A Review on the Potential Use of Medicinal Plants from the Apiaceae and the Rosaceae Families in Cardiovascular Diseases—Experimental Evidence and Traditional Applications
Abstract
1. Introduction
2. The Aim of the Study
3. Methods
4. Discussion
4.1. Activities
4.1.1. Antihypertensive, Lower Elevated Blood Pressure
4.1.2. Vasodilator, Vasorelaxant, and Anti-Vasospasm
4.1.3. Calcium Channel-Blocking Actions
4.1.4. Diuretic
4.1.5. Cardioprotective
4.1.6. Anti-Thrombotic (Prevent Blood Coagulation), Antiplatelet, Inhibits Platelet Aggregation, Anticoagulation
4.1.7. Antiarrhythmic
- Positive Inotropic Effects on Heart
- Negative Chronotropic Effect
4.1.8. Hypolipidemic (Cholesterol-Lowering Properties), Antihyperlipidemic, and Hypocholesterolemic Activity
4.1.9. Antioxidant Activity
4.2. Individual Plants
4.2.1. Crataegus (Hawthorn) sp.
4.2.2. Rubus idaeus
4.2.3. Ammi visnaga
4.2.4. Coriandrum sativum
4.2.5. Anethum graveolens
4.2.6. Apium graveolens
4.2.7. Petroselinum crispum
4.2.8. Prunus amygdalus
4.3. Active Compounds Responsible for Activity in Cardiovascular Diseases
5. Conclusions and Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 28 November 2023).
- World Heart Report 2023: Confronting the World’s Number One Killer; World Heart Federation: Geneva, Switzerland, 2023.
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: A narrative review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Thayabaranathan, T.; Kim, J.; Cadilhac, D.A.; Thrift, A.G.; Donnan, G.A.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; et al. Global stroke statistics 2022. Int. J. Stroke 2022, 17, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Peripheral Artery Disease Collaborators. Global burden of peripheral artery disease and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Glob. Health 2023, 11, e1553–e1565. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, Q.; Much, A.A.; Maor, E.; Segev, A.; Beinart, R.; Adawi, S.; Lu, Y.; Bragazzi, N.L.; Wu, J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: Results from the Global Burden of Disease Study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Brieler, J.; Breeden, M.A.; Tucker, J. Cardiomyopathy: An overview. Am. Fam. Physician 2017, 96, 640–646. [Google Scholar] [PubMed]
- Santangelo, G.; Bursi, F.; Faggiano, A.; Moscardelli, S.; Simeoli, P.S.; Guazzi, M.; Lorusso, R.; Carugo, S.; Faggiano, P. The Global Burden of Valvular Heart Disease: From Clinical Epidemiology to Management. J. Clin. Med. 2023, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Dolk, H.; Loane, M.; Garne, E. European Surveillance of Congenital Anomalies (EUROCAT) Working Group. Congenital heart defects in Europe: Prevalence and perinatal mortality, 2000 to 2005. Circulation 2011, 123, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Rajani, R.; Klein, J.L. Infective endocarditis: A contemporary update. Clin. Med. 2020, 20, 31–35. [Google Scholar] [CrossRef]
- Wang, X.; Bu, X.; Wei, L.; Liu, J.; Yang, D.; Mann, D.L.; Ma, A.; Hayashi, T. Global, Regional, and National Burden of Myocarditis from 1990 to 2017: A Systematic Analysis Based on the Global Burden of Disease Study 2017. Front. Cardiovasc. Med. 2021, 8, 692990. [Google Scholar] [CrossRef]
- Lazarou, E.; Tsioufis, P.; Vlachopoulos, C.; Tsioufis, C.; Lazaros, G. Acute pericarditis: Update. Curr. Cardiol. Rep. 2022, 24, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.A.; Johnson, C.O.; Colquhoun, S.M.; Karthikeyan, G.; Beaton, A.; Bukhman, G.; Forouzanfar, M.H.; Longenecker, C.T.; Mayosi, B.M.; Mensah, G.A.; et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N. Engl. J. Med. 2017, 377, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Majeed, F.; Taleb, A.; Zubair, H.M.; Shumzaid, M.; Farooq, M.A.; Baig, M.M.F.A.; Abbas, M.; Saeed, M.; Changxing, L. A Review of medicinal plants in cardiovascular disorders: Benefits and risks. Am. J. Chin. Med. 2020, 48, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.H. A review of medicinal plants used in therapy of cardiovascular diseases. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 572–591. [Google Scholar]
- Delbaere, Q.; Chapet, N.; Huet, F.; Delmas, C.; Mewton, N.; Prunier, F.; Angoulvant, D.; Roubille, F. Anti-inflammatory drug candidates for prevention and treatment of cardiovascular diseases. Pharmaceuticals 2023, 16, 78. [Google Scholar] [CrossRef]
- Wong, Z.W.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of the protective role of natural products on isoproterenol-induced myocardial infarction: A review. Biomed. Pharmacother. 2017, 94, 1145–1166. [Google Scholar] [CrossRef]
- Guzman, E.; Molina, J. The predictive utility of the plant phylogeny in identifying sources of cardiovascular drugs. Pharm. Biol. 2018, 56, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Dimmito, M.P.; Stefanucci, A.; Della Valle, A.; Scioli, G.; Cichelli, A.; Mollica, A. An overview on plants cannabinoids endorsed with cardiovascular effects. Biomed. Pharmacother. 2021, 142, 111963. [Google Scholar] [CrossRef]
- The World Flora Online. Available online: https://wfoplantlist.org/ (accessed on 25 November 2023).
- World Checklist of Vascular Plants. Facilitated by the Royal Botanic Garden, Kew. Available online: https://powo.science.kew.org (accessed on 25 November 2023).
