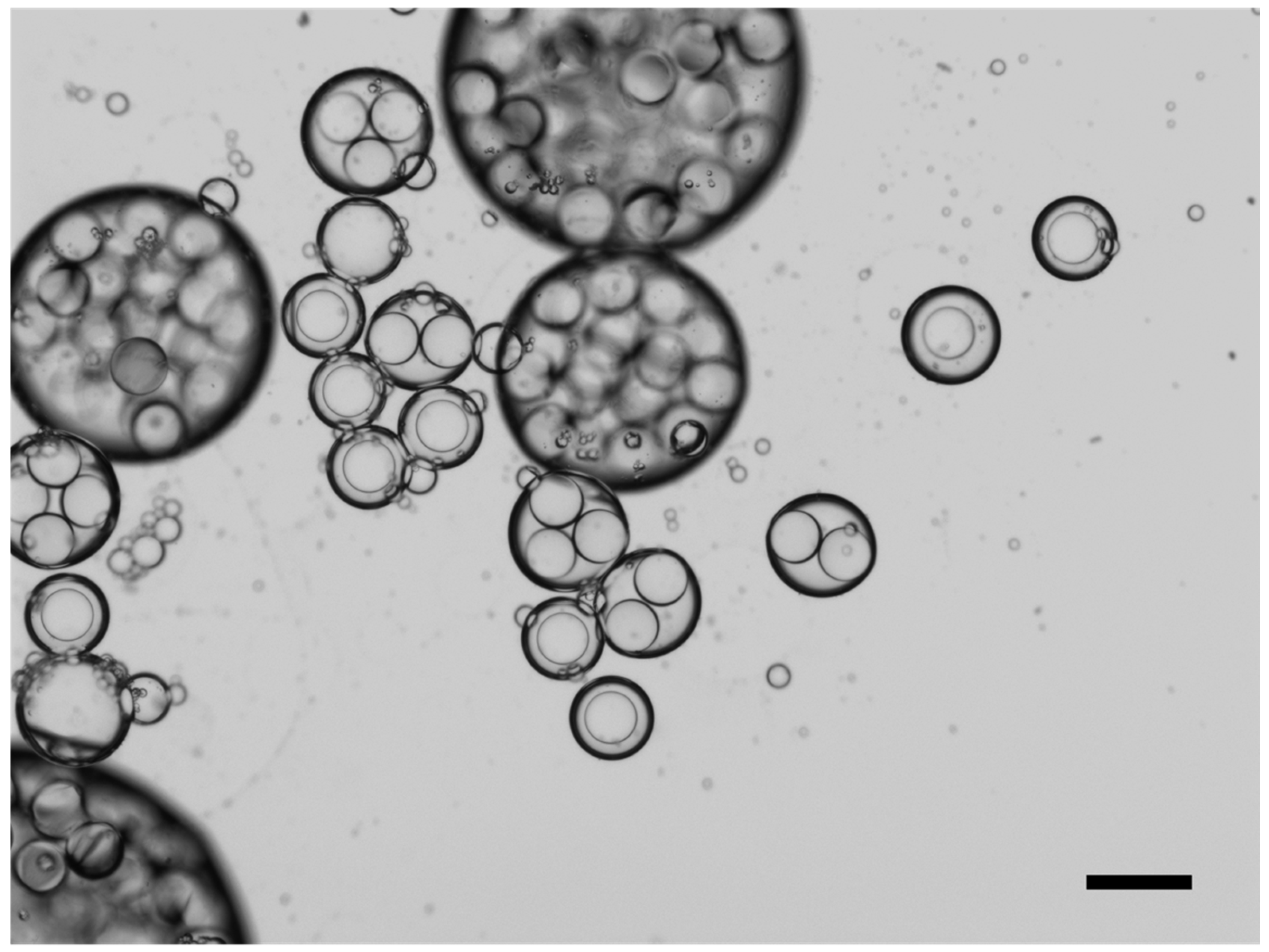
2.1. Microcapsule Formation Behavior
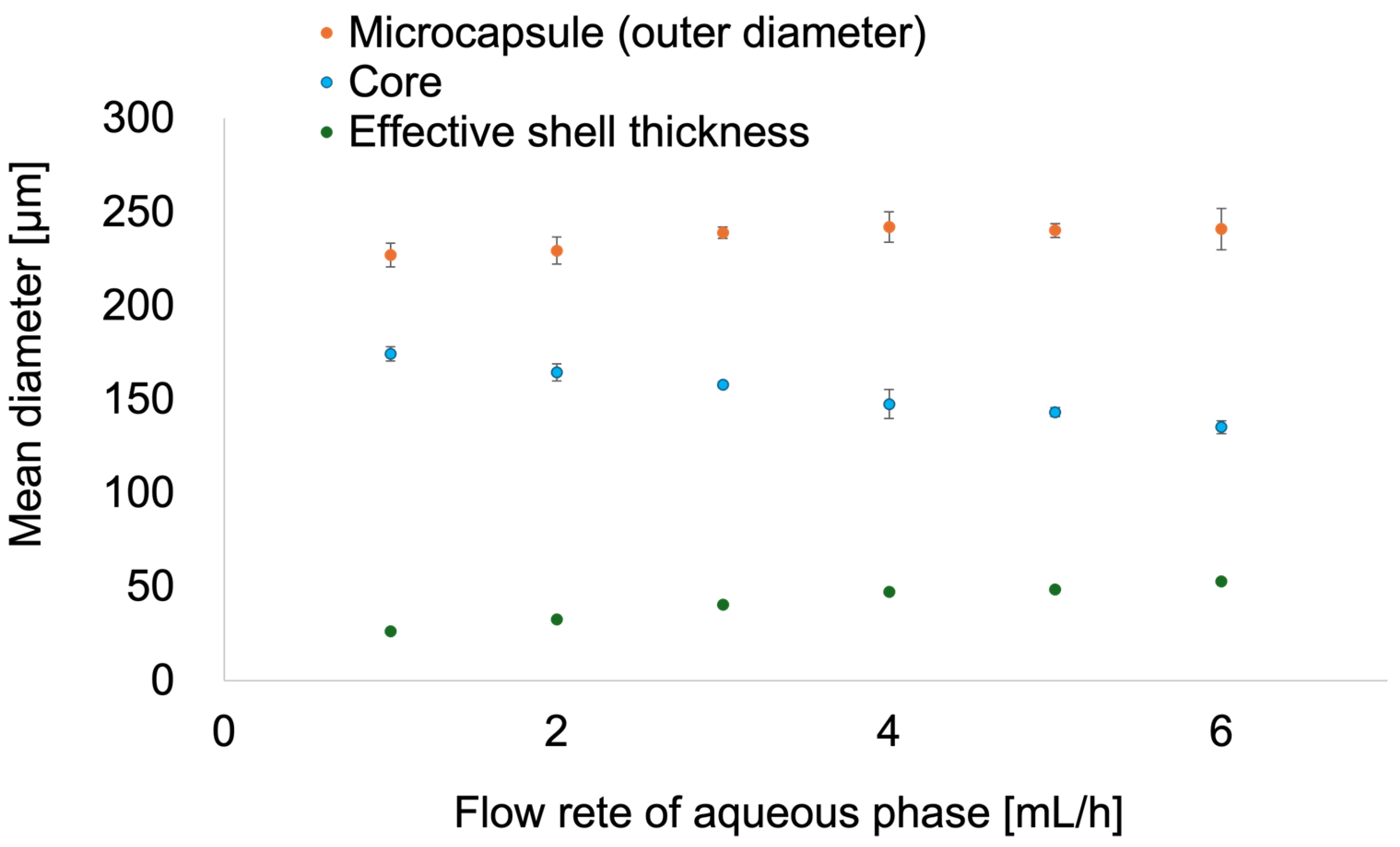
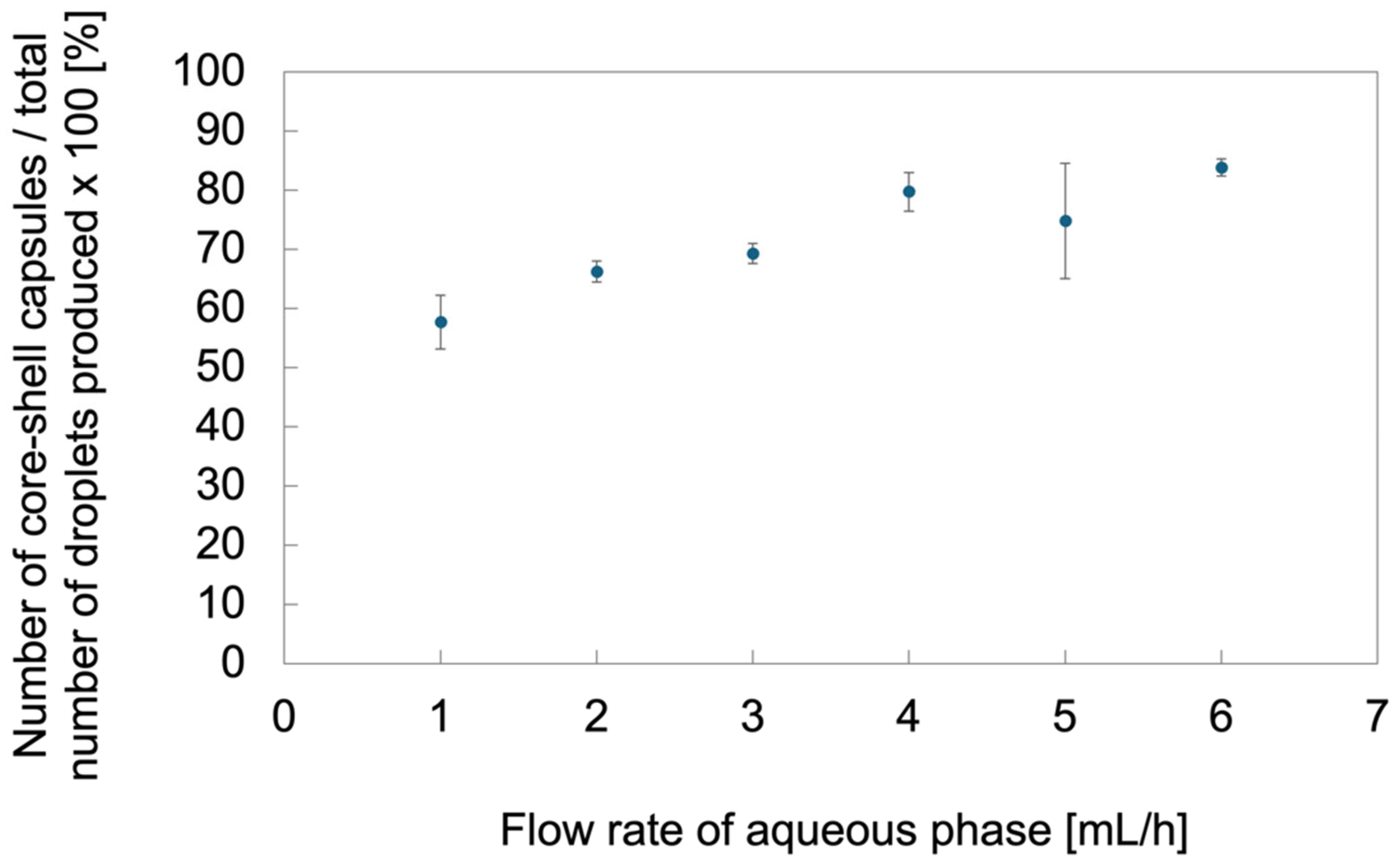
2.2. Effect of Flow Conditions on the Morphology and Dimensions of the Microcapsules
2.3. Characterization of Microcapsules
2.3.1. Characteristics in Response to Temperature Stimulation
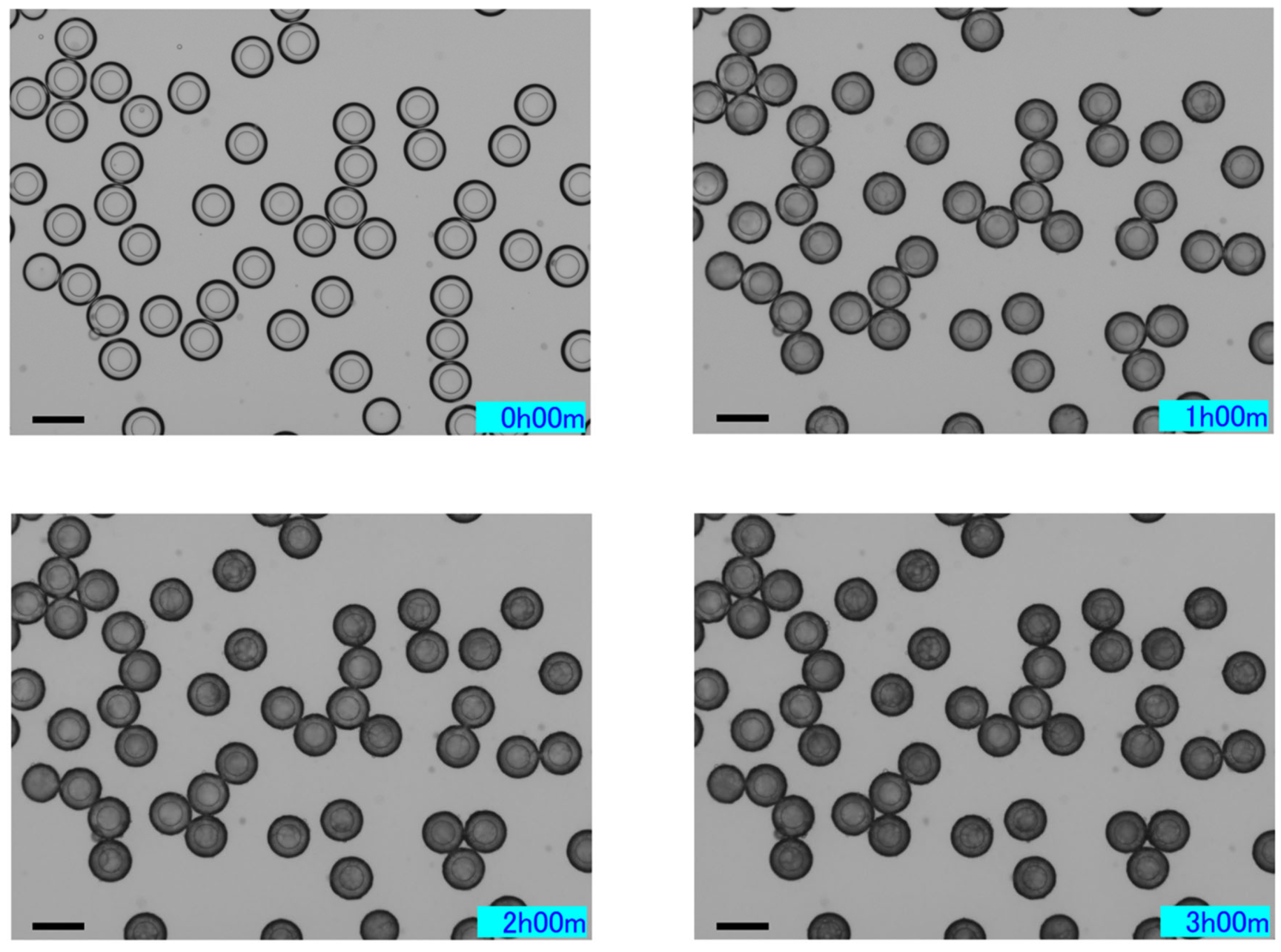
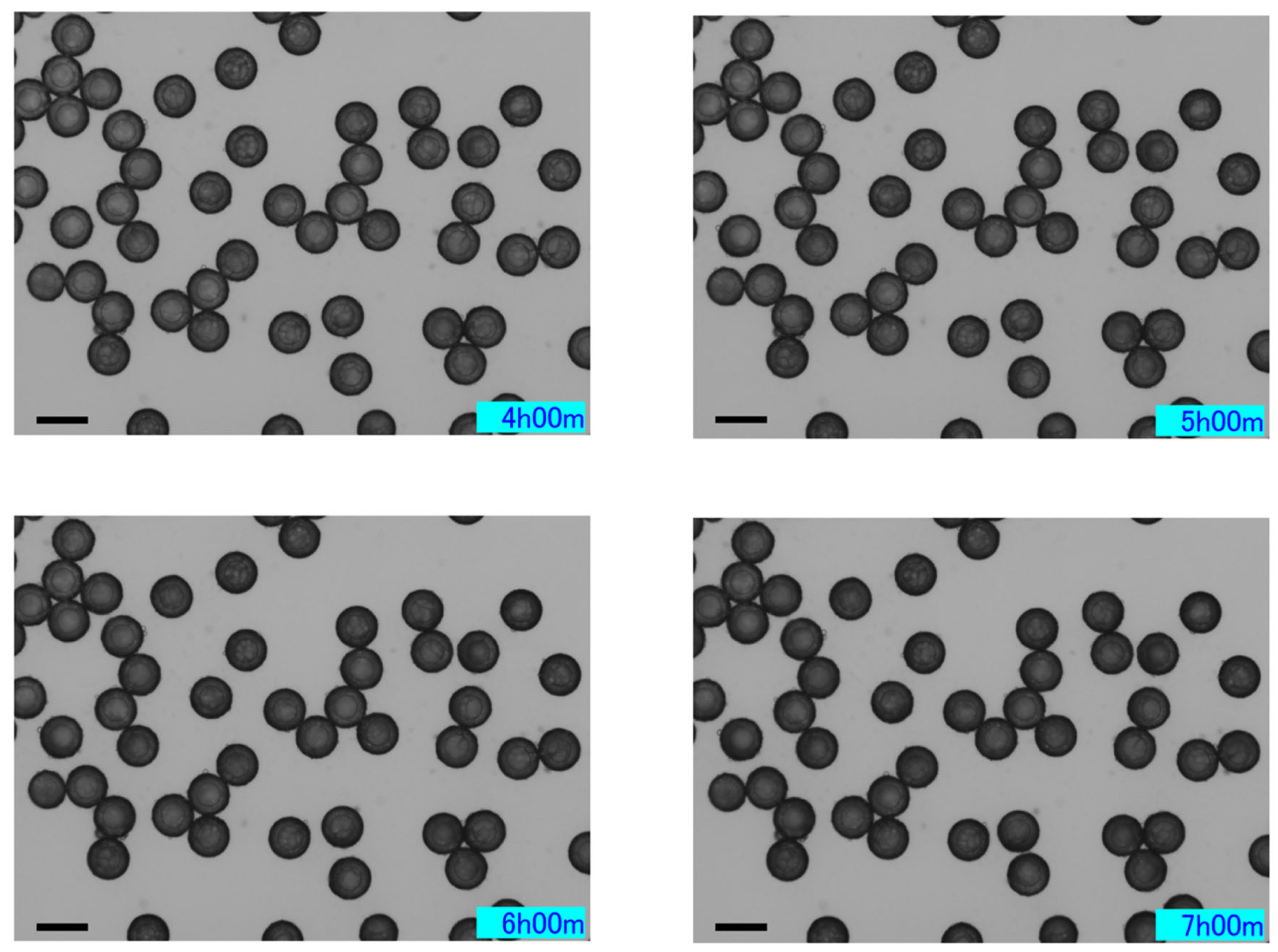
2.3.2. Characteristics over Time
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Microcapsule Formation
4.3. Characterization Methods for Microcapsules
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.X.; Liu, K.Y.; Zhao, M.N.; Wang, S.H.; Zhou, Z.X.; Shen, Y.Q.; Jiang, L.M. A pH-responsive fragrance release system based on pseudopeptide polymeric micelles. React. Funct. Polym. 2018, 132, 138–144. [Google Scholar] [CrossRef]
- Trachsel, A.; Paret, N.; Berthier, D.L.; Herrmann, A. Light-Induced Fragrance Release from 2-Oxoacetates: Impact of Compound Mixtures on the Efficiency of the Norrish Type II Photoreaction in Solution and in Encapsulation Systems. Chemphotochem 2022, 6, e202200045. [Google Scholar] [CrossRef]
