Increased Myo/Nog Cell Presence and Phagocytic Activity in Retinal Degeneration: Insights from a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Housing Conditions
2.2. Tissue Collection and Processing
2.3. Immunohistochemistry and Imaging
2.4. Statistical Analyses
3. Results
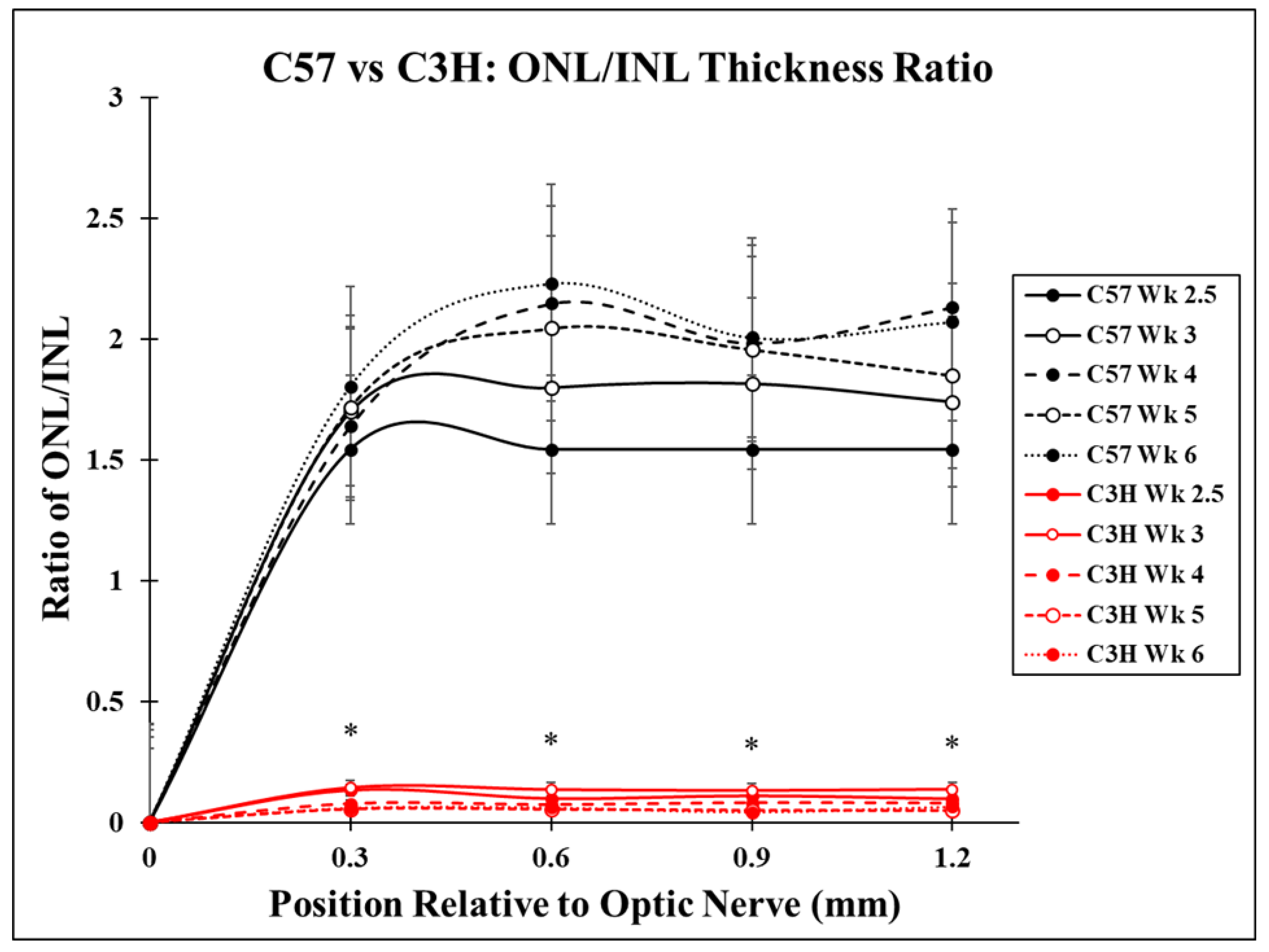
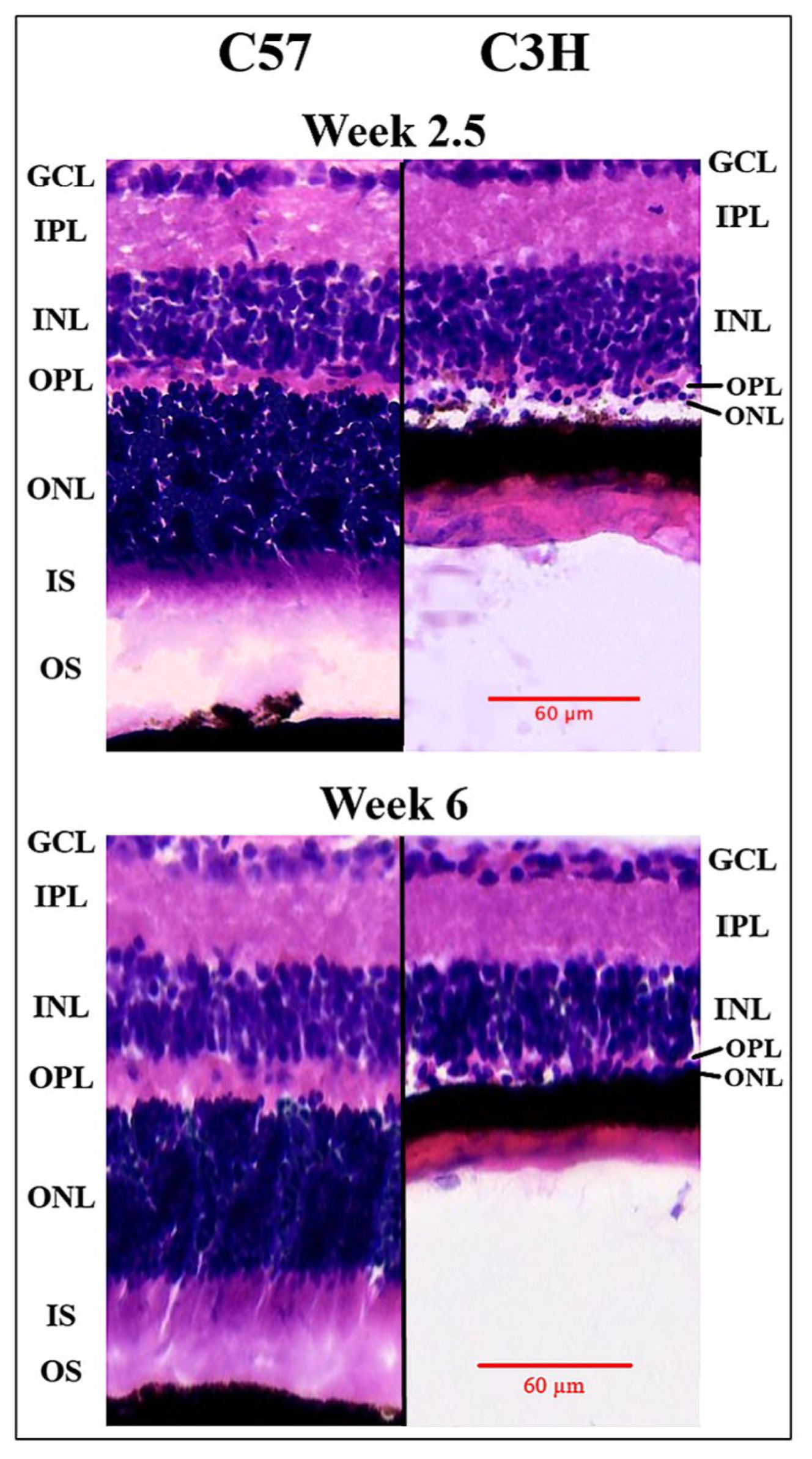
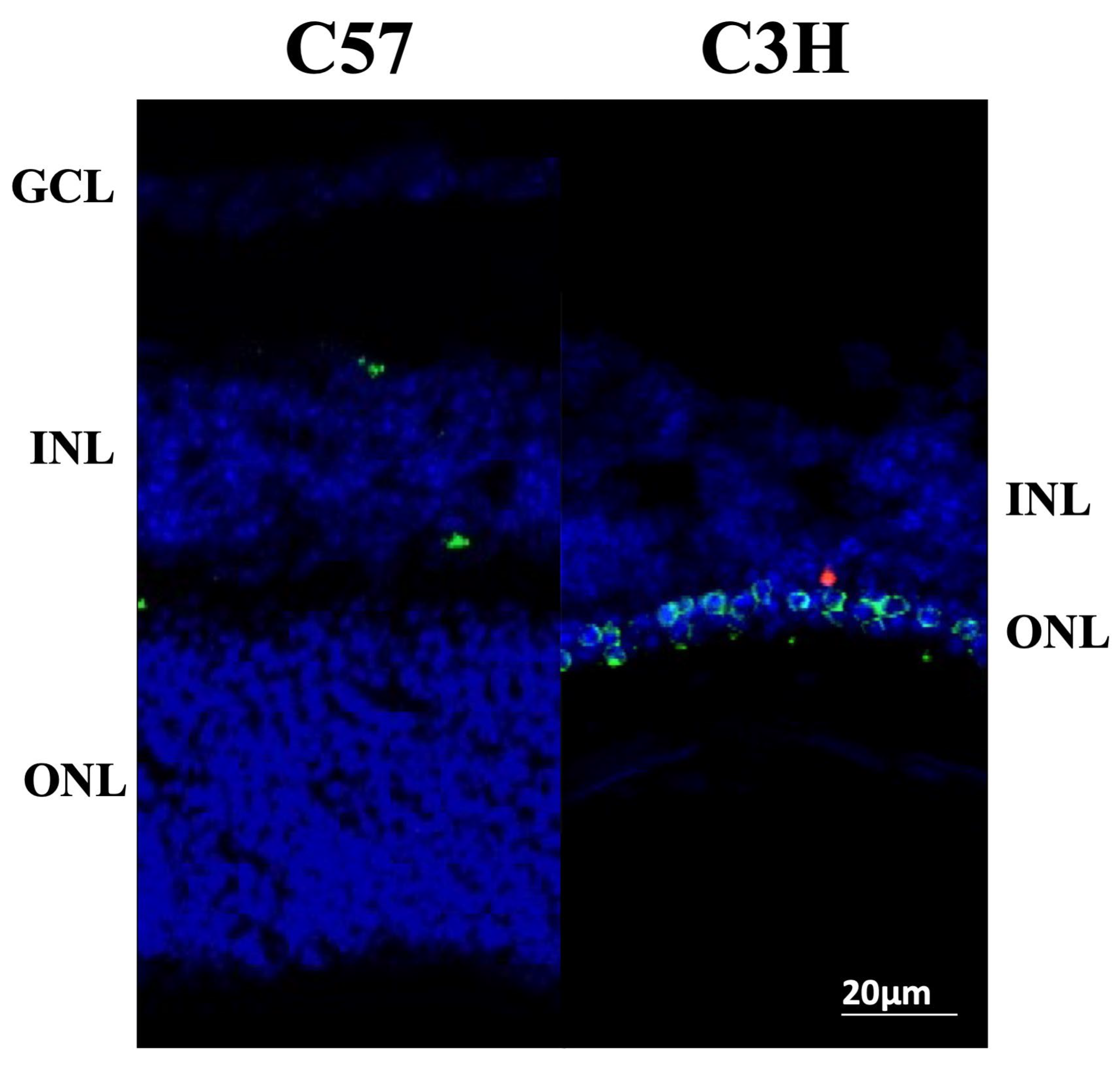
3.1. Photoreceptor Degeneration in the C3H Mice
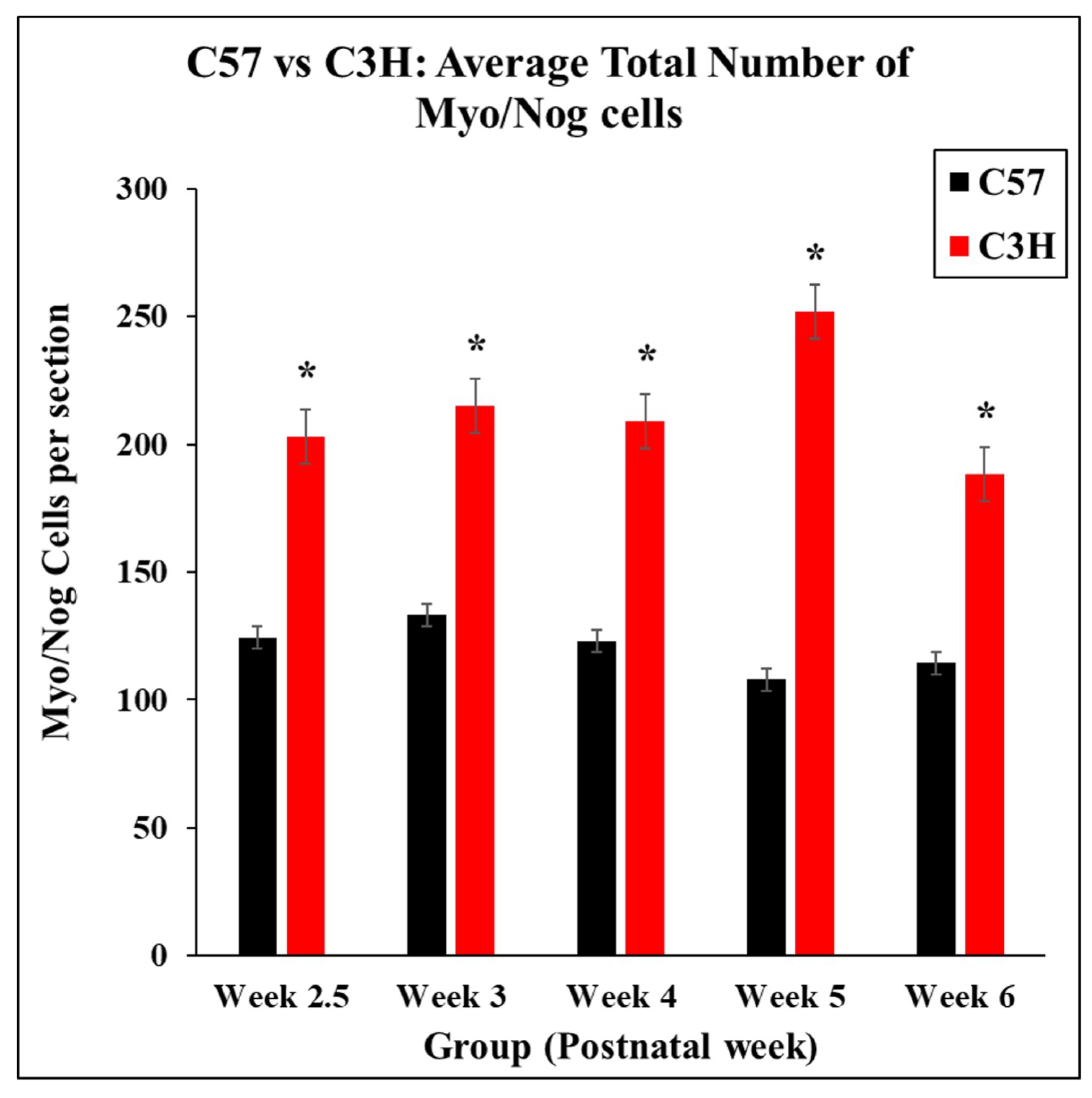
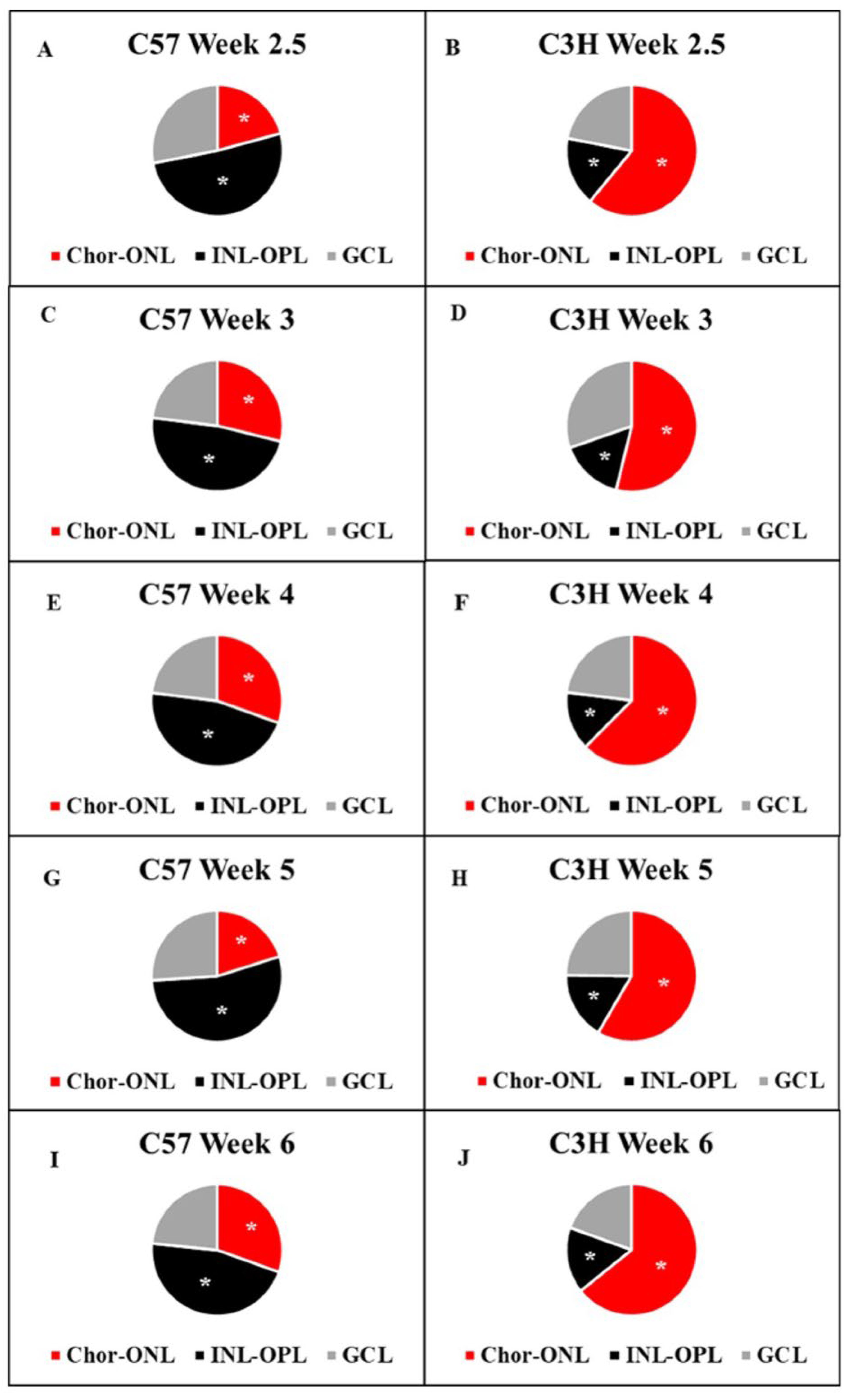
3.2. Distribution of Myo/Nog Cells Shift in Number and Location with Stress
3.3. Localization of Nuclear Remnants Inside Myo/Nog Cells Capable of Phagocytosis of Degenerating Photoreceptors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gerhart, J.; Elder, J.; Neely, C.; Schure, J.; Kvist, T.; Knudsen, K.; George-Weinstein, M. MyoD-positive epiblast cells regulate skeletal muscle differentiation in the embryo. J. Cell Biol. 2006, 175, 283–292. [Google Scholar] [CrossRef]
- Gerhart, J.; Baytion, M.; DeLuca, S.; Getts, R.; Lopez, C.; Niewenhuis, R.; Nilsen, T.; Olex, S.; Weintraub, H.; George-Weinstein, M. DNA dendrimers localize MyoD mRNA in presomitic tissues of the chick embryo. J. Cell Biol. 2000, 149, 825–834. [Google Scholar] [CrossRef]