- Amiri, M.S.; Joharchi, M.R. Ethnobotanical knowledge of Apiaceae family in Iran: A review. Avicenna J. Phytomed. 2016, 6, 621–635. [Google Scholar]
- Somova, L.I.; Shode, F.O.; Moodley, K.; Govender, Y. Cardiovascular and diuretic activity of kaurene derivatives of Xylopia aethiopica and Alepidea amatymbica. J. Ethnopharmacol. 2001, 77, 165–174. [Google Scholar] [CrossRef]
- Anrep, G.V.; Barsoum, G.S.; Kenawy, M.R.; Misrahy, G. Ammi visnaga in the treatment of anginal syndrome. Br. Heart J. 1946, 8, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Bhagavathula, A.S.; Al-Khatib, A.J.M.; Elnour, A.A.; Al Kalbani, N.M.S.; Shehab, A. Ammi visnaga in treatment of urolithiasis and hypertriglyceridemia. Pharmacogn. Res. 2015, 7, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Torres, A.I.; Zarzuelo, A. Cardiovascular effects of visnagin on rats. Planta Med. 2000, 66, 35–39. [Google Scholar] [CrossRef]
- Duarte, J.; Vallejo, I.; Perez Vizcaino, F.; Jimenez, R.; Zarzuelo, A.; Tamargo, J. Effects of visnadine on rat isolated vascular smooth muscles. Planta Med. 1997, 63, 233–236. [Google Scholar] [CrossRef]
- Erbring, H.; Uebel, H.; Vogel, G. Zur chemie, pharmakologie und toxikologie von visnadin. Arzneimittelforschung 1967, 17, 283–287. [Google Scholar]
- Galal, E.E.; Kandil, A.; Latif, M.A. Evaluation of cardiac inotropism of Ammi visnaga principles by the intra-ventricular technique. J. Drug Res. Egypt 1975, 7, 45–57. [Google Scholar]
- Khan, Z.A.; Assiri, A.M.; Al-Afghani, H.M.; Maghrabi, T.M. Inhibition of oxalate nephrolithiasis with Ammi visnaga (AI-Khillah). Int. Urol. Nephrol. 2001, 33, 605–608. [Google Scholar] [CrossRef]
- Rauwald, H.W.; Brehm, O.; Odenthal, K.P. The involvement of a Ca2+ channel blocking mode of action in the pharmacology of Ammi visnaga fruits. Planta Med. 1994, 60, 101–105. [Google Scholar] [CrossRef]
- Amtaghri, S.; Slaoui, M.; Eddouks, M. Ammodaucus leucotrichus acts as an antihypertensive and vasorelaxant agent through sGC and prostaglandin synthesis pathways. Cardiovasc. Hematol. Agents Med. Chem. 2023, 21, 177–192. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Abbasi, N. Hypolipidemic activity of Anethum graveolens in rats. Phytother. Res. 2008, 22, 372–375. [Google Scholar] [CrossRef]
- Harmala, P.; Vuorela, H.; Hiltunen, R.; Nyiredy, S.; Sticher, O.; Tornquist, K.; Kaltia, S. Strategy for the isolation and identification of coumarins with calcium antagonistic properties from the roots of Angelica archangelica. Phytochem. Anal. 1992, 3, 42. [Google Scholar] [CrossRef]
- Harmala, P.; Vuorela, H.; Tornquist, K.; Hiltunen, R. Choice of solvent in the extraction of Angelica archangelica roots with reference to calcium blocking activity. Planta Med. 1992, 58, 176. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Shin, M.; Ham, I.; Choi, H. Investigation of the mechanisms of Angelica dahurica root extract-induced vasorelaxation in isolated rat aortic rings. BMC Complement. Altern. Med. 2015, 15, 395. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Murakami, T.; Nishida, N.; Kageura, T.; Yoshikawa, M. Medicinal Foodstuffs. XX. Vasorelaxant Active Constituents from the roots of Angelica furcijuga KITAGAWA: Structures of Hyuganins A, B, C, and D. Chem. Pharm. Bull. 2000, 48, 1429. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Sakuma, S.; Sumiya, T.; Nishida, H.; Fujimoto, Y.; Baba, K.; Kozawa, M. The effects of xanthoangelol E on arachidonic acid metabolism in the gastric antral mucosa and platelet of the rabbit. Res. Commun. Chem. Pathol. Pharmacol. 1992, 77, 227. [Google Scholar] [PubMed]
- Matsuura, M.; Kimura, Y.; Nakata, K.; Baba, K.; Okuda, H. Artery relaxation by chalcones isolated from the roots of Angelica keiskei. Planta Med. 2001, 67, 230. [Google Scholar] [CrossRef]
- Ko, F.N.; Wu, T.S.; Liou, M.J.; Huang, T.F.; Teng, C.M. Vasorelaxation of rat thoracic aorta caused by osthole isolated from Angelica pubescens. Eur. J. Pharmacol. 1992, 219, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Shen, S.; Dai, X.; Zhang, T. Angelica sinensis polysaccharide ameliorates myocardial ischemia-reperfusion injury in rats by inhibiting TLR4/NF-κB. Trop. J. Pharm. Res. 2023, 22, 777–782. [Google Scholar] [CrossRef]
- Brankovic, S.; Kitic, D.; Radenkovic, M.; Veljkovic, S.; Kostic, M.; Miladinovic, B.; Pavlovic, D. Hypotensive and cardio-inhibitory effects of the aqueous and ethanol extracts of celery (Apium graveolens, Apiaceae). Acta Medica Median. 2010, 49, 13–16. [Google Scholar]
- Fitzpatrick, D.F.; Hirschfield, S.L.; Ricci, T.; Jantzen, P.; Coffey, R.G. Endothelium-dependent vasorelaxation caused by various plant extracts. J. Cardiovasc. Pharmacol. 1995, 26, 90–95. [Google Scholar] [CrossRef]
- Ko, F.-N.; Huang, T.-F.; Teng, C.-M. Vasodilatory action mechanisms of apigenin isolated from Apium graveolens in rat thoracic aorta. Biochim. Biophys. Acta 1991, 1115, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.; Imenshahidi, M.; Mohajeri, S. Antihypertensive effect of celery seed on rat blood pressure in chronic administration. J. Med. Food. 2013, 16, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Shayani Rad, M.; Moohebati, M.; MohammadEbrahimi, S.; Motamedshariaty, V.S.; Mohajeri, S.A. Safety evaluation and biochemical efficacy of celery seed extract (Apium graveolens) capsules in hypertensive patients: A randomized, triple-blind, placebo-controlled, cross-over, clinical trial. Inflammopharmacology 2022, 30, 1669–1684. [Google Scholar] [CrossRef] [PubMed]
- Shayani Rad, M.S.; Moohebati, M.; Mohajeri, S.A. Effect of celery (Apium graveolens) seed extract on hypertension: A randomized, triple-blind, placebo-controlled, cross-over, clinical trial. Phytother. Res. 2022, 36, 2889–2907. [Google Scholar] [CrossRef] [PubMed]
- Tsi, D.; Das, N.P.; Tan, B.K.H. Effects of aqueous celery (Apium graveolens) extract on lipid parameters of rats fed a high fat diet. Planta Med. 1995, 61, 18–21. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Therapeutic properties of medicinal plants: A review of plants with cardiovascular effects (part 1). Int. J. Pharmacol. Toxicol. 2015, 5, 163–176. [Google Scholar]
- Gnanapragasam, A.; Ebenezar, K.K.; Sathish, V.; Govindaraju, P.; Devaki, T. Protective effect of Centella asiatica on antioxidant tissue defense system against adriamycin induced cardiomyopathy in rats. Life Sci. 2004, 76, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Satake, T.; Kamiya, K.; An, Y.; Oishi, T.; Yamamoto, J. The anti-thrombotic active constituents from Centella asiatica. Biol. Pharm. Bull. 2007, 30, 935–940. [Google Scholar] [CrossRef]