- Nicastro, G.; Black, L.M.; Ravarino, P.; D’Agostino, S.; Faccio, D.; Tomasini, C.; Giuri, D. Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels. Int. J. Mol. Sci. 2022, 23, 3105. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, K.; Lee, Y.; Yang, K.; Choe, D.; Roh, Y.H. Thermoresponsive semi-interpenetrating gelatin-alginate networks for encapsulation and controlled release of scent molecules. Int. J. Biol. Macromol. 2022, 208, 1096–1105. [Google Scholar] [CrossRef]
- Theisinger, S.; Schoeller, K.; Osborn, B.; Sarkar, M.; Landfester, K. Encapsulation of a Fragrance via Miniemulsion Polymerization for Temperature-Controlled Release. Macromol. Chem. Phys. 2009, 210, 411–420. [Google Scholar] [CrossRef]
- Sansukcharearnpon, A.; Wanichwecharungruang, S.; Leepipatpaiboon, N.; Kerdcharoen, T.; Arayachukeat, S. High loading fragrance encapsulation based on a polymer-blend: Preparation and release behavior. Int. J. Pharm. 2010, 391, 267–273. [Google Scholar] [CrossRef]
- Sadovoy, A.V.; Lomova, M.V.; Antipina, M.N.; Braun, N.A.; Sukhorukov, G.B.; Kiryukhin, M.V. Layer-by-Layer Assembled Multilayer Shells for Encapsulation and Release of Fragrance. ACS Appl. Mater. Interfaces 2013, 5, 8948–8954. [Google Scholar] [CrossRef]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef]
- Zhang, H.; Tumarkin, E.; Peerani, R.; Nie, Z.; Sullan, R.M.A.; Walker, G.C.; Kumacheva, E. Microfluidic production of biopolymer microcapsules with controlled morphology. J. Am. Chem. Soc. 2006, 128, 12205–12210. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Shum, H.C.; Adams, L.L.A.; Sun, B.J.; Holtze, C.; Gu, Z.Z.; Weitz, D.A. Enhanced Encapsulation of Actives in Self-Sealing Microcapsules by Precipitation in Capsule Shells. Langmuir ACS J. Surf. Colloids 2011, 27, 13988–13991. [Google Scholar] [CrossRef]
- Abbaspourrad, A.; Datta, S.S.; Weitz, D.A. Controlling Release From pH-Responsive Microcapsules. Langmuir ACS J. Surf. Colloids 2013, 29, 12697–12702. [Google Scholar] [CrossRef] [PubMed]