- Gerhart, J.; Pfautz, J.; Neely, C.; Elder, J.; DuPrey, K.; Menko, A.S.; Knudsen, K.; George-Weinstein, M. Noggin producing, MyoD-positive cells are crucial for eye development. Dev. Biol. 2009, 336, 30–41. [Google Scholar] [CrossRef][Green Version]
- Gerhart, J.; Werner, L.; Mamalis, N.; Infanti, J.; Withers, C.; Abdalla, F.; Gerhart, C.; Bravo-Nuevo, A.; Gerhart, O.; Getts, L.; et al. Depletion of Myo/Nog Cells in the Lens Mitigates Posterior Capsule Opacification in Rabbits. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1813–1823. [Google Scholar] [CrossRef]
- Bravo-Nuevo, A.; Brandli, A.A.; Gerhart, J.; Nichols, J.; Pitts, M.; Sutera, C.K.; Assali, S.; Scheinfeld, V.; Prendergast, G.C.; Stone, J.; et al. Neuroprotective effect of Myo/Nog cells in the stressed retina. Exp. Eye Res. 2016, 146, 22–25. [Google Scholar] [CrossRef]
- Lecker, P.; Johal, K.; McGrath, A.; Spikes, J.; Bernstein, J.; MacPherson, V.; Brahmbhatt, R.; Fadl, N.; Weyback-Liogier, E.; Adams, S.; et al. Myo/Nog Cells Increase in Response to Elevated Intraocular Pressure and Mitigate Ganglion Cell Death in a Mouse Model of Glaucoma. Appl. Sci. 2023, 13, 12423. [Google Scholar] [CrossRef]
- Joseph-Pauline, S.; Morrison, N.; Braccia, M.; Payne, A.; Gugerty, L.; Mostoller, J.; Lecker, P.; Tsai, E.J.; Kim, J.; Martin, M.; et al. Acute Response and Neuroprotective Role of Myo/Nog Cells Assessed in a Rat Model of Focal Brain Injury. Front. Neurosci. 2021, 15, 780707. [Google Scholar] [CrossRef]