- Zhao, Y.; Shu, P.; Zhang, Y.; Lin, L.; Zhou, H.; Xu, Z.; Suo, D.; Xie, A.; Jin, X. Effect of Centella asiatica on oxidative stress and lipid metabolism in hyperlipidemic animal models. Oxidative Med. Cell. Longev. 2014, 2014, 154295. [Google Scholar] [CrossRef]
- Hussain, F.; Jahan, N.; Rahman, K.U.; Sultana, B.; Jamil, S. Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their Angiotensin-Converting Enzyme (ACE) inhibition potential. Oxidative Med. Cell. Longev. 2018, 2018, 4643736. [Google Scholar] [CrossRef]
- Jabeen, Q.; Bashir, S.; Lyoussi, B.; Gilani, A. Coriander fruit exhibits gut modulatory, blood pressure lowering and diuretic activities. J. Ethnopharmacol. 2009, 122, 123–130. [Google Scholar] [CrossRef]
- Patel, D.K.; Desai, S.N.; Gandhi, H.P.; Ranjitsinh, V.D.; Ramachandran, A.V. Cardio protective effect of Coriandrum sativum L. on isoproterenol induced myocardial necrosis in rats. Food Chem. Toxicol. 2012, 50, 3120–3125. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.M.; Selvam, A.; Mahismita, P. In vitro analysis of Coriandrum sativum on HL-1 cell line against FYCO1 as a potential therapeutic for cardiovascular disease. J. Cardiovasc. Dis. Res. 2022, 13, 1546–1560. [Google Scholar]
- Rana, R.; Mehmood, M.H.; Shaukat, B.; Shahid, S.; Malik, A.; Murtaza, B. Evaluation of the cardiovascular effects of Coriandrum sativum and Citrus limon to treat arsenic-induced endothelial damage and hypertension in rats. Life 2022, 12, 1842. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, P.; Jasuja, N.D.; Joshi, S.C. Ameliorative efficiency of Coriandrum sativum seed extract on atherosclerosis and oxidative stress in male albino hyperlipidemic rabbits. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 26–39. [Google Scholar]
- Zeb, F.; Safdar, M.; Fatima, S.; Khan, S.; Alam, S.; Muhammad, M.; Syed, A.; Habib, F.; Shakoor, H. Supplementation of garlic and coriander seed powder: Impact on body mass index, lipid profile and blood pressure of hyperlipidemic patients. Pak. J. Pharm. Sci. 2018, 31, 1935–1941. [Google Scholar]
- Afsheen, N.; Khalil Ur, R.; Jahan, N.; Khan, K.M.; Zia, M.A. Optimization of cardioprotective potential of various concentrations of medicinal plants by using response surface methodology. Pak. Vet. J. 2019, 39, 13–18. [Google Scholar] [CrossRef]
- Aissaoui, A.; Zizi, S.; Israili, Z.H.; Lyoussi, B. Hypoglycemic and hypolipidemic effects of Coriandrum sativum L. in Meriones shawi rats. J. Ethnopharmacol. 2011, 137, 652–661. [Google Scholar] [CrossRef]
- Chaudhary, S.K.; Maity, N.; Nema, N.K.; Bhadra, S.; Saha, B.P.; Mukherjee, P.K. Angiotensin converting enzyme inhibition activity of fennel and coriander oils from India. Nat. Prod. Commun. 2013, 8, 671–672. [Google Scholar] [CrossRef]
- Dhanapakiam, P.; Joseph, J.M.; Ramaswamy, V.K.; Moorthi, M.; Kumar, A.S. The cholesterol lowering property of coriander seeds (Coriandrum sativum): Mechanism of action. J. Environ. Biol. 2008, 29, 53–56. [Google Scholar]
- Dhyani, N.; Parveen, A.; Siddiqi, A.; Hussain, M.E.; Fahim, M. Cardioprotective efficacy of Coriandrum sativum (L.) seed extract in heart failure rats through modulation of endothelin receptors and antioxidant potential. J. Diet. Suppl. 2020, 17, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.A.; Kumar, T.; Murthy, P.B.; Pillai, K.S. Hypolipidemic effect of Coriandrum sativum L. in triton-induced hyperlipidemic rats. Indian J. Exp. Biol. 2004, 42, 909–912. [Google Scholar]
- Rehman, N.; Jahan, N.; ul-Rahman, K.; Khan, K.M.; Zafar, F. Anti-arrhythmic potential of Coriandrum sativum seeds in salt induced arrhythmic rats. Pak. Vet. J. 2016, 36, 465–471. [Google Scholar]
- Wang, X.; Liu, Y.; Wang, Y.; Dong, X.; Wang, Y.; Yang, X.; Tian, H.; Li, T. Protective effect of Coriander (Coriandrum sativum L.) on high-fructose and high-salt diet-induced hypertension: Relevant to improvement of renal and intestinal function. J Agric. Food Chem. 2022, 70, 3730–3744. [Google Scholar] [CrossRef] [PubMed]
- Shirke, S.S.; Jagtap, A.G. Effects of methanolic extract of Cuminum cyminum on total serum cholesterol in ovariectomized rats. Indian J. Pharmacol. 2009, 41, 92–93. [Google Scholar] [CrossRef]
- Gilani, A.H.; Shaheen, E.; Saeed, S.A.; Bibi, S.; Irfanullah Sadiq, M.; Faizi, S. Hypotensive action of coumarin glycosides from Daucus carota. Phytomedicine 2000, 7, 423–426. [Google Scholar] [CrossRef]
- Gilani, A.H.; Shaheen, F.; Saeed, S.A. Cardiovascular actions of Daucus carota. Arch. Pharm. Res. 1994, 17, 150–153. [Google Scholar] [CrossRef]
- Nicolle, C.; Cardinault, N.; Aprikian, O.; Busserolles, J.; Grolier, P.; Rock, E.; Demigne, C.; Mazur, A.; Scalbert, A.; Amouroux, P.; et al. Effect of carrot intake on cholesterol metabolism and on antioxidant status in cholesterol-fed rat. Eur. J. Nutr. 2003, 42, 254–261. [Google Scholar] [CrossRef]
- Noriega-Cisneros, R.; Peña-Montes, D.J.; Huerta-Cervantes, M.; Torres-Martínez, R.; Huerta, M.; Manzo-Avalos, S.; Salgado-Garciglia, R.; Saavedra-Molina, A. Eryngium carlinae ethanol extract corrects lipid abnormalities in Wistar rats with experimental diabetes. J. Med. Food. 2020, 23, 827–833. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.S.; Amin, R. The vascular action of aqueous extracts of Foeniculum vulgare leaves. J. Ethnopharmacol. 1988, 24, 213–218. [Google Scholar] [CrossRef]
- Tognolini, M.; Ballabeni, V.; Bertoni, S.; Bruni, R.; Impicciatore, M.; Barocelli, E. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol. Res. 2007, 56, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Z.; Bache, R.J. Coronary and systemic hemodynamic effects of tetramethylpyrazine in the dog. J. Cardiovasc. Pharmacol. 1985, 7, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Ajebli, M.; Eddouks, M. Antihypertensive activity of Petroselinum crispum through inhibition of vascular calcium channels in rats. J. Ethnopharmacol. 2019, 242, 112039. [Google Scholar] [CrossRef]
- Brankovic, S.; Djosev, S.; Kitic, D.; Radenkovic, M.; Veljkovic, S.; Nesic, M.; Pavlovic, D. Hypotensive and negative chronotropic and inotropic effects of the aqueous and ethanol extract from parsley leaves. J. Clin. Lipidol. 2008, 2, 408. [Google Scholar] [CrossRef]
- Chaves, D.S.; Frattani, F.S.; Assafim, M.; de Almeida, A.P.; Zingali, R.B.; Costa, S.S. Phenolic chemical composition of Petroselinum crispum extract and its effect on haemostasis. Nat. Prod. Commun. 2011, 6, 961–964. [Google Scholar] [CrossRef] [PubMed]
- El Rabey, H.; Al-Seeni, M.; Al-Ghamdi, H. Comparison between the hypolipidemic activity of parsley and carob in hypercholesterolemic male rats. BioMed Res. Int. 2017, 2017, 3098745. [Google Scholar] [CrossRef] [PubMed]