- Abbaspourrad, A.; Duncanson, W.J.; Lebedeva, N.; Kim, S.H.; Zhushma, A.P.; Datta, S.S.; Dayton, P.A.; Sheiko, S.S.; Rubinstein, M.; Weitz, D.A. Microfluidic Fabrication of Stable Gas-Filled Microcapsules for Acoustic Contrast Enhancement. Langmuir ACS J. Surf. Colloids 2013, 29, 12352–12357. [Google Scholar] [CrossRef]
- Abbaspourrad, A.; Carroll, N.J.; Kim, S.H.; Weitz, D.A. Polymer Microcapsules with Programmable Active Release. J. Am. Chem. Soc. 2013, 135, 7744–7750. [Google Scholar] [CrossRef]
- Grolman, J.M.; Inci, B.; Moore, J.S. pH-Dependent Switchable Permeability from Core Shell Microcapsules. ACS Macro Lett. 2015, 4, 441–445. [Google Scholar] [CrossRef]
- Zieringer, M.A.; Carroll, N.J.; Abbaspourrad, A.; Koehler, S.A.; Weitz, D.A. Microcapsules for Enhanced Cargo Retention and Diversity. Small 2015, 11, 2903–2909. [Google Scholar] [CrossRef]
- Lee, H.; Choi, C.H.; Abbaspourrad, A.; Wesner, C.; Caggioni, M.; Zhu, T.; Weitz, D.A. Encapsulation and Enhanced Retention of Fragrance in Polymer Microcapsules. ACS Appl. Mater. Interfaces 2016, 8, 4007–4013. [Google Scholar] [CrossRef]
