Terpenes as Potential Anti-Alzheimer’s Disease Agents
Abstract
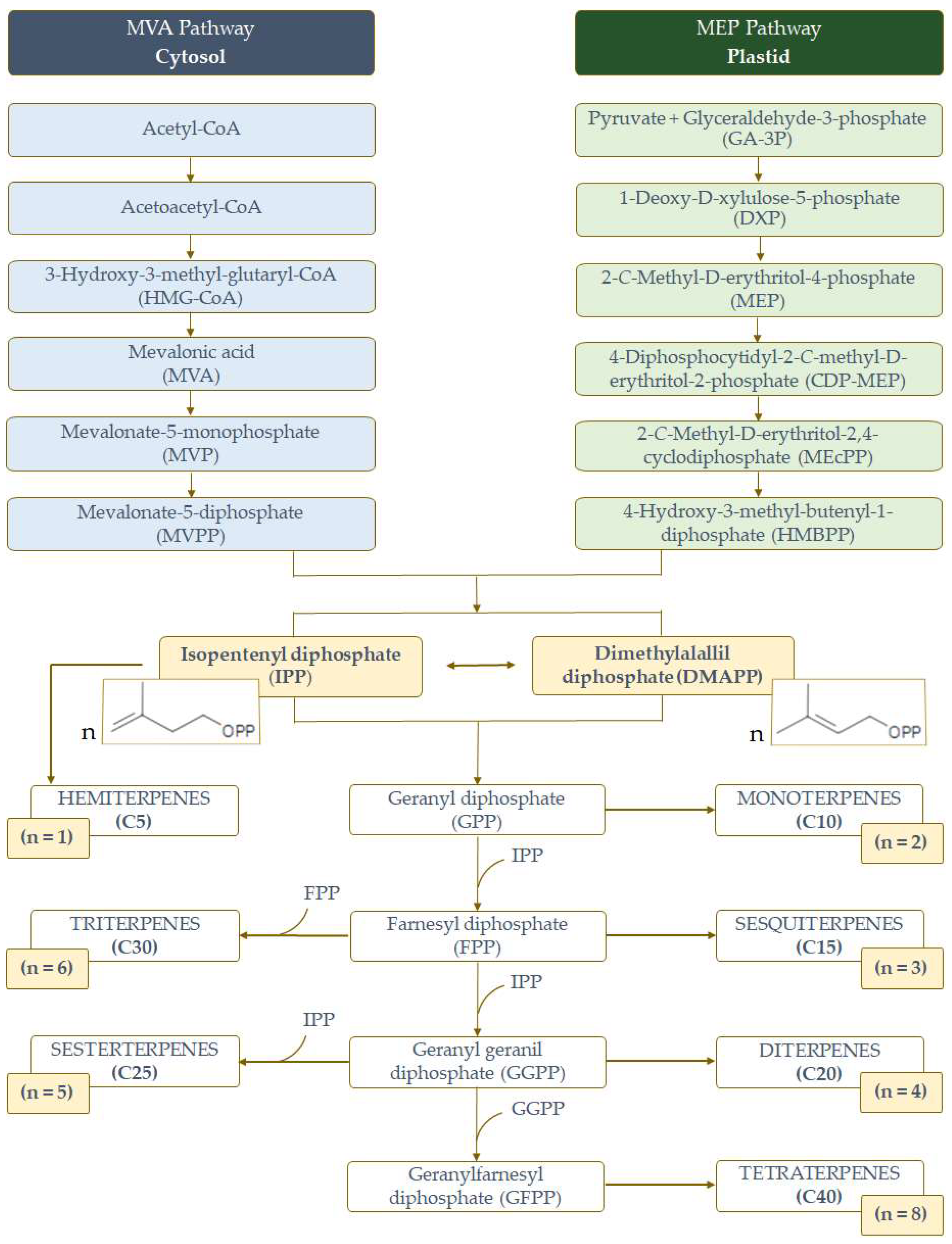
1. Introduction
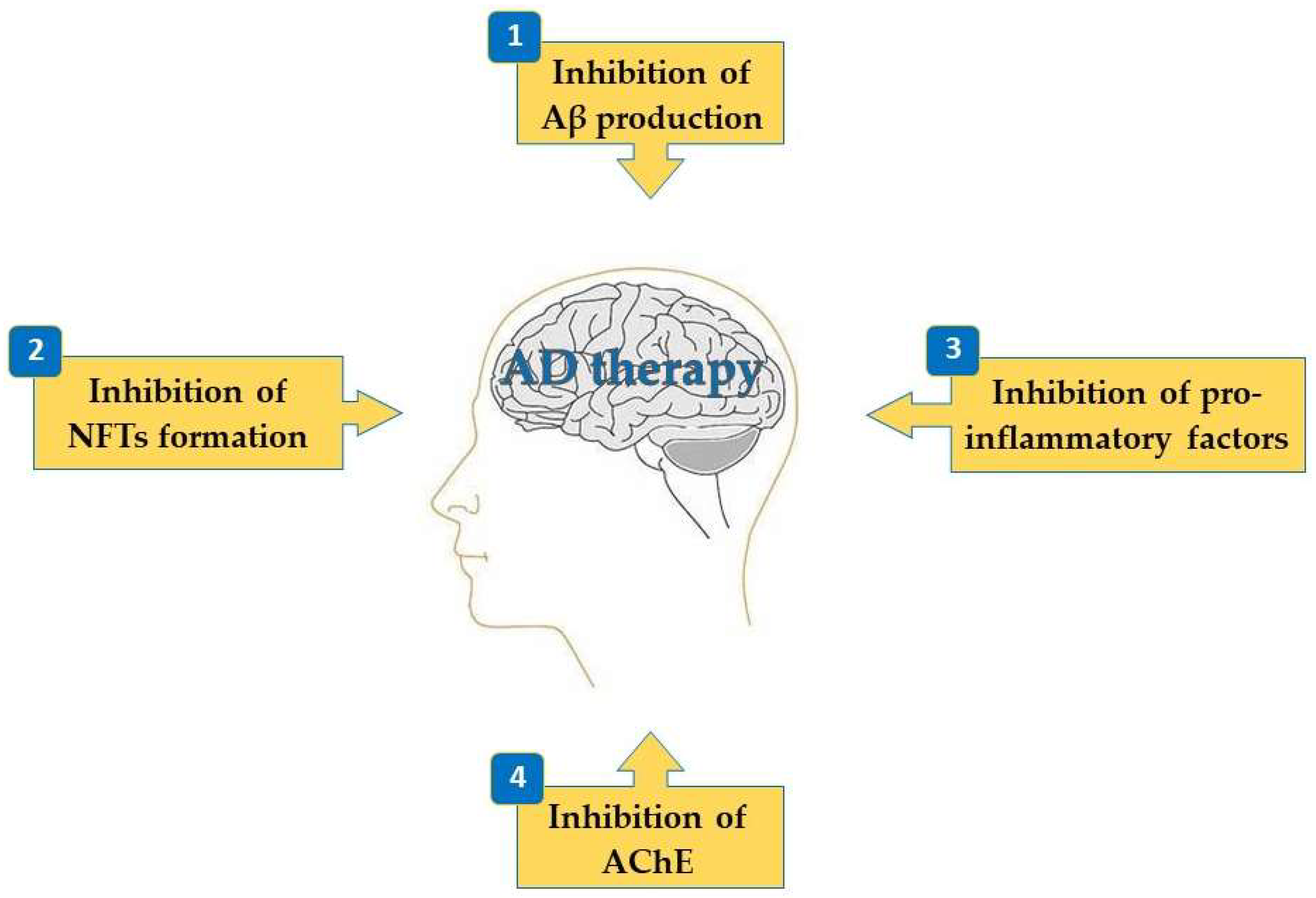
2. Activities of Terpenes on the Mechanisms Associated with AD
2.1. Inhibition of Aβ Production
2.1.1. Monoterpenes and Diterpenes
2.1.2. Triterpenes and Meroterpenes
2.2. Inhibition of NFTs Formation
2.2.1. Inhibition of GSK-3β
Diterpenes
2.3. Inhibition of Pro-Inflammatory Factors
2.3.1. Monoterpenes
2.3.2. Sesquiterpenes

2.3.3. Diterpenes
2.3.4. Triterpenes
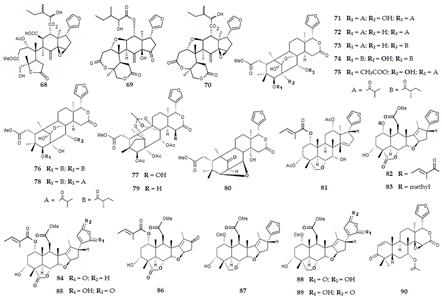
2.3.5. Tetraterpenes
2.4. Inhibition of Acetylcholinesterase (AChE)
2.4.1. Monoterpenes

2.4.2. Sesquiterpenes
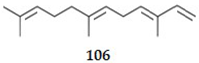
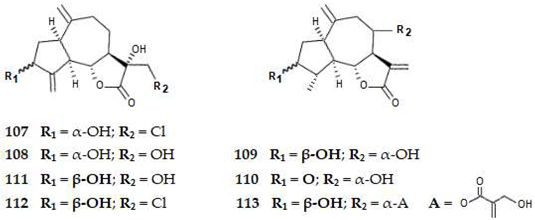
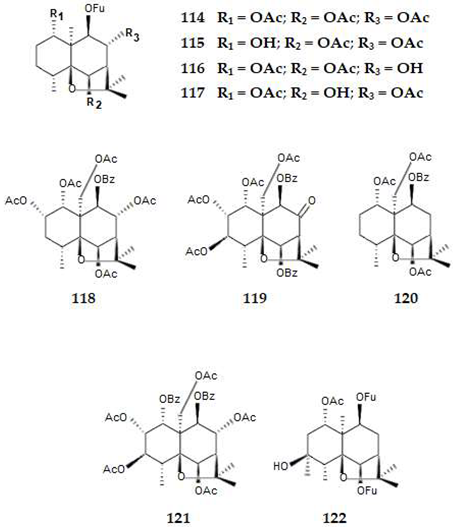
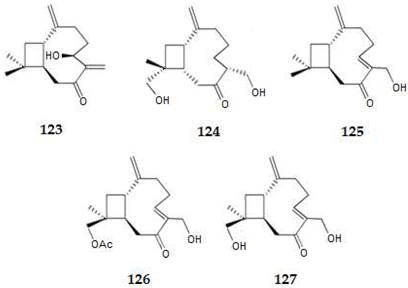
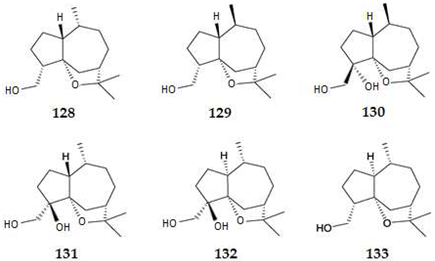
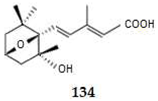
2.4.3. Diterpenes
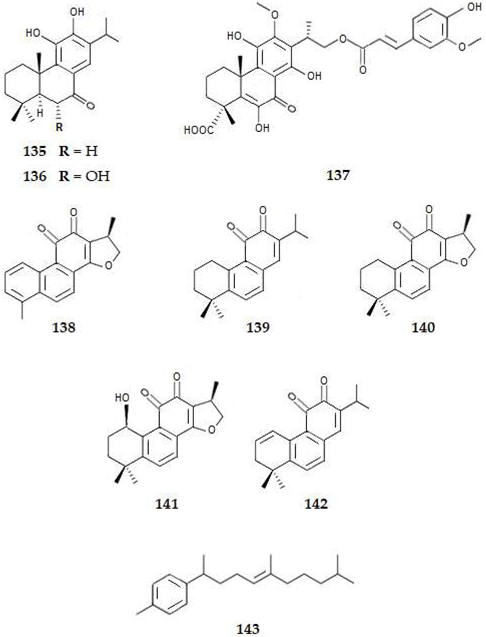
2.4.4. Triterpenes
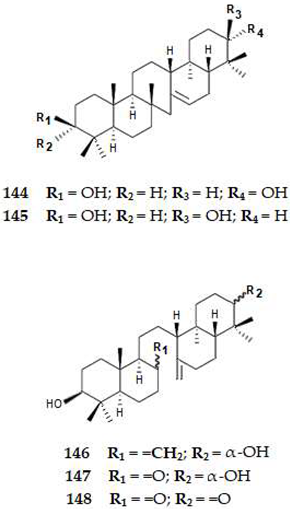
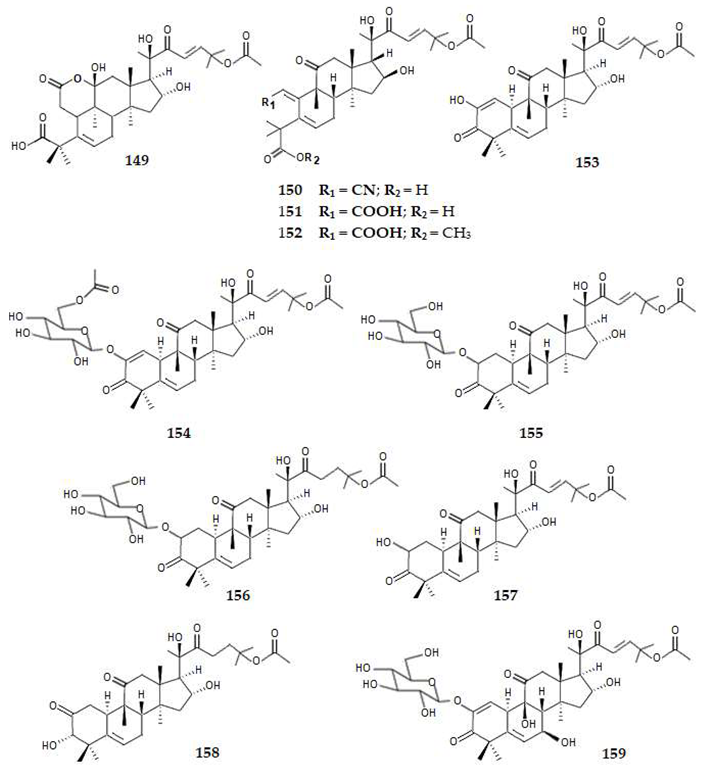
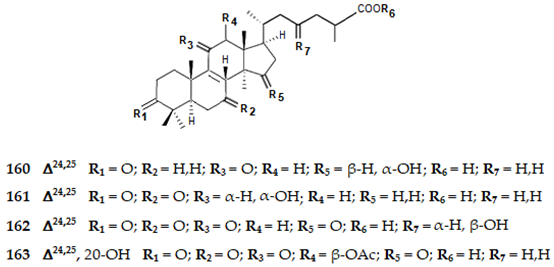


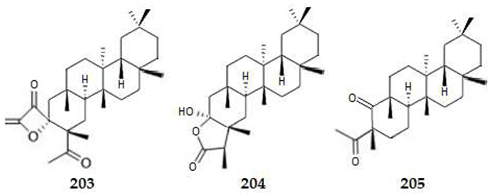
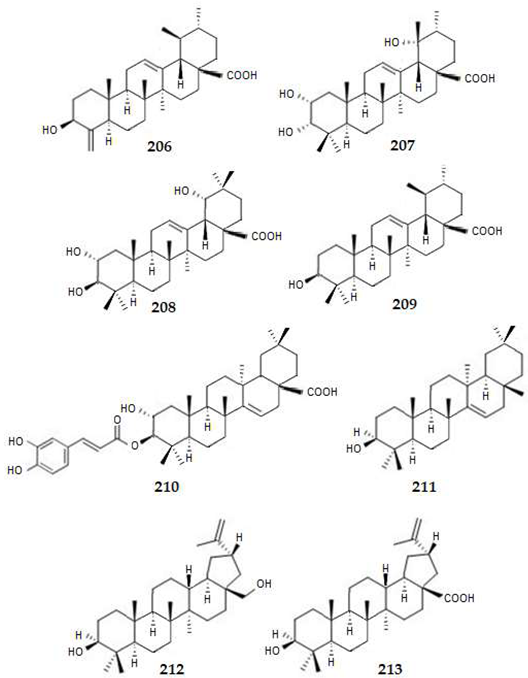
2.4.5. Meroterpenes
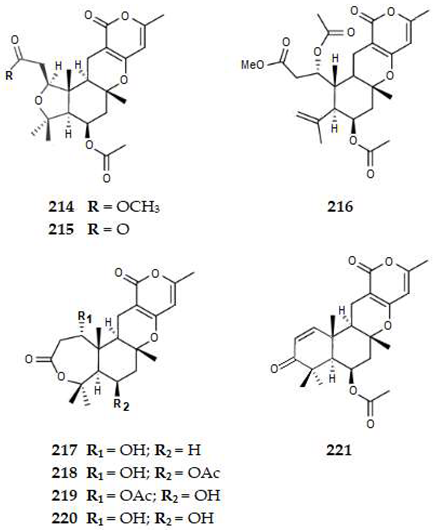
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Terpene (T) | Inhibition (I) | ||||||
|---|---|---|---|---|---|---|---|
| N. | Name | Class | Source | Log P 1 | Mechanism | IC50 (μM) or I (%) | Refs. |
| 1 | Linalool | MonoT | Plant | 2.97 | BACE1 | IN | [63] |
| P-IF (NO) | ND | [78,79] | |||||
| AChE | IN | [121] | |||||
| 2 | 2,3,4,4-Tetramethyl-5-methylene-cyclopent-2-enone | MonoT | Plant | 1.7 | BACE1 | 31.8% at 45 μM | [63] |
| 3 | Gracilin L | DiT | Sponge | ND | BACE1 | 24.6% at 1 μM | [64,65] |
| P-IF | ND | [101] | |||||
| 4 | Gracilin H | DiT | Sponge | 3.1 | BACE1 | <24% at 1 μM | [64,65] |
| P-IF | ND | [101] | |||||
| 5 | Tetrahydroaplysulphurin-1 | DiT | Sponge | 4.6 | BACE1 | <24% at 1 μM | [64,65] |
| GSK3β | 57.1% at 1 μM | [65] | |||||
| P-IF | ND | [101] | |||||
| 6 | Gracilin A | DiT | Sponge | 5.1 | BACE1 | IN | [64,65] |
| P-IF | ND | [101] | |||||
| 7 | Ginsenoside Rg1 | TriT | Plant | 2.7 | BACE1 | 6.2 | [64,66] |
| 8 | Ginsenoside Re | TriT | Plant | ND | BACE1 | ND | [64,67] |
| 9 | Ginsenoside Rg3 | TriT | Plant | 4.0 | BACE1 | ND | [64,68] |
| P-IF | ND | [102,103,104] | |||||
| 10 | Pseudoginsenoside-F11 | TriT | Plant | 1.9 | BACE1 | ND | [69,70] |
| 11 | Asperterpene A | MeroT | Fungus | ND | BACE1 | 0.08 | [73,74] |
| 12 | Asperterpene B | MeroT | Fungus | ND | BACE1 | 0.06 | [73,74] |
| 13 | Asperterpene E | MeroT | Fungus | 2.0 | BACE1 | 3.3 | [73,74] |
| 14 | Asperterpene F | MeroT | Fungus | 2.1 | BACE1 | 5.9 | [73,74] |
| 15 | Asperterpene J | MeroT | Fungus | 1.5 | BACE1 | 31.7 | [73,74] |
| 16 | Asperterpene D | MeroT | Fungus | 1.0 | BACE1 | >50.0 | [73,74] |
| 17 | Asperterpene G | MeroT | Fungus | 2.0 | BACE1 | >50.0 | [73,74] |
| 18 | Asperterpene H | MeroT | Fungus | 2.4 | BACE1 | >50.0 | [73,74] |
| 19 | Asperterpene I | MeroT | Fungus | 1.8 | BACE1 | >50.0 | [73,74] |
| 20 | Asperterpene K | MeroT | Fungus | 2.1 | BACE1 | >50.0 | [73,74] |
| 21 | Asperterpene L | MeroT | Fungus | 1.7 | BACE1 | >50.0 | [73,74] |
| 22 | Asperterpene M | MeroT | Fungus | 2.6 | BACE1 | >50.0 | [73,74] |
| 23 | Terretonin | MeroT | Fungus | 1.2 | BACE1 | >50.0 | [74] |
| 24 | Terretonin A | MeroT | Fungus | 2.2 | BACE1 | >50.0 | [74] |
| 25 | Terretonin D | MeroT | Fungus | 2.0 | BACE1 | >50.0 | [74] |
| 26 | Terretonin G | MeroT | Fungus | 1.5 | BACE1 | >50.0 | [74] |
| 27 | Terretonin H | MeroT | Fungus | 1.9 | BACE1 | >50.0 | [74] |
| 28 | Terreusterpene A | MeroT | Fungus | 1.8 | BACE1 | 6.0 | [75] |
| 29 | Terreusterpene B | MeroT | Fungus | 2.5 | BACE1 | 11.4 | [75] |
| 30 | Terreusterpene C | MeroT | Fungus | 1.5 | BACE1 | >40.0 | [75] |
| 31 | Terreusterpene D | MeroT | Fungus | 1.2 | BACE1 | 1.9 | [75] |
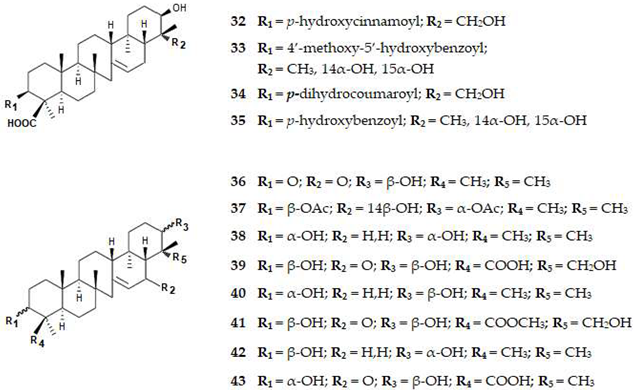
| 32 | 3β,21β,29-Trihydroxyserrat-14-en-24-oic acid-3b-yl-(70-hydroxycinnamate) | TriT | Plant | ND | BACE1 | 1.1 | [76] |
| AChE | >30.0 | [76,114] | |||||
| 33 | 3β,14α,15α,21β-Tetrahydroxyserratan-24-oic acid-3β-yl-(40 -methoxy-50-hydroxybenzoate) | TriT | Plant | ND | BACE1 | 1.0 | [76] |
| AChE | 10.0 | [76,114] | |||||
| 34 | 3β,21β,29-Trihydroxyserrat-14-en-3β-yl p-dihydrocoumarate | TriT | Plant | ND | BACE1 | >10.0 | [76] |
| AChE | 1.7 | [76,114] | |||||
| 35 | 3β,21β,29-Trihydroxyserrat-14-en-24-oic acid-3β-yl-(40-hydroxybenzoate) | TriT | Plant | ND | BACE1 | 0.3 | [76] |
| AChE | >30 | [76,114] | |||||
| 36 | 21β-Hydroxyserrat-14- en-3,16-dione | TriT | Plant | ND | BACE1 | 0.2 | [76] |
| AChE | 10.7 | [76,114] | |||||
| 37 | 3β,21α-Diacetoxyserratan-14β-ol | TriT | Plant | ND | BACE1 | >10 | [76] |
| AChE | 0.9 | [76,114] | |||||
| 38 | Serrat-14-en-3α,21α-diol | TriT | Plant | ND | BACE1 | >10.0 | [76] |
| AChE | >30.0 | [76,114] | |||||
| 39 | 3β,21β,29-Trihydroxy-16-oxoserrat-14-en-24-oic acid | TriT | Plant | ND | BACE1 | >10.0 | [76] |
| AChE | >30.0 | [76,114] | |||||
| 40 | Serrat-14-en-3α,21β-diol | TriT | Plant | ND | BACE1 | >10.0 | [76] |
| AChE | >30.0 | [76,114] | |||||
| 41 | 3β,21β,29-Trihydroxy-16-oxoserrat-14-en-24-methyl ester | TriT | Plant | ND | BACE1 | >10.0 | [76] |
| AChE | >30.0 | [76,114] | |||||
| 42 | 3β,21α-Dihydroxyserratan-14β-ol | TriT | Plant | ND | BACE1 | > 10.0 | [76] |
| AChE | >30.0 | [76,114] | |||||
| 43 | 3α,21β-Dihydroxy-16-oxoserrat-14-en-24-oic acid | TriT | Plant | ND | BACE1 | >10.0 | [76] |
| AChE | >30.0 | [76,114] | |||||
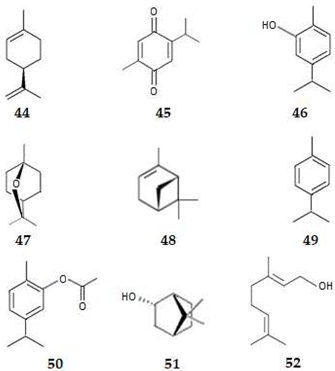
| 44 | (+)-Limonene | MonoT | Plant | 3.4 | P-IF | ND | [77] |
| 45 | Thymoquinone | MonoT | Plant | 2.0 | P-IF | ND | [80,81,82] |
| 46 | Carvacrol | MonoT | Plant | 3.1 | P-IF | ND | [83] |
| 47 | 1,8-Cineol | MonoT | Plant | 2.5 | P-IF | ND | [78,84] |
| AChE | IN | [121] | |||||
| 48 | α-Pinene | MonoT | Plant | 2.8 | P-IF | ND | [78,84] |
| AChE | IN | [121] | |||||
| 49 | p-Cymene | MonoT | Plant | 4.1 | P-IF | ND | [78,85] |
| AChE | IN | [121] | |||||