- Gadi, D.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Legssyer, A.; Bruel, A.; Berrabah, M.; Legrand, C.; Fauvel-Lafeve, F.; Mekhfi, H. Flavonoids purified from parsley inhibit human blood platelet aggregation and adhesion to collagen under flow. J. Complement. Integr. Med. 2012, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Gadi, D.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Legssyer, A.; Legrand, C.; Lafeve, F.F.; Mekhfi, H. Parsley extract inhibits in vitro and ex vivo platelet aggregation and prolongs bleeding time in rats. J. Ethnopharmacol. 2009, 125, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Kreydiyyeh, S.I.; Usta, J. Diuretic effect and mechanism of action of parsley. J. Ethnopharmacol. 2002, 79, 353–357. [Google Scholar] [CrossRef]
- Malheiros, J.; Simões, D.M.; Antunes, P.E.; Figueirinha, A.; Cotrim, M.D.; Fonseca, D.A. Vascular effects of polyphenols from Agrimonia eupatoria L. and role of isoquercitrin in its vasorelaxant potential in human arteries. Pharmaceuticals 2022, 15, 638. [Google Scholar] [CrossRef]
- Petkov, V. Plants with hypotensive, antiatheromatous and coronarodilatating action. Am. J. Chin. Med. 1979, 7, 197–236. [Google Scholar] [CrossRef] [PubMed]
- Takır, S.; Altun, I.H.; Sezgi, B.; Süzgeç-Selçuk, S.; Mat, A.; Uydeş-Doǧan, B.S. Vasorelaxant and blood pressure lowering effects of Alchemilla vulgaris: A comparative study of methanol and aqueous extracts. Pharmacogn. Mag. 2015, 11, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, M.B.; Aliev, O.I.; Andreeva, V.Y.; Vasilev, A.S.; Kalinkina, G.I. Effect of Alchemilla vulgaris extract on the structure and function of erythrocyte membranes during experimental arterial hypertension. Bull. Exp. Biol. Med. 2006, 141, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Khastkhodaei, S.; Sharifi, G.; Salahi, R.; Rahnamaeian, M.; Moattar, F. Clinical efficacy of Stragol™ herbal heart drop in ischemic heart failure of stable chest angina. Eur. J. Integr. Med. 2011, 3, e201–e207. [Google Scholar] [CrossRef]
- Nasa, Y.; Hashizume, H.; Hoque, A.N.; Abiko, Y. Protective effect of Crataegus extract on the cardiac mechanical dysfunction in isolated perfused working rat heart. Arzneimittelforschung 1993, 43, 945–949. [Google Scholar] [PubMed]
- Schossler, M.; Hφlzl, J.; Fricke, U. Myocardial effects of flavonoids from Crataegus species. Arzneimittelforschung 1995, 45, 842–845. [Google Scholar]
- Shatoor, A.S.; Soliman, H.; Al-Hashem, F.; Gamal, B.E.; Othman, A.; El-Menshawy, N. Effect of hawthorn (Crataegus aronia syn. Azarolus (L.)) on platelet function in albino Wistar rats. Thromb. Res. 2012, 130, 75–80. [Google Scholar] [CrossRef]
- Asgary, S.; Naderi, G.H.; Sadeghi, M.; Kelishadi, R.; Amiri, M. Antihypertensive effect of Iranian Crataegus curvisepala Lind.: A randomized, double-blind study. Drugs Exp. Clin. Res. 2004, 30, 221–225. [Google Scholar] [PubMed]
- Dalli, E.; Colomer, E.; Tormos, M.C.; Cosín-Sales, J.; Milara, J.; Esteban, E.; Sáez, G. Crataegus laevigata decreases neutrophil elastase and has hypolipidemic effect: A randomized, double-blind, placebo-controlled trial. Phytomedicine 2011, 18, 769–775. [Google Scholar] [CrossRef]
- Littleton, R.M.; Miller, M.; Hove, J.R. Whole plant based treatment of hypercholesterolemia with Crataegus laevigata in a zebrafish model. BMC Complement. Altern. Med. 2012, 12, 105. [Google Scholar] [CrossRef]
- Walker, A.F.; Marakis, G.; Simpson, E.; Hope, J.L.; Robinson, P.A.; Hassanein, M.; Simpson, H.C. Hypotensive effects of hawthorn for patients with diabetes taking prescription drugs: A randomised controlled trial. Br. J. Gen. Pract. 2006, 56, 437–443. [Google Scholar] [PubMed]
- Ornelas-Lim, C.; Luna-Vázquez, F.J.; Rojas-Molina, A.; Ibarra-Alvarado, C. Development of a quantified herbal extract of hawthorn Crataegus mexicana leaves with vasodilator effect. Saudi Pharm. J. 2021, 29, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Garjani, A.; Nazemiyeh, H.; Maleki, N.; Valizadeh, H. Effects of extracts from flowering tops of Crataegus meyeri A. Pojark. on ischaemic arrhythmias in anaesthetized rats. Phytother. Res. 2000, 14, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Topal, G.; Koç, E.; Karaca, C.; Altuğ, T.; Ergin, B.; Demirci, C.; Melikoğlu, G.; Meriçli, A.H.; Kucur, M.; Ozdemir, O.; et al. Effects of Crataegus microphylla on vascular dysfunction in streptozotocin-induced diabetic rats. Phytother. Res. 2013, 27, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Long, S.R.; Proteau, P.J.; Filtz, T.M. Hawthorn (Crataegus monogyna Jacq.) extract exhibits atropine-sensitive activity in a cultured cardiomyocyte assay. J. Nat. Med. 2008, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arslan, R.; Bor, Z.; Bektas, N.; Meriçli, A.H.; Ozturk, Y. Antithrombotic effects of ethanol extract of Crataegus orientalis in the carrageenan-induced mice tail thrombosis model. Thromb. Res. 2011, 127, 210–213. [Google Scholar] [CrossRef]
- Von Eiff, M.; Brunner, H.; Haegeli, A.; Kreuter, U.; Martina, B.; Meier, B. Hawthorn/passion flower extract and improvement in physical exercise capacity of patients with dyspnea class II of the NYHA functional classification. Acta Ther. 1994, 20, 47–63. [Google Scholar]
- Jayachandran, K.S.; Khan, M.; Selvendiran, K.; Devaraj, S.N.; Kuppusamy, P. Crataegus oxycantha extract attenuates apoptotic incidence in myocardial ischemia-reperfusion injury by regulating Akt and Hif-1 signaling pathways. J. Cardiovasc. Pharmacol. 2010, 56, 526–531. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Niranjali Devaraj, S. Cardioprotective effect of tincture of Crataegus on isoproterenol-induced myocardial infarction in rats. J. Pharm. Pharmacol. 2004, 56, 921–926. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Thirupurasundari, C.J.; Devaraj, S.N. Pretreatment with alcoholic extract of Crataegus oxycantha (AEC) activates mitochondrial protection during isoproterenol—Induced myocardial infarction in rats. Mol. Cell. Biochem. 2006, 292, 59–67. [Google Scholar] [CrossRef]
- Long, S.R.; Carey, R.A.; Crofoot, K.M.; Proteau, P.J.; Filtz, T.M. Effect of hawthorn (Crataegus oxycantha) crude extract and chromatographic fractions on multiple activities in a cultured cardiomyocyte assay. Phytomedicine 2006, 13, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Tauchert, M.; Gildor, A.; Lipinski, J. High-dose Crataegus (hawthorn) extract WS 1442 for the treatment of NYHA class II heart failure patients. Herz 1999, 24, 465–474. (In German) [Google Scholar] [CrossRef] [PubMed]