- Chang, B. Animal Models of Retinitis Pigmentosa (RP). In Animal Models of Ophthalmic Diseases; Chan, C.-C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 101–116. [Google Scholar]
- Paquet-Durand, F.; Silva, J.; Talukdar, T.; Johnson, L.E.; Azadi, S.; van Veen, T.; Ueffing, M.; Hauck, S.M.; Ekstrom, P.A. Excessive activation of poly(ADP-ribose) polymerase contributes to inherited photoreceptor degeneration in the retinal degeneration 1 mouse. J. Neurosci. 2007, 27, 10311–10319. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Johnson, L.; Ekstrom, P. Calpain activity in retinal degeneration. J. Neurosci. Res. 2007, 85, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Sanges, D.; Comitato, A.; Tammaro, R.; Marigo, V. Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc. Natl. Acad. Sci. USA 2006, 103, 17366–17371. [Google Scholar] [CrossRef] [PubMed]
- Portera-Cailliau, C.; Sung, C.H.; Nathans, J.; Adler, R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 1994, 91, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Nambu, H.; Yuge, K.; Shikata, N.; Tsubura, A.; Matsuzawa, A. Fas-independent apoptosis of photoreceptor cells in C3H mice. Exp. Anim. 1996, 45, 309–315. [Google Scholar] [CrossRef]