| 50 | Carvacryl acetate | MonoT | Plant | 3.0 | P-IF | ND | [78,86,87,88] |
| 51 | Borneol | MonoT | Plant | 2.7 | P-IF | ND | [78,86,87,88] |
| 52 | Geraniol | MonoT | Plant | 2.9 | P-IF | ND | [78,86,87,88] |
| 53 | Artemisinin | SesquiT | Plant | 2.8 | P-IF | ND | [89] |
| AChE | 103.9 | [125] | |||||
| 54 | Parthenolide | SesquiT | Plant | 2.3 | P-IF | ND | [91,92,93] |
| 55 | 7β-(3-Ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3,14-dehy-dro-Z-notonipetranone (ECN) | SesquiT | Plant | ND | P-IF | ND | [78,91] |
| 56 | α-Cyperone | SesquiT | Plant | 3.8 | P-IF | ND | [78,92,93,94] |
| 57 | Lactucopicrin | SesquiT | Plant | 1.1 | P-IF | ND | [78,92,93,94] |
| 58 | Bakkenolide B | SesquiT | Plant | 3.3 | P-IF | ND | [78,92,93,94] |
| 59 | Carnosic acid | DiT | Plant | 4.9 | P-IF | ND | [89,95,96] |
| 60 | Ginkgolide A | DiT | Plant | 0.6 | P-IF | ND | [97,98,99,100] |
| 61 | Ginkgolide B | DiT | Plant | 0.4 | P-IF | ND | [97,98,99,100] |
| 62 | Ginkgolide C | DiT | Plant | 1.4 | P-IF | ND | [97,98,99,100] |
| 63 | Ginsenoside Rd | TriT | Plant | 2.4 | P-IF | ND | [78,108,109] |
| 64 | Protopanaxatriol (PPT) | TriT | Plant | 5.9 | P-IF | ND | [78,108,109] |
| 65 | Gypenoside XVII | TriT | Plant | 1.9 | P-IF | ND | [78,111] |
| 66 | Methyl kulonate | TriT | Plant | 6.4 | P-IF (NO) | 4.6 | [112] |
| 67 | 3β-Hydroxy-24-nor-urs-4(23),12-dien-28-oic acid | TriT | Plant | ND | P-IF (NO) | 10.1 | [113] |
| 68 | Trichilia lactone D5 | TriT | Plant | ND | P-IF (NO) | >4.0 | [114,115] |
| AChE | 19.1 | [114,115] | |||||
| 69 | Rohituka 3 | TriT | Plant | 1.8 | P-IF (NO) | 3.0 | [114,115] |
| AChE | 34.2 | [114,115] | |||||
| 70 | Dregeanin DM4 | TriT | Plant | 2.6 | P-IF (NO) | 2.9 | [114,115] |
| AChE | 45.7 | [114,115] | |||||
| 71 | 2-Hydroxyxylorumphiin F | TriT | Plant | 3.9 | P-IF (NO) | 24.5 | [116] |
| 72 | Xylorumphiin E | TriT | Plant | 3.9 | P-IF (NO) | >50.0 | [116] |
| 73 | Xylorumphiin F | TriT | Plant | ND | P-IF (NO) | >50.0 | [116] |
| 74 | Xylorumphiin G | TriT | Plant | 3.9 | P-IF (NO) | >50.0 | [116] |
| 75 | Xylorumphiin H | TriT | Plant | ND | P-IF (NO) | >50.0 | [116] |
| 76 | Xylorumphiin I | TriT | Plant | 3.9 | P-IF (NO) | 31.3 | [116] |
| 77 | Xylorumphiin J | TriT | Plant | 3.9 | P-IF (NO) | >50.0 | [116] |
| 78 | Xyloccensin X | TriT | Plant | 2.5 | P-IF (NO) | >50.0 | [116] |
| 79 | Xyloccensin E | TriT | Plant | 1.5 | P-IF (NO) | >50.0 | [116] |
| 80 | Xyloccensin K | TriT | Plant | 1.9 | P-IF (NO) | >50.0 | [116] |
| 81 | Trichilinin B | TriT | Plant | 5.4 | P-IF (NO) | 29.2 | [112] |
| 82 | 3-Deacetyl-28-oxosalannin | TriT | Plant | 3.1 | P-IF (NO) | >100.0 | [112] |
| 83 | 3-Deacetyl-4’-demethyl-28-oxosalannin | TriT | Plant | ND | P-IF (NO) | >100.0 | [112] |
| 84 | 3-Deacetyl-28-oxosalannolactone | TriT | Plant | ND | P-IF (NO) | 86.0 | [112] |
| 85 | 3-Deacetyl-28-oxoisosalanninolide | TriT | Plant | ND | P-IF (NO) | >100.0 | [112] |
| 86 | 3-Deacetyl-17-defurano-17,28-dioxosalannin | TriT | Plant | ND | P-IF (NO) | >100.0 | [112] |
| 87 | Ohchinin | TriT | Plant | 4.1 | P-IF (NO) | 28.7 | [112] |
| 88 | 23-Hydroxyohchininolide | TriT | Plant | ND | P-IF (NO) | 58.6 | [112] |
| 89 | 21-Hydroxyisoohchinolide | TriT | Plant | ND | P-IF (NO) | 87.3 | [112] |
| 90 | Gedunin | TriT | Plant | 4.2 | P-IF | ND | [78,117] |
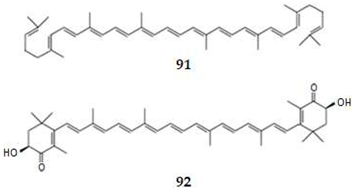
| 91 | Lycopene | TetraT | Plant | 15.6 | P-IF | ND | [78,118] |
| 92 | Astaxanthin | TetraT | Algae | 10.3 | P-IF | ND | [78,119,120] |
| 93 | Carvone | MonoT | Plant | 2.4 | AChE | 2900 | [121] |
| 94 | Ocimene | MonoT | Plant | 4.3 | AChE | 4700 | [121] |
| 95 | Menthone | MonoT | Plant | 2.7 | AChE | 9000 | [121] |
| 96 | Pulegone | MonoT | Plant | 2.8 | AChE | 9000 | [121] |
| 97 | Citral | MonoT | Plant | 3.0 | AChE | IN | [121] |
| 98 | Citronellal | MonoT | Plant | 3.0 | AChE | IN | [121] |
| 99 | Terpinene-4-ol | MonoT | Plant | 2.2 | AChE | IN | [121] |
| 100 | β-Myrcene | MonoT | Plant | 4.3 | AChE | IN | [121] |
| 101 | ϒ-Terpinene | MonoT | Plant | 2.8 | AChE | IN | [121] |
| 102 | Isopulegol | MonoT | Plant | 3.0 | AChE | IN | [121] |
| 103 | Menthol | MonoT | Plant | 3.0 | AChE | IN | [121] |
| 104 | α-Terpinene | MonoT | Plant | 4.2 | AChE | IN | [121] |
| 105 | α-Phellandrene | MonoT | Plant | 3.2 | AChE | IN | [121] |
| 106 | Farnesene | SesquiT | Plant | 5.7 | AChE | ND | [121] |
| 107 | Cornigeraline A | SesquiT | Plant | ND | AChE | 20.5 | [114,122] |
| 108 | Sibthorpine | SesquiT | Plant | ND | AChE | 35.8 | [114] |
| 109 | 3-Hydroxy-grosheimin | SesquiT | Plant | ND | AChE | 30.5 | [114] |
| 110 | Grosheimin | SesquiT | Plant | 0.8 | AChE | 61.8 | [114] |
| 111 | Solstitalin A | SesquiT | Plant | 0.0 | AChE | 25.7 | [114] |
| 112 | 13-Chlorosolstitialine | SesquiT | Plant | ND | AChE | 62.1 | [114] |
| 113 | Cynaropicrin | SesquiT | Plant | 0.6 | AChE | 31.3 | [114] |
| 114 | 1α,6β,8α-Triacetoxy-9β-furoyloxy-β-agarofuran | SesquiT | Plant | ND | AChE | 248 | [114,123] |
| 115 | 1α-Hydroxy-6β,8α-diacetoxy-9β-furoyloxy-β-agarofuran | SesquiT | Plant | ND | AChE | 738 | [114,123] |
| 116 | 1α,6β-Diacetoxy-8α-hydroxy- 9β-furoyloxy-β-agarofuran | SesquiT | Plant | ND | AChE | 161 | [114,123] |
| 117 | 1α-Acetoxy-6β,8α-dihydroxy-9β-furoyloxy-β-agarofuran | SesquiT | Plant | ND | AChE | 312 | [114,123] |
| 118 | 1α,2α,6β,8α-Pentaacetoxy-9β-benzoyloxy-β-agarofuran | SesquiT | Plant | ND | AChE | 122 | [114,123] |
| 119 | 1α,2α,3β,15-Tetraacetoxy-6β,9β-dibenzoyl-8-oxo-β-agarofuran | SesquiT | Plant | ND | AChE | 463 | [114,123] |
| 120 | 1α,6β,15-Triacetoxy-9-benzoyloxy-β-agarofuran | SesquiT | Plant | ND | AChE | 695 | [114,123] |
| 121 | 2α,3β,6β,8α,15-Pentaacetoxy-1α,9β-benzoyloxy-β-agarofuran | SesquiT | Plant | ND | AChE | 482 | [114,123] |
| 122 | 1α-Acetoxy,6β,9β-difuroyloxy-4β-hydroxy-β-agarofuran | SesquiT | Plant | ND | AChE | 738 | [114,123] |
| 123 | Pulicaryenne A | SesquiT | Plant | ND | AChE | 214.9 | [124] |
| 124 | (1S,6R, 9S, 11R)-13,14-Dihydroxycaryophyll-2(15)-en-7-one | SesquiT | Plant | ND | AChE | 40. 0 | [124] |
| 125 | (5Z)-14-Hydroxycaryophyllen-7-one | SesquiT | Plant | ND | AChE | 108.3 | [124] |