- Thirupurasundari, C.J.; Jayalakshmi, R.; Niranjali Devaraj, S. Liver architecture maintenance by tincture of Crataegus against isoproterenol-induced myocardially infarcted rats. J. Med. Food 2005, 8, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, N.A.; Thiruchenduran, M.; Devaraj, S.N. Anti-inflammatory and anti-apoptotic effects of Crataegus oxyacantha on isoproterenol-induced myocardial damage. Mol. Cell. Biochem. 2012, 367, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.E.; Poindexter, B.J.; Bickm, R.J.; Dasgupta, A. A comparison of the effects of commercially available Hawthornz preparations on calcium transients of isolated cardiomyocytes. J. Med. Food 2008, 11, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Ahmad, F. Role of natural herbs in the treatment of hypertension. Pharmacogn. Rev. 2011, 5, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; An, Y.; Zhao, C.; Han, L.; Boakye-Yiadom, M.; Wang, W.; Zhang, Y. Regulation effects of Crataegus pinnatifida leaf on glucose and lipids metabolism. J. Agric. Food Chem. 2011, 59, 4987–4994. [Google Scholar] [CrossRef]
- al Makdessi, S.; Sweidan, H.; Dietz, K.; Jacob, R. Protective effect of Crataegus oxyacantha against reperfusion arrhythmias after global no-flow ischemia in the rat heart. Basic Res. Cardiol. 1999, 94, 71–77. [Google Scholar] [CrossRef]
- Anselm, E.; Socorro, V.F.M.; Dal-Ros, S.; Schott, C.; Bronner, C.; Schini-Kerth, V.B. Crataegus special extract WS 1442 causes endothelium-dependent relaxation via a redox-sensitive Src- and Akt-dependent activation of endothelial NO synthase but not via activation of estrogen receptors. J. Cardiovasc. Pharmacol. 2009, 53, 253–260. [Google Scholar] [CrossRef]
- Bödigheimer, K.; Chase, D. Effectiveness of hawthorn extract at a dosage of 3 × 100 mg per day. Multicentre double-blind trial with 85 NYHA stage II heart failure patients. Münch. Med. Wschr. 1994, 136, S7–S11. [Google Scholar]
- Brixius, K.; Willms, S.; Napp, A.; Tossios, P.; Ladage, D.; Bloch, W.; Mehlhorn, U.; Schwinger, R.H. Crataegus special extract WS 1442 induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at serine 1177. Cardiovasc. Drugs Ther. 2006, 20, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Eichstädt, H.; Störk, T.; Möckel, M.; Danne, O.; Funk, P.; Köhler, S. Wirksamkeit und Vertraeglichkeit von Crataegus-Extract WS 1442 bei herzinsuffizienten Patienten mit eingeschraenkter linksventrikulaerer Funktion [Efficacy and safety of Crataegus special extract WS 1442 in patients suffering from heart failure with impaired left ventricular ejection fraction]. Perfusion 2001, 14, 212–217. [Google Scholar]
- Fuchs, S.; Bischoff, I.; Willer, E.A.; Bräutigam, J.; Bubik, M.F.; Erdelmeier, C.A.; Koch, E.; Faleschini, M.T.; De, M.M.; Bauhart, M. The dual edema-preventing molecular mechanism of the Crataegus extract WS 1442 can be assigned to distinct phytochemical fractions. Planta Med. 2016, 83, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Fürst, R.; Zirrgiebel, U.; Totzke, F.; Zahler, S.; Vollmar, A.M.; Koch, E. The Crataegus extract WS® 1442 inhibits balloon catheter-induced intimal hyperplasia in the rat carotid artery by directly influencing PDGFR-β. Atherosclerosis 2010, 211, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Halver, J.; Wenzel, K.; Sendker, J.; Carrillo García, C.; Erdelmeier, C.A.J.; Willems, E.; Mercola, M.; Symma, N.; Könemann, S.; Koch, E.; et al. Crataegus extract WS®1442 stimulates cardiomyogenesis and angiogenesis from stem cells: A possible new pharmacology for Hawthorn? Front. Pharmacol. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Holubarsch, C.J.; Colucci, W.S.; Meinertz, T.; Gaus, W.; Tendera, M. The efficacy and safety of Crataegus extract WS 1442 in patients with heart failure: The SPICE trial. Eur. J. Heart Fail. 2008, 10, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Holubarsch, C.J.; Colucci, W.S.; Meinertz, T.; Gaus, W.; Tendera, M. Survival and prognosis: Investigation of Crataegus extract WS 1442 in congestive heart failure (SPICE)—Rationale, study design and study protocol. Eur. J. Heart Fail. 2000, 2, 431–437. [Google Scholar] [CrossRef]
- Hwang, H.S.; Bleske, B.E.; Ghannam, M.M.J.; Converso, K.; Russell, M.W.; Hunter, J.C.; Boluyt, M.O. Effects of hawthorn on cardiac remodeling and left ventricular dysfunction after 1 month of pressure overload-induced cardiac hypertrophy in rats. Cardiovasc. Drugs Ther. 2008, 22, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Leuchtgens, H. The Crataegus special extract WS 1442 in patients with cardiac insufficiency NYHA II. A placebo-controlled double blind study. Fortschr. Med. 1993, 111, 352–354. [Google Scholar]
- Muller, A.; Linke, W.; Zhao, Y.; Klaus, W. Crataegus extract prolongs action potential duration in guinea-pig papillary muscle. Phytomedicine 1996, 3, 257–261. [Google Scholar] [CrossRef]
- Peters, W.; Drueppel, V.; Kusche-Vihrog, K.; Schubert, C.; Oberleithner, H. Nanomechanics and sodium permeability of endothelial surface layer modulated by hawthorn extract WS 1442. PLoS ONE 2012, 7, e29972. [Google Scholar] [CrossRef] [PubMed]
- Poepping, S.; Rose, H.; Ionescu, I.; Fischer, Y.; Kammermeier, H. Effect of a hawthorn extract on contraction and energy turnover of isolated rat cardiomyocytes. Arzneimittelforschung 1995, 45, 1157–1161. [Google Scholar]
- Schmidt, U.; Kuhn, U.; Ploch, M.; Hübner, W.-D. Efficacy of the Hawthorn (Crataegus) preparation LI 132 in 78 patients with chronic congestive heart failure defined as NYHA functional class II. Phytomedicine 1994, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, R.H.; Pietsch, M.; Frank, K.; Brixius, K. Crataegus special extract WS 1442 increases force of contraction in human myocardium cAMP-independently. J. Cardiovasc. Pharmacol. 2000, 35, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Tauchert, M. Efficacy and safety of Crataegus extract WS 1442 in comparison with placebo in patients with chronic stable New York Heart Association class-III heart failure. Am. Heart J. 2002, 143, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Veveris, M.; Koch, E.; Chatterjee, S.S. Crataegus special extract WS 1442 improves cardiac function and reduces infarct size in a rat model of prolonged coronary ischemia and reperfusion. Life Sci. 2004, 74, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Weikl, A.; Assmus, K.D.; Neukum-Schmidt, A.; Schmitz, J.; Zapfe, G.; Noh, H.S.; Siegrist, J. Crataegus-Spezialextrakt WS 1442. Objektiver Wirksamkeitsnachweis bei Patienten mit Herzinsuffizienz (NYHA II) [Crataegus Special Extract WS 1442. Assessment of objective effectiveness in patients with heart failure (NYHA II)]. Fortschr. Med. 1996, 114, 291–296. (In Germany) [Google Scholar] [PubMed]