- Arandjelovic, S.; Ravichandran, K.S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015, 16, 907–917. [Google Scholar] [CrossRef]
- Gerhart, J.; Gugerty, L.; Lecker, P.; Abdalla, F.; Martin, M.; Gerhart, O.; Gerhart, C.; Johal, K.; Bernstein, J.; Spikes, J.; et al. Myo/Nog cells are nonprofessional phagocytes. PLoS ONE 2020, 15, e0235898. [Google Scholar] [CrossRef]
- Brandli, A.; Gerhart, J.; Sutera, C.K.; Purushothuman, S.; George-Weinstein, M.; Stone, J.; Bravo-Nuevo, A. Role of Myo/Nog Cells in Neuroprotection: Evidence from the Light Damaged Retina. PLoS ONE 2017, 12, e0169744. [Google Scholar] [CrossRef] [PubMed]
- Stuart, L.M.; Ezekowitz, R.A. Phagocytosis: Elegant complexity. Immunity 2005, 22, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Vandenabeele, P. Phagocytosis of Dying Cells: From Molecular Mechanisms to Human Diseases; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Penberthy, K.K.; Ravichandran, K.S. Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 2016, 269, 44–59. [Google Scholar] [CrossRef]
- Gerhart, J.; Bowers, J.; Gugerty, L.; Gerhart, C.; Martin, M.; Abdalla, F.; Bravo-Nuevo, A.; Sullivan, J.T.; Rimkunas, R.; Albertus, A.; et al. Brain-specific angiogenesis inhibitor 1 is expressed in the Myo/Nog cell lineage. PLoS ONE 2020, 15, e0234792. [Google Scholar] [CrossRef]