- Ling, S.D.; Geng, Y.H.; Chen, A.; Du, Y.A.; Xu, J.H. Enhanced single-cell encapsulation in microfluidic devices: From droplet generation to single-cell analysis. Biomicrofluidics 2020, 14, 061508. [Google Scholar] [CrossRef]
- Torza, S.; Mason, S.G. Three-phase interactions in shear and electrical fields. J. Colloid Interface Sci. 1970, 33, 67–83. [Google Scholar] [CrossRef]
- Xu, J.-H.; Ge, X.-H.; Chen, R.; Luo, G.-S. Microfluidic preparation and structure evolution of double emulsions with two-phase cores. RSC Adv. 2014, 4, 1900–1906. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive Forces at Interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Chesters, A.K. The Modeling of Coalescence Processes in Fluid Liquid Dispersions—A Review of Current Understanding. Chem. Eng. Res. Des. 1991, 69, 259–270. [Google Scholar]
- Fukuyama, M.; Mizuguchi, T.; Santivongskul, P.; Ono, Y.; Kasuya, M.; Inagawa, A.; Hibara, A. Kinetic description of water transport during spontaneous emulsification induced by Span 80. Nanoscale 2024, 16, 4056–4062. [Google Scholar] [CrossRef] [PubMed]
- Penttilä, P.A.; Vierros, S.; Utriainen, K.; Carl, N.; Rautkari, L.; Sammalkorpi, M.; Österberg, M. Phospholipid-Based Reverse Micelle Structures in Vegetable Oil Modified by Water Content, Free Fatty Acid, and Temperature. Langmuir ACS J. Surf. Colloids 2019, 35, 8373–8382. [Google Scholar] [CrossRef]
- Hirama, H.; Wada, S.; Shimamura, J.; Komazaki, Y.; Inoue, T.; Torii, T. Surface modification of a glass microchannel for the formation of multiple emulsion droplets. Microfluid. Nanofluid. 2017, 21, 91. [Google Scholar] [CrossRef]
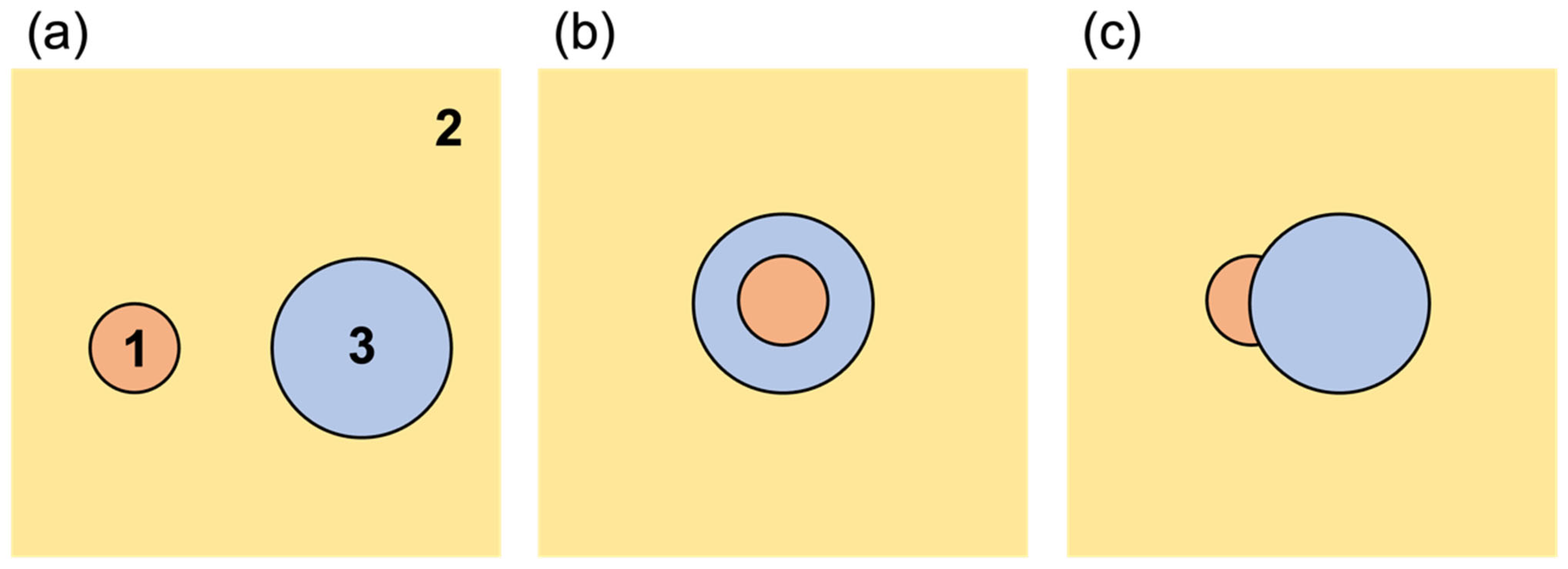
| Droplet State | S1 | S2 | S3 | Description |
|---|---|---|---|---|
| Non-engulfing | <0 | >0 | <0 | Phase 1 does not spread on Phase 2, and Phase 3 does not spread on Phase 2. The droplets exist independently. |
| Complete engulfing | <0 | <0 | >0 | Phase 3 completely engulfs Phase 1, forming a stable O/W/O or W/O/W emulsion floating in Phase 2. |