- Zapfe, G. Clinical efficacy of Crataegus extract WS® 1442 in congestive heart failure NYHA class II. Phytomedicine 2001, 8, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Vautaw, B.M.; Gillespie, B.; Aaronson, K.D. Hawthorn extract randomized blinded chronic heart failure (HERB CHF) trial. Eur. J. Heart Fail. 2009, 11, 990–999. [Google Scholar] [CrossRef]
- Asher, G.N.; Viera, A.J.; Weaver, M.A.; Rosalie, D.; Melissa, C.; Hinderliter, A.L. Effect of Hawthorn standardized extract on flow mediated dilation in prehypertensive and mildly hypertensive adults: A randomized, controlled cross-over trial. BMC Complement. Altern. Med. 2012, 12, 26. [Google Scholar] [CrossRef]
- Koçyildiz, Z.C.; Birman, H.; Olgaç, V.; Akgün-Dar, K.; Melikoğlu, G.; Meriçli, A.H. Crataegus tanacetifolia leaf extract prevents L-NAME-induced hypertension in rats: A morphological study. Phytother. Res. 2006, 20, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.T.; Badrealam, K.F.; Shibu, M.A.; Kuo, C.H.; Huang, C.Y.; Chen, B.C.; Lin, Y.M.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Eriobotrya japonica ameliorates cardiac hypertrophy in H9c2 cardiomyoblast and in spontaneously hypertensive rats. Environ. Toxicol. 2018, 33, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Moazen, S.; Amani, R.; Homayouni Rad, A.; Shahbazian, H.; Ahmadi, K.; Taha Jalali, M. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: A randomized double-blind controlled trial. Ann. Nutr. Metab. 2013, 63, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Mudnic, I.; Modun, D.; Brizic, I.; Vukovic, J.; Generalic, I.; Katalinic, V.; Bilusic, T.; Ljubenkov, I.; Boban, M. Cardiovascular effects in vitro of aqueous extract of wild strawberry (Fragaria vesca L.) leaves. Phytomedicine 2009, 16, 462–469. [Google Scholar] [CrossRef]
- Ali, F.; Shahid, F.; Shahid, T. Aqueous extract of Malus domestica inhibits human platelet aggregation induced by multiple agonists. Phytopharm. Commun. 2023, 03, 27–37. [Google Scholar] [CrossRef]
- Usta, C.; Bedel, A.; Kurtoğlu, A.U.; Kurtoğlu, E. Effects of crabapple (Malus sylvestris) on blood glucose and lipid levels in diabetic rats. J. Food Nutr. Res. 2016, 4, 148–151. [Google Scholar] [CrossRef]
- Li, S.C.; Liu, Y.H.; Liu, J.F.; Chang, W.H.; Chen, C.M.; Chen, C.Y. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 474–479. [Google Scholar] [CrossRef]
- Liu, J.F.; Liu, Y.H.; Chen, C.M.; Chang, W.H.; Chen, C.Y. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: A randomized crossover controlled feeding trial. Eur. J. Nutr. 2013, 52, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Baniasad, A.; Khajavirad, A.; Hosseini, M.; Shafei, M.N.; Aminzadah, S.; Ghavi, M. Effect of hydro-alcoholic extract of Rosa damascena on cardiovascular responses in normotensive rat. Avicenna J. Phytomed. 2015, 5, 319–324. [Google Scholar]
- Mullen, W.; McGinn, J.; Lean, M.E.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef]
- VandenAkker, N.E.; Vendrame, S.; Tsakiroglou, P.; Klimis-Zacas, D. Red raspberry (Rubus idaeus) consumption restores the impaired vasoconstriction and vasorelaxation in the aorta of the obese zucker rat, a model of the metabolic syndrome. J. Berry Res. 2021, 11, 89–101. [Google Scholar] [CrossRef]
- Khan, V.; Sharma, S.; Bhandari, U.; Sharma, N.; Rishi, V.; Haque, S.E. Suppression of isoproterenol-induced cardiotoxicity in rats by raspberry ketone via activation of peroxisome proliferator activated receptor-α. Eur. J. Pharmacol. 2019, 842, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Liu, J.; Ufur, H.; He, G.; Liqian, H.; Chen, P. The antihypertensive effect of ethyl acetate extract from red raspberry fruit in hypertensive rats. Pharmacogn. Mag. 2011, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Odukoya, J.O.; Odukoya, J.O.; Mmutlane, E.M.; Ndinteh, D.T. Ethnopharmacological study of medicinal plants used for the treatment of cardiovascular diseases and their associated risk factors in sub-Saharan Africa. Plants 2022, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Jouad, H.; Haloui, M.; Rhiouani, H.; Hilaly, J.E.; Eddouks, M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez–Boulemane). J. Ethnopharmacol. 2001, 77, 175–182. [Google Scholar] [CrossRef]
- Idm’hand, E.; Msanda, F.; Cherifi, K. Medicinal uses, phytochemistry and pharmacology of Ammodaucus leucotrichus. Clin. Phytosci. 2020, 6, 6. [Google Scholar] [CrossRef]
- Mootoosamy, A.; Fawzi Mahomoodally, M. Ethnomedicinal application of native remedies used against diabetes and related complications in Mauritius. J. Ethnopharmacol. 2014, 151, 413–444. [Google Scholar] [CrossRef]
- Samaha, A.A.; Fawaz, M.; Salami, A.; Baydoun, S.; Eid, A.H. Antihypertensive indigenous lebanese plants: Ethnopharmacology and a clinical trial. Biomolecules 2019, 9, 292. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Srinivas, P.; Krishnamurthy, N. Effect of enzymes on extraction of volatiles from celery seeds. Food Chem. 2010, 120, 230–234. [Google Scholar] [CrossRef]
- Poddar, S.; Sarkar, T.; Choudhury, S.; Chatterjee, S.; Ghosh, P. Indian traditional medicinal plants: A concise review. Int. J. Bot. Stud. 2020, 5, 174–190. [Google Scholar]
- Siddique, Y.H.; Naz, F.; Jyoti, S.; Fatima, A.; Khanam, S.; Ali, F.; Mujtaba, S.F.; Faisal, M. Effect of Centella asiatica leaf extract on the dietary supplementation in transgenic Drosophila model of Parkinson’s disease. Parkinsons. Dis. 2014, 014, 262058. [Google Scholar] [CrossRef] [PubMed]
- Verma, T.; Sinha, M.; Bansal, N.; Yadav, S.R.; Shah, K.; Chauhan, N.S. Plants used as antihypertensive. Nat. Prod. Bioprospect. 2021, 11, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Neamsuvan, O.; Komonhiran, P.; Boonming, K. Medicinal plants used for hypertension treatment by folk healers in Songkhla province, Thailand. J. Ethnopharmacol. 2018, 214, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Lozoya, X. Mexican medicinal plants used for treatment of cardiovascular diseases. Am. J. Chin. Med. 1980, 8, 86–95. [Google Scholar] [CrossRef]
- Ody, P.; Blumenthal, M. The Complete Medicinal Herbal: A Practical Guide to the Healing Properties of Herbs, with More than 250 Remedies for Common Ailments; Kindersley: New York, NY, USA, 1993. [Google Scholar]
- Jiangsu New Medical College (Ed.) A Compendium of Chinese Medicines; Shanghai Scientific and Technological I Press: Hong Kong, China, 1979. [Google Scholar]