- Park, D.; Tosello-Trampont, A.C.; Elliott, M.R.; Lu, M.; Haney, L.B.; Ma, Z.; Klibanov, A.L.; Mandell, J.W.; Ravichandran, K.S. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 2007, 450, 430–434. [Google Scholar] [CrossRef]
- Patel, V.; Dial, K.; Wu, J.; Gauthier, A.G.; Wu, W.; Lin, M.; Espey, M.G.; Thomas, D.D.; Ashby, C.R., Jr.; Mantell, L.L. Dietary Antioxidants Significantly Attenuate Hyperoxia-Induced Acute Inflammatory Lung Injury by Enhancing Macrophage Function via Reducing the Accumulation of Airway HMGB1. Int. J. Mol. Sci. 2020, 21, 977. [Google Scholar] [CrossRef]
- Gauthier, A.G.; Lin, M.; Zefi, S.; Kulkarni, A.; Thakur, G.A.; Ashby, C.R., Jr.; Mantell, L.L. GAT107-mediated α7 nicotinic acetylcholine receptor signaling attenuates inflammatory lung injury and mortality in a mouse model of ventilator-associated pneumonia by alleviating macrophage mitochondrial oxidative stress via reducing MnSOD-S-glutathionylation. Redox Biol. 2023, 60, 102614. [Google Scholar] [CrossRef]
- Yimam, M.; Horm, T.; O’Neal, A.; Jiao, P.; Hong, M.; Brownell, L.; Jia, Q.; Lin, M.; Gauthier, A.; Wu, J.; et al. A Standardized Botanical Composition Mitigated Acute Inflammatory Lung Injury and Reduced Mortality through Extracellular HMGB1 Reduction. Molecules 