| 126 | (1S,6R, 9S, 11R)-13,14-Dihydroxycaryophyll-2(15)-en-7-one | SesquiT | Plant | ND | AChE | 101.2 | [124] |
| 127 | (1S,5Z,9R,11S)-12,14-Dihydroxycaryophylla-2(15),5-dien-7-one | SesquiT | Plant | ND | AChE | 25.8 | [124] |
| 128 | Quinanol A | SesquiT | Plant | ND | AChE | 63.1% at 45 μM or 100.8 μM | [126] |
| 129 | Quinanol B | SesquiT | Plant | ND | AChE | 15.0% at 45 μM | [126] |
| 130 | Quinanol C | SesquiT | Plant | ND | AChE | 19.1% at 45 μM | [126] |
| 131 | Quinanol D | SesquiT | Plant | ND | AChE | <10% at 45 μM | [126] |
| 132 | Quinanol E | SesquiT | Plant | ND | AChE | <10% at 45 μM | [126] |
| 133 | Sinenofuranol | SesquiT | Plant | 3.1 | AChE | 24.2% at 45 μM | [126] |
| 134 | Megatigma-7,9-diene-1,4-epoxy-2-hydroxy-10-carboxylic acid | SesquiT | Plant | ND | AChE | 9.3 | [127] |
| 135 | 12-O-Demethylcryptojaponol | DiT | Plant | 5.3 | AChE | 50.8 | [114,128] |
| 136 | 6α-Hydroxydemethylcryptojaponol | DiT | Plant | 4.7 | AChE | 19.2 | [114,128] |
| 137 | Lycocasuarinone A | DiT | Plant | ND | AChE | 26.8 | [127] |
| 138 | 15,16-Dihydrotanshinone | DiT | Plant | 3.2 | AChE | 65.2% at 35.8 µM | [129] |
| 139 | Miltirone | DiT | Plant | 4.9 | AChE | IN | [129] |
| 140 | Cryptotanshinone | DiT | Plant | 3.8 | AChE | IN | [129] |
| 141 | 1β-Hydroxy-cryptotanshinone | DiT | Plant | ND | AChE | IN | [129] |
| 142 | 1,2-Didehydromiltirone | DiT | Plant | 4.6 | AChE | IN | [129] |
| 143 | Pentylcurcumene | DiT | Plant | ND | AChE | 268.6 | [130] |
| 144 | Serrat-14-en-3β,21α-diol | TriT | Plant | 7.5 | AChE | >50.0 | [127] |
| 145 | Serrat-14-en-3β,21β-diol | TriT | Plant | 7.5 | AChE | >50.0 | [127] |
| 146 | α-Onocerin | TriT | Plant | 7.4 | AChE | >50.0 | [127] |
| 147 | 26-Nor-8-oxo-α-onocerin | TriT | Plant | 6.0 | AChE | >50.0 | [127] |
| 148 | 26-Nor-8-oxo-21-one-α-onocerin | TriT | Plant | 6.0 | AChE | 1.0 | [127] |
| 149 | Colocynthenin A | TriT | Plant | 2.7 | AChE | 2.6 | [114,131] |
| 150 | Colocynthenin B | TriT | Plant | 2.7 | AChE | >10 | [114,131] |
| 151 | Colocynthenin C | TriT | Plant | ND | AChE | 3.1 | [114,131] |
| 152 | Colocynthenin D | TriT | Plant | ND | AChE | >10.0 | [114,131] |
| 153 | Cucurbitacin E | TriT | Plant | 3.2 | AChE | >10.0 | [114,131] |
| 154 | 6′-Acetyl-2-O-β-D-glucocucurbitacin E | TriT | Plant | ND | AChE | >10.0 | [114,131] |
| 155 | Arvenin I | TriT | Plant | 1.0 | AChE | >10.0 | [114,131] |
| 156 | Arvenin II | TriT | Plant | 1.1 | AChE | >10.0 | [114,131] |
| 157 | Cucurbitacin B | TriT | Plant | 2.6 | AChE | >10.0 | [114,131] |
| 158 | 23,24-Dihydrocucurbitacin B | TriT | Plant | 2.7 | AChE | >10.0 | [114,131] |
| 159 | Colocynthoside A | TriT | Plant | 0.3 | AChE | >10.0 | [114,131] |
| 160 | Ganolucidic acid E | TriT | Plant | 5.0 | AChE | 13.8 | [114,132] |
| 161 | 11β-Hydroxy-3,7-dioxo-5α-lanosta-8,24(E)-dien-26-oic acid | TriT | Plant | ND | AChE | 10.8 | [114,132] |
| 162 | 23S-Hydroxy-3,7,11,15-tetraoxo-lanost-8,24(E)-diene-26-oic acid | TriT | Plant | ND | AChE | >200.0 | [114,132] |
| 163 | Ganoderic acid X1 | TriT | Plant | ND | AChE | >200.0 | [114,132] |
| 164 | Ganoderenic acid D | TriT | Plant | 2.0 | AChE | >200.0 | [114,132] |
| 165 | Ganoderenic acid H | TriT | Plant | 2.7 | AChE | >200.0 | [114,132] |
| 166 | Ganoderic acid K | TriT | Plant | 2.2 | AChE | >200.0 | [114,132] |
| 167 | 3β,7β-Dihydroxy-11,15,23-trioxo-lanost-8,16-dien-26-oic acid | TriT | Plant | 1.9 | AChE | >200.0 | [114,132] |
| 168 | Ganoderic acid E | TriT | Plant | 2.3 | AChE | >200.0 | [114,132] |
| 169 | 12-Acetoxy-3,7,11,15,23-pentaoxolanost-8,20-dien-26-oic acid | TriT | Plant | ND | AChE | >200.0 | [114,132] |
| 170 | 7β-Hydroxy-3,11,15,23-tetraoxo-27ξ-lanost-8,16-dien-26-oic acid | TriT | Plant | ND | AChE | >200.0 | [114,132] |
| 171 | Ganoderenic acid B | TriT | Plant | 2.4 | AChE | >200.0 | [114,132] |
| 172 | Ganoderenic acid F | TriT | Plant | 2.4 | AChE | >200.0 | [114,132] |
| 173 | Ganodernoid D | TriT | Plant | 2.1 | AChE | >200.0 | [114,132] |
| 174 | Ganodernoid F | TriT | Plant | 2.1 | AChE | >200.0 | [114,132] |
| 175 | Ganoderenic acid C | TriT | Plant | 2.7 | AChE | >200.0 | [114,132] |
| 176 | 3β-Hydroxyganodernoid D | TriT | Plant | 2.4 | AChE | >200.0 | [114,132] |
| 177 | Ganoderic acid H | TriT | Plant | 2.6 | AChE | >200.0 | [114,132] |
| 178 | 12β-Hydroxyganoderenic acid F | TriT | Plant | 1.9 | AChE | >200.0 | [114,132] |
| 179 | Methylganoderate F | TriT | Plant | ND | AChE | >200.0 | [114,132] |
| 180 | Ganoderic acid J | TriT | Plant | 2.6 | AChE | >200.0 | [114,132] |
| 181 | Ganoderic acid F | TriT | Plant | 2.3 | AChE | >200.0 | [114,132] |
| 182 | Ganoderic acid AP | TriT | Plant | ND | AChE | >200.0 | [114,132] |
| 183 | Ganoderic acid B | TriT | Plant | 2.2 | AChE | >200.0 | [114,132] |
| 184 | Ganoderic acid C | TriT | Plant | 2.5 | AChE | >200.0 | [114,132] |
| 185 | Ganoderic acid D | TriT | Plant | 1.9 | AChE | >200.0 | [114,132] |
| 186 | Ganoderic acid G | TriT | Plant | 1.7 | AChE | >200.0 | [114,132] |
| 187 | Ganoderic acid N | TriT | Plant | 0.7 | AChE | >200.0 | [114,132] |
| 188 | Methyl ganoderate G | TriT | Plant | 2.9 | AChE | >200.0 | [114,132] |
| 189 | Ganoderic acid Am1 | TriT | Plant | ND | AChE | 183.0 | [114,132] |
| 190 | Methyl ganoderate C | TriT | Plant | 2.2 | AChE | 148.0 | [114,132] |
| 191 | Ganodernoid C1 | TriT | Plant | ND | AChE | 142.0 | [114,132] |
| 192 | 12β-Hydroxyganodernic acid F | TriT | Plant | ND | AChE | 102.0 | [114,132] |
| 193 | Methyl ganoderate E | TriT | Plant | 2.6 | AChE | 45.8 | [114,132] |
| 194 | Ganoderic acid C6 | TriT | Plant | 2.0 | AChE | 147.5 | [114,132] |
| 195 | Methyl ganoderic acid C6 | TriT | Plant | ND | AChE | 145.2 | [114,132] |
| 196 | Gaodernoid A | TriT | Plant | ND | AChE | 149.0 | [114,132] |
| 197 | Gaodernoid B2 | TriT | Plant | ND | AChE | 102.4 | [114,132] |
| 198 | Ganoderlactone G | TriT | Plant | ND | AChE | 130.5 | [114,132] |
| 199 | Lucidone A | TriT | Plant | 1.2 | AChE | >200.0 | [114,132] |
| 200 | Lucidone D | TriT | Plant | 2.4 | AChE | >200.0 | [114,132] |
| 201 | Ganodernoid B | TriT | Plant | 1.5 | AChE | >200.0 | [114,132] |