- Kwan, C.Y.; Gaspar, V.; Shi, A.G.; Wang, Z.L.; Chen, M.C.; Ohta, A.; Cragoe, E.J., Jr.; Daniel, E.E. Vascular effects of tetramethylpyrazine: Direct interaction with smooth muscle alpha-adrenoceptors. Eur. J. Pharmacol. 1991, 198, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.; Roumysa, B. Associations between the use of herbal therapy and sociodemographic factors. Spat. DD-Peer Rev. J. Complement. Med. Drug Discov. 2013, 3, 59. [Google Scholar]
- Balogun, F.O.; Ashafa, A.O.T. A review of plants used in South African traditional medicine for the management and treatment of hypertension. Planta Med. 2019, 85, 312–334. [Google Scholar] [CrossRef] [PubMed]
- Gunjan, M.; Naing, T.W.; Saini, R.S.; Ahmad, A.; Naidu, J.R.; Kumar, I. Marketing trends & future prospects of herbal medicine in the treatment of various disease. World J. Pharm. Res. 2015, 4, 132–155. [Google Scholar]
- Mahleyuddin, N.N.; Moshawih, S.; Ming, L.C.; Zulkifly, H.H.; Kifli, N.; Loy, M.J.; Sarker, M.M.R.; Al-Worafi, Y.M.; Goh, B.H.; Thuraisingam, S.; et al. Coriandrum sativum L.: A review on ethnopharmacology, phytochemistry, and cardiovascular benefits. Molecules 2022, 27, 209. [Google Scholar] [CrossRef]
- Erarslan, Z.B.; Kültür, Ş. Leaf anatomical traits of Crataegus orientalis Pall. ex M.Bieb. (Rosaceae) from Turkey. J. Res. Pharm. 2019, 23, 804–811. [Google Scholar] [CrossRef]
- Popović, Z.; Smiljanić, M.; Kostić, M.; Nikić, P.; Jankovi, S. Wild flora and its usage in traditional phytotherapy (Deliblato Sands, Serbia, South East Europe. Indian J. Tradit. Knowl. 2014, 13, 9–35. [Google Scholar]
- Blumenthal, M.; Busse, W.R.; Goldberg, A.; Gruenwald, J.; Hall, T.; Riggins, C.W.; Rister, R.S. The complete German Commission E monographs. In Therapeutic Guide to Herbal Medicines; American Botanical Council, Integrative Medicine Communications: Austin, TX, USA; Boston, MA, USA, 1998. [Google Scholar] [CrossRef]
- Miller, A.L. Botanical influences on cardiovascular disease. Altern. Med. Rev. 1998, 3, 422–431. [Google Scholar] [PubMed]
- Jerie, P. Milestones of cardiovascular pharmacotherapy: Salicylates and aspirin. Cas. Lek. Ceskych 2006, 145, 901–904. [Google Scholar]
- Seleteng Kose, L.; Moteetee, A.; Van Vuuren, S. Ethnobotanical survey of medicinal plants used in the Maseru district of Lesotho. J. Ethnopharmacol. 2015, 170, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Cavero, R.; Calvo, M. Medicinal plants used for respiratory affections in Navarra and their pharmacological validation. J. Ethnopharmacol. 2014, 158, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity potential of Prunus spinosa L. flower extracts: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. Front Pharmacol. 2017, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.N.K.; Mannan, M.A.; Ahmed, M.N. Medicinal plants used by the traditional medical practitioners of Barendra and Shamatat (Rajshahi & Khulna division) region in Bangladesh for treatment of cardiovascular disorders. J. Med. Plants 2014, 2, 9–14. [Google Scholar]
- Nazni, P.; Dharmaligam, R. Isolation and separation of phenolic compound from coriander flowers. Int. J. Agric. Food Sci. 2014, 4, 13–21. [Google Scholar]
- Michel, J.; Abd Rani, N.Z.; Husain, K. A review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Front. Pharmacol. 2020, 11, 852. [Google Scholar] [CrossRef]
- Senejoux, F.; Demougeot, C.; Cuciureanu, M.; Miron, A.; Cuciureanu, R.; Berthelot, A.; Girard-Thernier, C. Vasorelaxant effects and mechanisms of action of Heracleum sphondylium L. (Apiaceae) in rat thoracic aorta. J. Ethnopharmacol. 2013, 147, 536–539. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Ram, M.; Nikfar, S.; Rahimi, R. Bioactive foods and medicinal plants for cardiovascular complications of type II diabetes: Current clinical evidence and future perspectives. Evid.-Based Complement. Altern. Med. 2021, 2021, 6681540. [Google Scholar] [CrossRef] [PubMed]
- Mendel, B.; Christianto, C.; Setiawan, M.; Prakoso, R.; Siagian, S.N. A comparative effectiveness systematic review and meta analysis of drugs for the prophylaxis of junctional ectopic tachycardia. Curr. Cardiol. Rev. 2022, 18, e030621193817. [Google Scholar] [CrossRef] [PubMed]
- Mitkowski, P.; Witkowski, A.; Stępińska, J.; Banach, M.; Jankowski, P.; Gąsior, M.; Wita, K.; Bartuś, S.; Burchardt, P.; Farkowski, M.M.; et al. Position of the Polish Cardiac Society on therapeutic targets for LDL cholesterol concentrations in secondary prevention of myocardial infarctions. Kardiol. Pol. 2023, 81, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Schmidt, K.; Ernst, E. Hawthorn extract for treating chronic heart failure: Meta-analysis of randomized trials. Am. J. Med. 2003, 114, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Guo, R.; Ernst, E. Hawthorn extract for treating chronic heart failure. Cochrane Database Syst. Rev. 2008, 23, CD005312. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Malek, F.A. Standardized extracts from hawthorn leaves and flowers in the treatment of cardiovascular disorders preclinical and clinical studies. Planta Med. 2011, 77, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Loew, D. Phytotherapy in heart failure. Phytomedicine 1997, 4, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Usai-Satta, P.; Monica, F. Clinical effectiveness of hawthorn, lemon balm and magnesium on patients with distress disorders: Preliminary data by a survey of Italian gastroenterologists. J. Complement. Altern. Med. Res. 2023, 23, 46–51. [Google Scholar] [CrossRef]
- Aguilera-Rodríguez, F.R.; Zamora-Perez, A.L.; Gutiérrez-Hernández, R.; Quirarte-Báez, S.M.; Reyes Estrada, C.A.; Ortiz-García, Y.M.; Lazalde-Ramos, B.P. Teratogen potential evaluation of the aqueous and hydroalcoholic leaf extracts of Crataegus oxyacantha in pregnancy rats. Plants 2023, 12, 2388. [Google Scholar] [CrossRef]
- Fogacci, F.; Degli Esposti, D.; Di Micoli, A.; Fiorini, G.; Veronesi, M.; Borghi, C.; Cicero, A.F.G. Effect of dietary supplementation with Diuripres® on blood pressure, vascular health, and metabolic parameters in individuals with high-normal blood pressure or stage I hypertension: The CONDOR randomized clinical study. Phytother. Res. 2023, 37, 4851–4861. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Ijaz, M.; Buabeid, M.; Kharaba, Z.J.; Yaseen, H.S.; Murtaza, G. Therapeutic effects and safe uses of plant-derived polyphenolic compounds in cardiovascular diseases: A review. Drug Des. Devel. Ther. 2021, 15, 4713–4732. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.P.; Bharate, S.S. Analytical methods for furanochromone natural product, khellin and its inspired drug candidates, amiodarone and sodium cromoglycate. Crit. Rev. Anal. Chem. 2022, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.Z.; Wu, Y.; Ke, J.J.; He, X.H.; Wang, Y.L. Quercetin postconditioning attenuates myocardial ischemia/reperfusion injury in rats through the PI3K/Akt pathway. Braz. J. Med. Biol. Res. 2013, 46, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Suchal, K.; Malik, S.; Gamad, N.; Malhotra, R.K.; Goyal, S.N.; Chaudhary, U.; Bhatia, J.; Ojha, S.; Arya, D.S. Kaempferol attenuates myocardial ischemic injury via inhibition of MAPK signaling pathway in experimental model of myocardial ischemia-reperfusion injury. Oxidative Med. Cell. Longev. 2016, 2016, 7580731. [Google Scholar] [CrossRef] [PubMed]