| Partial engulfing | <0 | <0 | <0 | Phase 3 partially engulfs Phase 1, with part of Phase 1 exposed to Phase 2. |
| Symbol | Phase | Composition | Surface Tension (Mean ± SD) (mN/m) |
|---|---|---|---|
| σW | Aqueous phase | 0.5 wt% agarose/2.0 wt% PVA in water | 39.6 ± 0.64 |
| σO1 | Oil phase 1 | 1 vol% benzaldehyde in mineral oil | 29.2 ± 0.05 |
| σO2 | Oil phase 2 | Mineral oil | 29.3 ± 0.15 |
| Symbol | Interface | Interfacial Tension (mN/m) |
|---|---|---|
| γWO1 | Aqueous/oil phase 1 | 0.79 |
| γWO2 | Aqueous/oil phase 2 | 0.77 |
| γO1O2 | Oil phase 1/oil phase 2 | 8.5 × 10−5 |
| Symbol | Spreading Coefficient (mN/m) |
|---|---|
| SO1 | −0.016 |
| SO2 | 0.016 |
| SW | −1.6 |
| No. | Liquid Properties | Thermal Stimulation | Measured Sensor Intensity (Mean ± SD) |
|---|---|---|---|
| 1 | Microcapsule | With | 7.1 ± 0.2 * |
| 2 | Microcapsule | Without | 6.7 ± 0.4 |
| 3 | Simply stirred solution | With | 9.3 ± 0.1 |