| 202 | Ganodermanondiol | TriT | Plant | 5.8 | AChE | >200.0 | [114,132] |
| 203 | Norfriedelin A | TriT | Plant | 7.6 | AChE | 10.3 | [114,133] |
| 204 | Norfriedelin B | TriT | Plant | 8.3 | AChE | 28.7 | [114,133] |
| 205 | Norfriedelin C | TriT | Plant | 7.9 | AChE | >50.0 | [114,133] |
| 206 | 3β-Hydroxy-24-nor-urs-4(23)-12-dien-28-oic acid | TriT | Plant | ND | AChE | 10.0 | [113,114] |
| 207 | Euscaphic acid | TriT | Plant | 5.0 | AChE | 35.9 | [114,134] |
| 208 | Arjunic acid | TriT | Plant | 5.2 | AChE | 37.5 | [114,134] |
| 209 | Ursolic acid | TriT | Plant | 7.3 | AChE | 21.5 | [114,134] |
| 210 | 2-Hydroxy-3α-O-caffeoyltaraxar-14-en-28-oic acid | TriT | Plant | ND | AChE | 13.5 | [114,135] |
| 211 | Taraxerol | TriT | Plant | 9.3 | AChE | ND | [114,135] |
| 212 | Betulin | TriT | Plant | 8.3 | AChE | 28.5 | [114,135] |
| 213 | Betulinic acid | TriT | Plant | 8.2 | AChE | 24.2 | [114,135] |
| 214 | Asperversin A | MeroT | Fungus | 2.3 | AChE | >40.0 | [136] |
| 215 | Asperversin B | MeroT | Fungus | 2.2 | AChE | >40.0 | [136] |
| 216 | Asperversin C | MeroT | Fungus | 3.4 | AChE | >40.0 | [136] |
| 217 | Asperversin D | MeroT | Fungus | 2.4 | AChE | >40.0 | [136] |
| 218 | Asperversin E | MeroT | Fungus | 2.0 | AChE | >40.0 | [136] |
| 219 | Asperversin F | MeroT | Fungus | 2.6 | AChE | >40.0 | [136] |
| 220 | Asperdemin | MeroT | Fungus | 1.4 | AChE | >40.0 | [136] |
| 221 | Asperversin G | MeroT | Fungus | 3.3 | AChE | 13.6 | [136] |
References
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar]
- Isik, A.T. Late onset Alzheimer’s diseasae in older people. Clin. Interv. Aging 2010, 5, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, C.; van Broeckhoven, C.; Sleegers, K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. 2016, 18, 421–430. [Google Scholar] [CrossRef]
- Hauser, P.S.; Narayanaswami, V.; Ryan, R.O. Apolipoprotein E: From lipid transport to neurobiology. Prog. Lipid Res. 2011, 50, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kanekiyo, T.; Xu, H.; Bu, G. Apoliprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Ver. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The role of apoliprotein E in Alzheimer’s disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yamada, K.; Liddelow, S.A.; Smith, S.T.; Zhan, L.; Lun, W.; Tsai, R.M.; Spina, S.; Grinberg, L.T.; Rojas, J.C.; et al. ApoEε4 markedly exacerbates tau-mediated neurodegeneration in a mouse mode tauopathy. Nature 2017, 549, 523–527. [Google Scholar] [CrossRef]
- Bekris, L.M.; Yu, C.; Bird, T.D.; Tsuang, T.W. Genetics of Alzheimer’s disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213–227. [Google Scholar] [CrossRef]
- Martins, M.; Silva, R.; Pinto, M.M.M.; Sousa, E. Marine natural products, multitarget therapy and repurposed agents in Alzheimer’s disease. Pharmaceuticals 2020, 13, 242. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef]
- Macauley, S.L.; Holtzman, D.M. Recent advances from the bench toward the bedside in Alzheimer’s disease. EBioMedicine 2015, 2, 94–95. [Google Scholar] [CrossRef]
- Takashima, A. Tau aggregation is a therapeutic target for Alzheimer’s disease. Curr. Alzheimer Res. 2010, 7, 665–669. [Google Scholar] [CrossRef]
- Coman, H.; Nemes, B. New therapeutic targets in Alzheimer’s disease. Int. J. Gerontol. 2017, 11, 2–6. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment combinatoirs for Alzheimer’s disease: Current and future pharmacotherapy options. J. Alzheimer’s Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
- Fish, P.V.; Steadman, D.; Bayle, E.D.; Whiting, P. New approaches for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2019, 29, 125–133. [Google Scholar] [CrossRef]
- Desai, A.; Mitchison, T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Ballatore, C.; Brunden, K.R.; Huryn, D.M.; Trojanowski, J.Q.; Lee, V.M.-Y.; Smith, A.B., III. Microtubule stabilizing agents as potential treatment for Alzheimer’s disease and related neurodegenerative tauopathies. J. Med. Chem. 2012, 55, 8979–8996. [Google Scholar] [CrossRef]
- White, J.A., II; Banerjee, R.; Gunawardena, S. Axonal transport and neurodegeneration: How marine drugs can be used for the development of therapeutics. Mar. Drugs 2016, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Kosik, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef] [PubMed]
- Naini, S.; Soussi-Yanicostas, N. Tau hyperphosphorylation and oxidative stress, a critical vicious circle in neurodegenerative tauopathies? Oxidative Med. Cell. Longev. 2015, 2015, 151979. [Google Scholar]
- Kolarova, M.; García-Sierra, F.; Bartos, A.; Ricny, J.; Ripava, D.; Ripava, D. Structure and pathology of tau protein in Alzheimer disease. Int. J. Alzheimer’s Dis. 2012, 2012, 731526. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Yardin, C.; Terra, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef]
- Citron, M. Alzheimer’s disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yin, H.; Kuret, J. Casein kinase 1 delta phosphorylates tau and disrupts its binding to microtubules. J. Biol. Chem. 2004, 279, 15938–15945. [Google Scholar] [CrossRef] [PubMed]
- Llorach-Pares, L.; Nonell-Canals, A.; Avila, C.; Sanchez-Martinez, M. Kororamides, convolutamines, and indole derivatives as possible tau and dual-specifity kinase inhibitors for Alzheimer’s disease: A computational study. Mar. Drugs 2018, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Karthikeyan, C.; Moorthy, H.N.; Waiker, D.K.; Jain, A.K.; Trivedi, P. Human CDC2-like kinase 1 (CLK1): A novel target for Alzheimer’s disease. Curr. Drug Targets 2014, 15, 539–550. [Google Scholar] [CrossRef]
- Tell, V.; Hilgeroth, A. Recent developments of protein kinase inhibitors as potential AD therapeutics. Front. Cell. Neurosci. 2013, 7, 189. [Google Scholar] [CrossRef]
- Dolan, P.J.; Johnson, G.V.W. The role of tau kinases in Alzheimer’s disease. Curr. Opin. Drug Discov. Dev. 2010, 13, 595–603. [Google Scholar]