2023, 28, 6560. [Google Scholar] [CrossRef] [PubMed]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Huang, X.; Gao, S.; Duan, J.; Zhang, Y.; Zhang, M. Microglia in retinal diseases: From pathogenesis towards therapeutic strategies. Biochem. Pharmacol. 2024, 230, 116550. [Google Scholar] [CrossRef]
- Newton, F.; Megaw, R. Mechanisms of Photoreceptor Death in Retinitis Pigmentosa. Genes 2020, 11, 1120. [Google Scholar] [CrossRef]
- Dong, S.; Zhen, F.; Xu, H.; Li, Q.; Wang, J. Leukemia inhibitory factor protects photoreceptor cone cells against oxidative damage through activating JAK/STAT3 signaling. Ann. Transl. Med. 2021, 9, 152. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Gerhart, J.; George-Weinstein, M. Myo/Nog Cells: The Jekylls and Hydes of the Lens. Cells 2023, 12, 1725. [Google Scholar] [CrossRef]
- Crispin, M.; Gerhart, J.; Heffer, A.; Martin, M.; Abdalla, F.; Bravo-Nuevo, A.; Philp, N.J.; Kuriyan, A.E.; George-Weinstein, M. Myo/Nog Cells Give Rise to Myofibroblasts During Epiretinal Membrane Formation in a Mouse Model of Proliferative Vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2023, 64, 1. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crowley, D.; Murad, S.; Helm, C.; Souza, R.; Coughlan, S.; Serpico, S.; Sugarman, E.; Margulies, K.; Heist, B.; Mitchell, K.D.; et al. Increased Myo/Nog Cell Presence and Phagocytic Activity in Retinal Degeneration: Insights from a Mouse Model. Appl. Sci. 2025, 15, 5486. https://doi.org/10.3390/app15105486