| 4 | Simply stirred solution | Without | 8.8 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirama, H.; Miyauchi, H.; Matsuo, Y.; Hayase, M. Monodisperse Generation of Fragrance-Loaded Microcapsules with Hydrophilic Polymer Shells Using Microfluidic Devices. Appl. Sci. 2025, 15, 5650. https://doi.org/10.3390/app15105650
Hirama H, Miyauchi H, Matsuo Y, Hayase M. Monodisperse Generation of Fragrance-Loaded Microcapsules with Hydrophilic Polymer Shells Using Microfluidic Devices. Applied Sciences. 2025; 15(10):5650. https://doi.org/10.3390/app15105650
Chicago/Turabian StyleHirama, Hirotada, Hiromasa Miyauchi, Yuki Matsuo, and Masanori Hayase. 2025. "Monodisperse Generation of Fragrance-Loaded Microcapsules with Hydrophilic Polymer Shells Using Microfluidic Devices" Applied Sciences 15, no. 10: 5650. https://doi.org/10.3390/app15105650
APA StyleHirama, H., Miyauchi, H., Matsuo, Y., & Hayase, M. (2025). Monodisperse Generation of Fragrance-Loaded Microcapsules with Hydrophilic Polymer Shells Using Microfluidic Devices. Applied Sciences, 15(10), 5650. https://doi.org/10.3390/app15105650