- Stotani, S.; Giordanetto, F.; Medda, F. DYRKlA inhibition as potential treatment for Alzheimer’s disease. Future Med. Chem. 2016, 8, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Branca, C.; Shaw, D.M.; Belfiore, R.; Gokhale, V.; Shaw, A.Y.; Foley, C.; Smith, B.; Hulme, C.; Dunckley, T.; Meechoovet, B.; et al. Dyrkl inhibition improves Alzheimer’s disease-like pathology. Aging Cell 2017, 16, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; Killick, R.; Lovestone, S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008, 104, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Martín, M.; Jurado, J.; Hemández, F.; Avila, J. GSK-3, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 2014, 7, 46. [Google Scholar] [CrossRef]
- Hemández, F.; Gómez de Barreda, E.; Fuster-Matanzo, A.; Lucas, J.J.; Avila, J. GSK3: A possible link between beta amyloid peptide and tau protein. Exp. Neurol. 2010, 223, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Hemández, F.; Lucas, J.J.; Avila, J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimers Dis. 2013, 33 (Suppl. S1), S141–S144. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P. Innate immune activation in neurodegenerative disease. Nat. Rev. lmmunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Schain, M.; Kreisl, W.C. Neuroinflammation in neurodegenerative disorders: A review. Curr. Neurol. Neurosci. Rep. 2017, 17, 25. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Cowan, M.; Petri, W.A. Microglia: Immune regulators of neurodevelopment. Front. Lmmunol. 2018, 9, 2576. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell. Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 44–468. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Cheng, J.; Hou, L. Drug development for Alzheimer’s disease: Microglia induced neuroinflammation as a target? Int. J. Mol. Sci. 2019, 20, 558. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Wang, X.; Liu, C.; Zhang, H.L. Pharmacological targeting of microglial activation: New therapeutic approach. Front. Cell. Neurosci. 2019, 13, 514. [Google Scholar] [CrossRef]
- Anglister, L.; Stiles, J.R.; Salpetert, M.M. Acetylcholinesterase density and turnover number at frog neuromuscular–junctions, with modeling of their role in synaptic function. Neuron 1994, 12, 783–794. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Ren, Y.; Howes, M. Acetylcholinesterase inhibitors from plants and fungi. J. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Alvarez, A.; Perez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–889. [Google Scholar] [CrossRef]
- Alvarez, A.; Alarcón, R.; Opazo, C.; Campos, E.O.; Muñoz, F.J.; Calderón, F.H.; Dajas, F.; Gentry, M.K.; Doctor, B.P.; De Mello, F.G.; et al. Stable complexes involving acetylcholinesterase and amyloid-beta peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer’s fibrils. J. Neurosci. 1998, 18, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Genereux, J.C.; Wiseman, R.L. Endoplasmic reticulum quality control and systemic amyloid disease: Impacting protein stability from the inside out. IUBMB Life 2015, 67, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, X.H.; Guan, J.; Ge, S.; Wu, M.B.; Lin, J.P. Advancement of multi-target drug discoveries and promising applications in the field of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 169, 200–223. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Lim, Y.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.-S. Terpenes from forests and human health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, L.Q.; Tang, X.C.; Zhang, H.Y. Retrospect and prospect of active principles from Chinese herbs in the treatment of dementia. Acta Pharmacol. Sin. 2010, 31, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Sergio, A.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic applications of terpenes on inflammatory diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef] [PubMed]
- Geris, R.; Simpson, T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009, 26, 1063–1094. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Bodor, N.; Buchwald, P. Retrometabolic Drug Design and Targeting; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 9–38. [Google Scholar]
- Leeson, P.D.; Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007, 11, 881–890. [Google Scholar] [CrossRef]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef]
- Muralidharan, A.; Josyula, V.R.; Hariharapura, R.C. Exploring the potential of marine microbes in clinical management of Alzheimer’s disease: A road map for bioprospecting and identifying promising isolates. Life Sci. 2018, 208, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Videira, R.; Castanheira, P.; Grãos, M.; Salgueiro, L.; Faro, C.; Cavaleiro, C. A necrodane monoterpenoid from Lavandula luisieri essential oil as a cell-permeable inhibitor of BACE-1, the β-secretase in Alzheimer’s disease. Flavour Fragr. J. 2013, 28, 380–388. [Google Scholar] [CrossRef]
- Naushad, M.; Durairajan, S.S.K.; Bera, A.K.; Senapati, S.; Li, M. Natural compounds with anti-BACE1 as promising therapeutic drugs for treating Alzheimer’s disease. Planta Med. 2019, 85, 1316–1325. [Google Scholar] [PubMed]
- Leirós, M.; Alonso, E.; Rateb, M.E.; Houssen, W.E.; Ebel, R.; Jaspars, M.; Alfonso, A.; Botana, L.M. Gracilins: Sponginella-derived promising compounds for Alzheimer’s didease. Neuropharmacology 2015, 93, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Du, G.-H. Ginsenoside Rg1 inhibits β-secretase activity in vitro and protects against Aβ-induced cytotoxicity in PC12 cells. J. Asian Nat. Prod. Res. 2009, 11, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Su, P.; Zhang, S.; Guo, L.; Zhang, H.; Liang, Y.; Qin, C.; Zhang, W. Ginsenoside Re reduces Aβ production by activating PPARγ to inhibit BACE1 in N2a/APP695 cells. Eur. J. Pharmacol. 2016, 793, 101–110. [Google Scholar] [CrossRef]
- Chen, F.; Eckman, E.A.; Eckman, C.B. Reductions in levels of the Alzheimer’s amyloid β peptide after oral administration of ginsenosides. FASEB J. 2006, 20, 1269–1271. [Google Scholar] [CrossRef]