Crowley D, Murad S, Helm C, Souza R, Coughlan S, Serpico S, Sugarman E, Margulies K, Heist B, Mitchell KD, et al. Increased Myo/Nog Cell Presence and Phagocytic Activity in Retinal Degeneration: Insights from a Mouse Model. Applied Sciences. 2025; 15(10):5486. https://doi.org/10.3390/app15105486
Chicago/Turabian StyleCrowley, Diana, Samantha Murad, Courtney Helm, Rachel Souza, Sarah Coughlan, Scott Serpico, Eric Sugarman, Kyle Margulies, Brian Heist, Kathryn D. Mitchell, and et al. 2025. "Increased Myo/Nog Cell Presence and Phagocytic Activity in Retinal Degeneration: Insights from a Mouse Model" Applied Sciences 15, no. 10: 5486. https://doi.org/10.3390/app15105486
APA StyleCrowley, D., Murad, S., Helm, C., Souza, R., Coughlan, S., Serpico, S., Sugarman, E., Margulies, K., Heist, B., Mitchell, K. D., Sutera, C. K., Martin, M., Font, C., Woodruff, M., Tsai, E.-J., Brahmbhatt, R., Lecker, P., Gorski, G., Benalcazar, J., ... Bravo-Nuevo, A. (2025). Increased Myo/Nog Cell Presence and Phagocytic Activity in Retinal Degeneration: Insights from a Mouse Model. Applied Sciences, 15(10), 5486. https://doi.org/10.3390/app15105486