- Laws, J.S., 3rd; Smid, S.D. Evaluating Cannabis sativa L.’s neuroprotection potential: From bench to bedside. Phytomedicine 2022, 107, 154485. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Jingyu, Y.; Chen, L.; Jun, X.; Shi, Q.; Xue, Y.; Chunfu, W. Pseudoginsenoside-F11 alleviates cognitive deficits and Alzheimer’s disease-type pathologies in SAMP8 mice. Pharmacol. Res. 2019, 139, 512–523. [Google Scholar]
- Fischbach, M.A.; Walsh, C.T. Assembly-line enzymologty for polyketide and nonribosomal peptide antibiotics: Logic, machinery, and mechanisms. Chem. Rev. 2006, 106, 3468–3496. [Google Scholar] [CrossRef]
- Itoh, T.; Tokunaga, K.; Matsuda, Y.; Fujii, I.; Abe, I.; Ebizuka, Y.; Kushiro, T. Reconstitution of a fungal meroterpenoid biosynthesis reveals the involvement of a novel family of terpene cyclases. Nat. Chem. 2010, 2, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Bao, J.; Wang, J.; Zhu, H.; Xue, Y.; Wang, X.; Li, H.; Sun, W.; Gao, W.; Lai, Y.; et al. Asperterpenes A and B, two unprecedented meroterpenoids from Aspergillus terreus with BACE1 inhibitory activities. Chem. Sci. 2016, 7, 6563. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Liu, M.; Zhou, Q.; Gao, W.; Chen, C.; Lai, Y.; Hu, Z.; Xue, Y.; Zhang, J.; Li, D.; et al. BACE1 Inhibitory meroterpenoids from Aspergillus terreus. J. Nat. Prod. 2018, 81, 1937–1945. [Google Scholar] [CrossRef]
- Qi, C.; Qiao, Y.; Gao, W.; Liu, M.; Zhou, Q.; Chen, C.; Lai, Y.; Xue, Y.; Zhang, J.; Li, D.; et al. New 3,5-dimethylorsellinic acid-based meroterpenoids with BACE1 and AchE inhibitory activities from Aspergillus terreus. Org. Biomol. Chem. 2018, 16, 9046. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; To, D.C.; Tran, M.H.; Oh, S.H.; Kim, J.A.; Ali, M.Y.; Woo, M.-H.; Choi, J.S.; Min, B.S. Isolation of cholinesterase and β-secretase 1 inhibiting compounds from Lycopodiella cernua. Bioorg. Med. Chem. 2015, 23, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.-S.; Koo, B.-S.; et al. Neuroprotective effects of limonene (+) against Aβ42-induced neurotoxicity in a Drosophila Model of Alzheimer’s Disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Pesce, M.; Patruno, A.; Moradi, S.Z.; Iranpanah, A.; Farzaei, M.H.; Sobarzo-Sanchez, E. Attenuation of NrF2/Keap1/ARE in Alzheimer’s disease by plant secondary metabolites: A mechanistic review. Molecules 2020, 25, 4926. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Shin, M.; Park, Y.; Choi, B.; Jang, S.; Lim, C.; Yun, H.S.; Lee, I.-S.; Won, S.-Y. Linalool alleviates Aβ42-Induced neurodegeneration via suppressing ROS production and inflammation in fly and rat models of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2021, 2021, 8887716. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Oesch, F. The immunosuppressive activity of artemisin-type drugs towards inflammatory and auto immune diseases. Med. Res. Rev. 2021, 41, 3023–3061. [Google Scholar] [CrossRef]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammatory by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef]
- Krylova, N.G.; Drobysh, M.S.; Semenkova, G.N.; Kulahava, T.A.; Pinchuk, S.V.; Shadyro, O.I. Cytotoxic and antiproliferative effects of thymoquinone on rat C6 glioma cells depend on oxidative stress. Mol. Cell. Biochem. 2019, 462, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Luo, Q.; Qiao, H.; Ding, H.; Cao, Y.; Yu, J.; Liu, R.; Zhang, Q.; Zhu, H.; Qu, I. The neuroprotective effects of ethanol-induced hippocampal neurons impairment via the antioxidative and antipoptotic pathways. Oxidative Med. Cell. Longev. 2017, 2017, 4079425. [Google Scholar] [PubMed]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. In vitro neuroprotective potential of the monoterpenes α-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. Naturforsch. C J. Biosci. 2016, 71, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.M.; Carvalho, R.B.F.; Costa, I.H.F.; Oliveira, G.A.L.; Souza, A.A.; Lima, S.G.; Freitas, R.M. Evaluation of p-cymene, a natural antioxidante. Pharm. Biol. 2015, 53, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.F.; Costa, L.M.; Almeida, A.A.C.; Silva, O.A.; Cerqueira, G.S.; Sousa, D.P.; Freitas, R.M. Is there a correlation between in vitro antioxidant potential and in vivo effect of carvacryl acetate against oxidative stress in mice hippocampus? Neurochem. Res. 2014, 39, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Pak, S.C.; Koo, B.-S.; Jeon, S. Borneol alleviates oxidative stress via upregulationof Nrf2 and Bcl-2 in SH-SY5Y cells. Pharm. Biol. 2013, 51, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.N.; Muralidhara, M. Analysis of the antioxidant activity of geraniol employing various in vitro models. Relevance to neurodegeneration in diabetic neuropathy. Asian J. Pharm. Clin. Res. 2017, 10, 10–105. [Google Scholar] [CrossRef]
- Olajide, O.A.; Sarker, S.D. Alzheimer’s disease: Natural products as inhibitors of neuroinflammation. Inflammopharmacalogy 2020, 28, 1439–1455. [Google Scholar] [CrossRef] [PubMed]
- Souček, M.; Herout, V.; Šorm, F. On terpenes CXVIII. Constitution of parthenolide. Collect. Czech. Chem. Commun. 1961, 26, 803–810. [Google Scholar] [CrossRef]
- Lee, J.; Songa, K.; Huhb, E.; Oh, M.S.; Kima, Y.S. Neuroprotection against 6-OHDA toxicity in PC12 cells and mice through the Nrf2 pathway by a sesquiterpenoid from Tussilago farfara. Redox Biol. 2018, 18, 6–15. [Google Scholar] [CrossRef]
- Ramu Venkatesan, R.; Subedi, L.; Yeo, E.-J.; Kim, S.Y. Lactucopicrin ameliorates oxidative stress mediated by scopolamineinduced neurotoxicity through activation of the NRF2 pathway. Neurochem. Int. 2016, 99, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.X.; He, D.W.; Chen, G.X.; Ran, X.; Guo, W.J.; Kan, X.C.; Wang, W.; Liu, D.F.; Fu, S.P.; Liu, J.X. α-Cyperone inhibits LPS-induced inflammation in BV-2 cells through activation of Akt/Nrf2/HO-1 and suppression of the NF-κB pathway. Food Funct. 2018, 9, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Choi, M.H.; Park, G.; Choi, Y.-W. Petasites japonicus bakkenolide B inhibits lipopolysaccharide-induced pro-inflammatory cytokines via AMPK/Nrf2 induction in microglia. Int. J. Molec. Med. 2018, 41, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Yan, W.; Gu, Z.; Li, Y.; Chen, L.; He, B. Anti-neuroinflammatory potential of natural products in the treatment of Alzheimer’s disease. Molecules 2023, 28, 1486. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Trudler, D.; Oh, C.K.; Lipton, S.A. Potential therapeutic use of the Rosemary diterpene carnosic acid for Alzheimer’s disease, Parkinson’s disease, and long-COVID through NRF2 activation to counteract the NLRP3 inflammasome. Antioxidants 2022, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Hebert, M.; Bellavance, G.; Barriault, L. Total synthesis of ginkgolide C and formal synthesis of ginkgolides A and B. J. Am. Chem. Soc. 2022, 144, 17792–17796. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.G.; Lu, X.; Gao, W.; Li, P.; Yang, H. Structure, synthesis, biosynthesis, and activity of the characteristic compounds from Ginkgo biloba L. Nat. Prod. Rep. 2022, 39, 474–511. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.T.; Yin, H.; Xu, B.L.; Yang, T.T.; Li, H.Q.; Sun, Y.; Liu, G.Z. Protective effects of ginkgolide on a cellular model of Alzheimer’s disease via suppression of the NF-kappaB signaling pathway. Appl. Biochem. Biotechnol. 2022, 194, 2448–2464. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Yang, Y.; Wang, J.; Chen, J.; Li, J.; Di, L. Optimization of the preparation conditions of borneol-modified ginkgolide liposomes by response surface methodology and study of their blood brain barrier permeability. Molecules 2018, 23, 303. [Google Scholar] [CrossRef]
- Leirós, M.; Sánchez, J.A.; Alonso, E.; Rateb, M.E.; Houssen, W.E.; Ebel, R.; Jaspars, M.; Alfonso, A.; Botana, L.M. Spongionella secondary matabolites protect mitochondrial function in cortical neurons against oxidative stress. Mar. Drugs 2014, 12, 700–718. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Park, J.; Kim, S.H.; Kwon, S.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Ginsenoside Rg3 alleviates lipopolysacaharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomol. Ther. 2013, 21, 381–390. [Google Scholar] [CrossRef]
- Ahn, J.W.; Jang, S.K.; Jo, B.R.; Kim, H.S.; Park, J.Y.; Park, H.Y.; Yoo, Y.M.; Joo, S.S. A therapeutic intervention for Alzheimer’s disease using ginsenoside Rg3: Its role in M2 microgial activation and non-amyloidogenesis. J. Physiol. Pharmacol. 2021, 72, 185–193. [Google Scholar]
- Li, J.; Huang, Q.; Chen, J.; Qi, H.; Liu, J.; Chen, J.; Zhao, D.; Wang, Z.; Li, X. Neuroprotective potentials of Panax Ginseng against Alzheimer’s disease: A review of preclinical and clinical evidences. Front. Pharmacol. 2021, 12, 688490. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bai, X.; Yu, S.; Zhao, W.; Qiao, J.; Liu, Y.; Zhao, D.; Wang, J.; Wang, S. Ginsenoside Re inhibits ROS/ASK-1 dependent mitochondrial apoptosis pathway and activation of Nrf2-antioxidant response in beta-amyloid-challenged SH-SY5Y cells. Molecules 2019, 24, 2687. [Google Scholar] [CrossRef]
- Wang, Y.; Kan, H.; Yin, Y.; Wu, W.; Hu, W.; Mingming Wang, M.; Weiping Li, W.; Li, W. Protective effects of ginsenoside Rg1 on chronic restraint stress induced learning and memory impairments in male mice. Pharmacol. Biochem. Behav. 2014, 120, 73–78. [Google Scholar] [CrossRef]
- Kovac, S.; Angelova, P.R.; Holmström, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta 2015, 1850, 794–801. [Google Scholar] [CrossRef]
- Du, J.; Cui, C.-H.; Park, S.C.; Kim, J.-K.; Yu, H.-S.; Jin, F.-X.; Sun, C.; Kim, S.-C.; Im, W.-T. Identification and characterization of a ginsenoside-transforming β-glucosidase from Pseudonocardia sp. Gsoil 1536 and its application for enhanced production of minor ginsenoside Rg2(S). PLoS ONE 2014, 9, e96914. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Ly, J.; Dong, L.; Jiang, N.; Wang, Y.; Wang, Q.; Li, Y.; Chen, S.; Fan, B.; Wang, F.; et al. Neuroprotective effects of 20(S)-protopanaxatriol (PPT) on scopolamine-induced cognitive deficits in mice. Phytother. Res. 2018, 32, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.S.; Lee, Y.C.; Rhee, Y.K.; Lee, S.Y. Consumer acceptance of ginseng food products. J. Food Sci. 2011, 76, S516–S522. [Google Scholar]
- Meng, X.; Wang, M.; Sun, G.; Ye, J.; Zhou, Y.; Dong, X.; Wang, T.; Lu, S.; Sun, X. Attenuation of Aβ25–35-induced parallel autophagic and apoptotic cell death by gypenoside XVII through the estrogen receptor-dependent activation of Nrf2/ARE pathways. Toxicol. Appl. Pharmacol. 2014, 279, 63–75. [Google Scholar] [CrossRef]
- Pan, X.; Matsumotoa, M.; Nishimotoa, Y.; Ogihara, E.; Zhang, J.; Ukiya, M.; Tokuda, H.; Koikec, K.; Akihisad, M.; Akihisa, T. Cytotoxic and nitric oxide production-inhibitory activities of limonoids and other compounds from the leaves and bark of Melia azedarach. Chem. Biodiv. 2014, 11, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Ma, R.-J.; Yang, L.; Li, J.-Y.; Hou, B.; Zhou, J. Triterpenoids and iridoids from Patrinia scabiosaefolia. Fitoterapia 2017, 119, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Min, S.L.S.; Liew, S.Y.; Chear, N.J.Y.; Goh, B.H.; Tan, W.-N.; Khaw, K.Y. Plant terpenoids as the promising source of cholinesterase inhibitors for anti-AD therapy. Biology 2022, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; Tsamo, A.T.; Melong, R.; Mkounga, P.; Nkengfack, A.E.; McGaw, L.J.; Elof, J.N. Cytotoxicity, nitric oxide and acetylcholinesterase inhibitory activity of three limonoids isolated from Trichilia welwitschii (Meliaceae). Biol. Res. 2015, 48, 57. [Google Scholar] [CrossRef] [PubMed]
- Sarigaputi, C.; Sommit, D.; Teerawatananond, T.; Pudhom, K. Weakly Anti-inflammatory limonoids from the seeds of Xylocarpus rumphii. J. Nat. Prod. 2014, 77, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Tom, S.; Rane, A.; Katewa, A.S.; Chamoli, M.; Matsumoto, R.R.; Andersen, J.K.; Chinta, S.J. Gedunin inhibits oligomeric Aβ1–42-induced microglia activation via modulation of Nrf2-NF-κB signaling. Mol. Neurobiol. 2019, 56, 7851–7862. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.; Wang, Z.; Cui, Y.; Tan, X.; Yuan, T.; Liu, Q.; Liu, Z.; Liu, X. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J. Nutr. Biochem. 2018, 56, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sánchez, E. The neuroprotective effects of astaxanthin: Therapeutic targets and clinical perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Abbaszadeh, F.; Dargahic, L.; Jorjania, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Targowska-Duda, K.; Klimek, K.; Ginalska, G.; Jóźwiak, K.; Waksmundzka-Hajnos, M.; Cieśla, L. Volatile terpenoids as potential drug leads in Alzheimer’s disease. Open Chem. 2017, 15, 332–343. [Google Scholar] [CrossRef]
- Hegazy, M.E.; Ibrahim, A.Y.; Mohamed, T.A.; Shahat, A.A.; El Halawany, A.M.; Abdel-Azim, N.S.; Alsaid, M.S.; Pare, P.W. Sesquiterpene lactones from Cynara cornigera: Acetyl cholinesterase inhibition and in silico ligand docking. Planta Med. 2016, 82, 138–146. [Google Scholar]
- Alarcón, J.; Cespedes, C.L.; Muñoz, E.; Balbontin, C.; Valdes, F.; Gutierrez, M.; Astudillo, L.; Seigler, S. Dihydroagarofuranoid sesquiterpenes as acetylcholinesterase inhibitors from Celastraceae plants: Maytenus disticha and Euonymus japonicus. J. Agric. Food Chem. 2015, 63, 10250–10256. [Google Scholar] [CrossRef]
- Afifa Zardi-Bergaoui, A.; Znati, M.; Harzallah-Skhiri, F.; Jannet, H.B. Caryophyllene Sesquiterpenes from Pulicaria vulgaris Gaertn.: Isolation, structure determination, bioactivity and structure. Chem. Biodivers. 2019, 16, e1800483. [Google Scholar]
- Chougouo, R.D.K.; Nguekeu, Y.M.M.; Dzoyem, J.P.; Awouafack, M.D.; Kouamouo, J.; Tane, P.; Mcgaw, L.J.; Eloff, J.N. Anti-inflammatory and acetylcholinesterase activity of extract, fractions, and five compounds isolated from the leaves and twigs of Artemisia annua growing in Cameroon. Springerplus 2016, 5, 1525. [Google Scholar] [CrossRef]
- Yang, D.-L.; Li, W.; Dong, W.-H.; Wang, J.; Mei, W.-L.; Dai, H.-F. Five new 5,11-epoxyguaiane sesquiterpenes in agarwood “Qi-Nan” from Aquilaria sinensis. Fitoterapia 2016, 112, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Li, D.; Li, X.-M.; Li, D.; Zhou, G.; Xu, K.-P.; Kang, F.-H.; Zou, Z.-X.; Xu, P.-S.; et al. Anti-cholinesterase activities of constituents isolated from Lycopodiastrum casuarinoides. Fitoterapia 2019, 139, 104366. [Google Scholar] [CrossRef]
- Murata, T.; Seldenge, E.; Oikawa, S.; Ageishi, K.; Batkhuu, J.; Sasaki, K.; Yoshizaki, F. Cholinesterase-inhibitory diterpenoids and chemical constituents from aerial parts of Caryopteris mongolica. J. Nat. Med. 2015, 69, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Senol, F.S.; Ślusarczyk, S.; Matkowski, A.; Pérez-Garrido, A.; Girón-Rodríguez, F.; Cerón-Carrasco, J.P.; den-Haan, H.; Peña-García, J.; Pérez-Sánchez, H.; Domaradzki, K.; et al. Selective in vitro and in silico butyrylcholinesterase inhibitory activity of diterpenes and rosmarinic acid isolated from Perovskia atriplicifolia Benth. and Salvia glutinosa L. Phytochemistry 2017, 133, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Dash, U.C.; Kanhar, S.; Dixit, A.; Dandapat, J.; Sahoo, A.K. Isolation, identification, and quantification of pentylcurcumene from Geophila repens: A new class of cholinesterase inhibitor for Alzheimer’s disease. Bioorg. Chem. 2019, 88, 102947. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.; Chen, X.; Chen, S.-X.; Gan, L.-S.; Tao Yuan, T. Colocynthenins A−D, ring-A seco-cucurbitane triterpenoids from the fruits of Citrullus colocynthis. J. Nat. Prod. 2018, 81, 2115–2119. [Google Scholar] [CrossRef]
- Wei, J.-C.; Wang, A.-H.; Wei, Y.-L.; Huo, X.-K.; Tian, X.-G.; Feng, L.; Ma, X.-C.; Wang, C.; Huang, S.-S.; Jia, J.-M. Chemical characteristics of the fungus Ganoderma lucidum and their inhibitory effects on acetylcholinesterase. J. Asian Natural Products Res. 2018, 20, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Q.; Peng, X.-R.; Xu-Yang Li, X.-Y.; Li, T.-Z.; Zhang, W.-M.; Shi, L.; Han, J.; Qiu, M.-H. Norfriedelins A-C with acetylcholinesterase inhibitory activity from acerola tree (Malpighia emarginata). Org. Lett. 2013, 15, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Ado, M.A.; Maulidiani, M.; Ismail, I.S.; Ghazali, H.M.; Shaari, K.; Abas, F. Acetylcholinesterase and α-glucosidase inhibitory compounds from Callicarpa maingayi. Nat. Prod. Res. 2021, 35, 2992–2996. [Google Scholar] [CrossRef] [PubMed]
- Jamila, N.; Khairuddean, M.; Yeong, K.K.; Osman, H.; Murugaiyah, V. Cholinesterase inhibitory triterpenoids from the bark of Garcinia hombroniana. J. Enzym. Inhib. Med. Chem. 2015, 30, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, W.; Deng, M.; Qi, C.; Chen, C.; Zhu, H.; Luo, Z.; Wang, J.; Xue, Y.; Zhang, Y. Aperversins A and B, two novel meroterpenoids with an unusual 5/6/6/6 ring from the marine-derived fungus Aspergillus versicolor. Mar. Drugs 2018, 16, 177. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodeghenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, X.; He, S.; Jiang, H.; Feng, F.; Liu, W.; Qu, W.; Sun, H. Rational design of multitarget-directed ligands: Strategies and emerging paradigms. J. Med. Chem. 2019, 62, 888–8914. [Google Scholar] [CrossRef]
- Kabir, A.; Muth, A. Polypharmacology: The science of multi-targeting molecules. Pharmacol. Res. 2022, 176, 106055. [Google Scholar] [CrossRef]
- Lima, E.; Medeiros, J. Marine organisms as alkaloid biosynthesizers of potential anti-Alzheimer agents. Mar. Drugs 2022, 20, 75. [Google Scholar] [CrossRef]
- Prati, F.; Uliassi, E.; Bolognesi, M. Two disease, one approach: Multitarget drug discovery in Alzheimer’s and neglected tropical diseases. Med. Chem. Comm. 2014, 5, 853–861. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, E.; Medeiros, J. Terpenes as Potential Anti-Alzheimer’s Disease Agents. Appl. Sci. 2024, 14, 3898. https://doi.org/10.3390/app14093898
Lima E, Medeiros J. Terpenes as Potential Anti-Alzheimer’s Disease Agents. Applied Sciences. 2024; 14(9):3898. https://doi.org/10.3390/app14093898
Chicago/Turabian StyleLima, Elisabete, and Jorge Medeiros. 2024. "Terpenes as Potential Anti-Alzheimer’s Disease Agents" Applied Sciences 14, no. 9: 3898. https://doi.org/10.3390/app14093898
APA StyleLima, E., & Medeiros, J. (2024). Terpenes as Potential Anti-Alzheimer’s Disease Agents. Applied Sciences, 14(9), 3898. https://doi.org/10.3390/app14093898