- Jalili, C.; Moradi, S.; Mirzababaei, A.; Mohammadi, H.; Heydarzadeh, F.; Miraghajani, M.; Lazaridi, A.V. Effects of Anethum graveolens (dill) and its derivatives on controlling cardiovascular risk factors: A systematic review and meta-analysis. J. Herb. Med. 2021, 30, 100516. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Z.; Wang, Y.; Geng, J.; Han, S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug Dev. Res. 2019, 80, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Mobasseri, M.; Ostadrahimi, A.; Jafarabadi, M.A.; Mahluji, S. Anethum graveolens supplementation improves insulin sensitivity and lipid abnormality in type 2 diabetic patients. Pharm. Sci. 2014, 20, 40–45. [Google Scholar]
- Buwa, C.C.; Mahajan, U.B.; Patil, C.R.; Goyal, S.N. Apigenin attenuates B-receptor-stimulated myocardial injury via safe guarding cardiac functions and escalation of antioxidant defense system. Cardiovasc. Toxicol. 2016, 16, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an important source of antioxidants and their applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Papakyriakopoulou, P.; Skaltsa, H.; Valsami, G.; Kadoglou, N.P.E. Bioactives from natural products with potential cardioprotective properties: Isolation, identification, and pharmacological actions of apigenin, quercetin, and silibinin. Molecules 2023, 28, 2387. [Google Scholar] [CrossRef]
- El-Assri, E.M.; Hajib, A.; Choukri, H.; Gharby, S.; Lahkimi, A.; Eloutassi, N.; Bouia, A. Nutritional quality, lipid, and mineral profiling of seven Moroccan Apiaceae family seeds. S. Afr. J. Bot. 2023, 160, 23–35. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P.; Pulcinelli, F.M. Synergism among flavonoids in inhibiting platelet aggregation and H2O2 production. Circulation 2002, 105, 53–54. [Google Scholar] [CrossRef][Green Version]
- Karthick, M.; Stanely Mainzen Prince, P. Preventive effect of rutin, a bioflavonoid, on lipid peroxides and antioxidants in isoproterenol-induced myocardial infarction in rats. J. Pharm. Pharmacol. 2006, 58, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Khanna, D. Green tea catechins: Defensive role incardiovascular disorders. Chin. J. Nat. Med. 2013, 11, 345–353. [Google Scholar] [CrossRef]
- Anandh Babu, P.; Sabitha, K.; Shyamaladevi, C. Green tea extract impedes dyslipidaemia and development of cardiac dysfunction in streptozotocin-diabetic rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.S. (-) Epicatechin prevents alterations in lysosomal glycohydrolases, cathepsins and reduces myocardial infarct size in isoproterenol-induced myocardial infarcted rats. Eur. J. Pharmacol. 2013, 706, 63–66. [Google Scholar] [CrossRef]
- Agrawal, Y.O.; Sharma, P.K.; Shrivastava, B.; Arya, D.S.; Goyal, S.N. Hesperidin blunts streptozotocin-isoproternol induced myocardial toxicity in rats by altering of PPAR-g receptor. Chem. Biol. Interact. 2014, 219, 211–220. [Google Scholar] [CrossRef]
- Akila, P.; Vennila, L. Chlorogenic acid a dietary polyphenol attenuates isoproterenol induced myocardial oxidative stress in rat myocardium: An in vivo study. Biomed. Pharmacother. 2016, 84, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, J.; Tse, H.F.; Le, X.C.; Rong, J. Plant natural products calycosin and gallic acid synergistically attenuate neutrophil infiltration and subsequent injury in isoproterenol-induced myocardial infarction: A possible role for leukotriene B4 12-hydroxydehydrogenase? Oxidative Med. Cell. Longev. 2015, 2015, 434052. [Google Scholar] [CrossRef]
- Li, L.; Gao, P.; Tang, X.; Liu, Z.; Cao, M.; Luo, R.; Li, X.; Wang, J.; Lin, X.; Peng, C.; et al. CB1R-stabilized NLRP3 inflammasome drives antipsychotics cardiotoxicity. Signal. Transduct. Target Ther. 2022, 7, 190. [Google Scholar] [CrossRef]
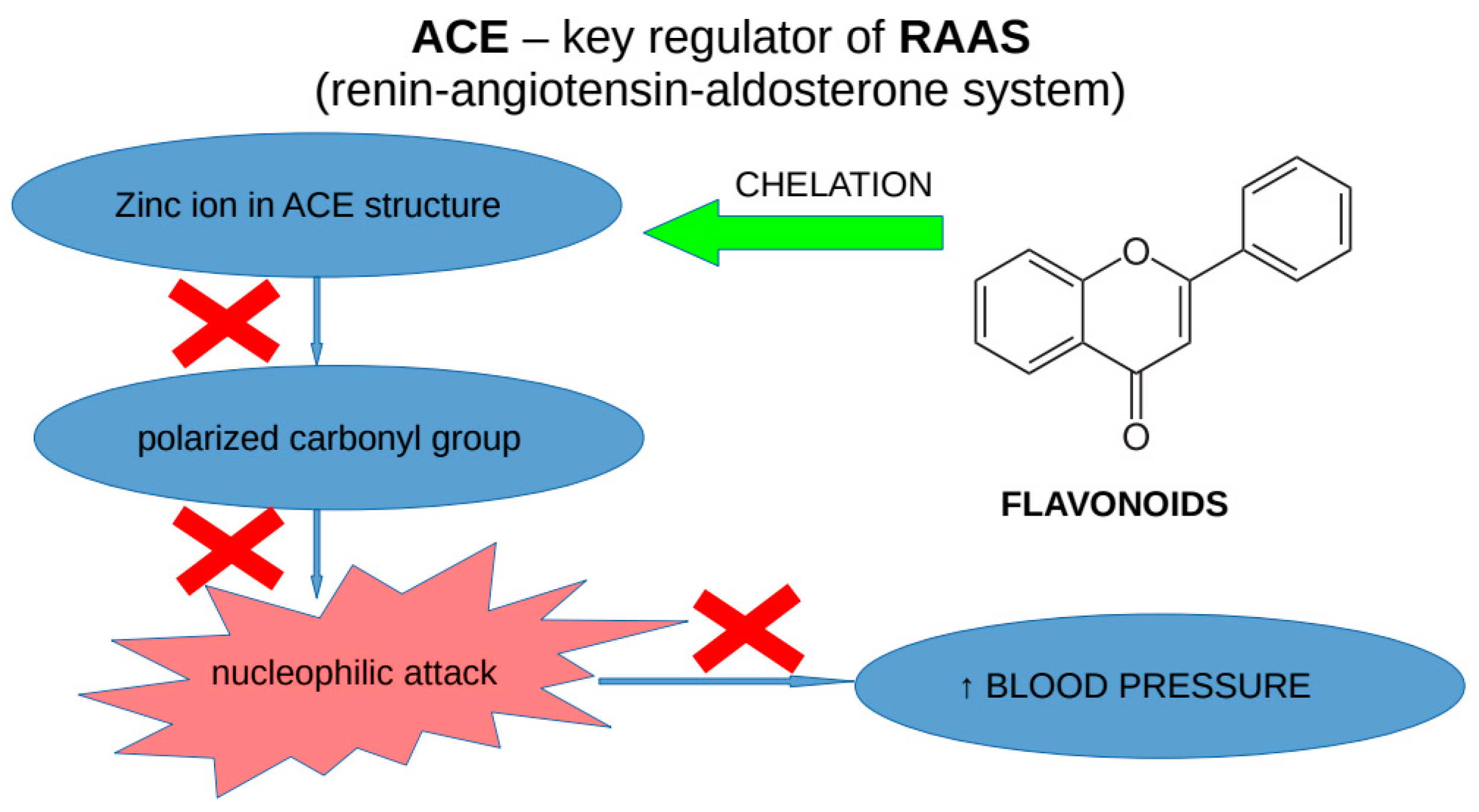
| Plant Species | Tested Material | Cardiovascular Activity | Experimental Model | Concentration Used | References |
|---|---|---|---|---|---|
| Alepidea amatymbica | fresh rhizomes/ hexane, dichloromethane, and methanol extracts | hypotensive, vasorelaxant, diuretic | male Wistar rats | 20 mg/kg b.w. (body weight) | [24] |
| Ammi visnaga | fruits | hypotensive, negative chronotropic effect | dogs | intravenous administration of khellin (20–30 mg/kg) | [25] |
| Ammi visnaga | seeds/water infusion | increasing HDL cholesterol level | 50-year-old man | 10 g seeds boiling in 200 mL of water for 10 days twice daily | [26] |
| Ammi visnaga | fruits (visnagin) | vasorelaxant (suppresses vascular smooth muscle contraction, dilates peripheral and coronary vessels, increases coronary circulation) | male Wistar rats | intravenous administration of visnagin (0.3–5 mg/kg) | [27] |
| Ammi visnaga | fruits (visnadin) | vasorelaxant, bronchodilatory, spasmolytic | male Wistar rats | 10−6 M–10−4 M | [28] |
| Ammi visnaga | fruits (visnadin) | increasing coronary blood flow | isolated guinea pig hearts | 60.0 μg/mL and 120.0 μg/mL of visnadin | [29] |
| Ammi visnaga | fruits (visnadin, dihydrosamidin, khellin, samidin) | positive inotropic effects on heart | dogs | 4.7 mg/kg/day for 7 days intramuscularly | [30] |
| Ammi visnaga | seeds/aqueous extract | diuretic | male Wistar albino rats | 500 mg/kg | [31] |
| Ammi visnaga | fruits/chloroform and methanol extract | inhibition of contractions of aorta induced by potassium chloride; calcium channel-blocking actions | rabbit and guinea pig aorta | 31.6, 100, and 316 µg/mL | [32] |
| Ammodaucus leucotrichus | fruits/aqueous extract | hypotensive, vasorelaxant | rats, isolated thoracic aortas | 60 and 100 mg/kg b.w. orally for 6 h or over 7 days | [33] |
| Anethum graveolens | powder and essential oils | hypolipidemic (cholesterol-lowering properties), cardioprotective | male Wistar rats | 45, 90, and 180 mg/kg orally for 2 weeks | [34] |
| Angelica archangelica | roots/chloroform extract | calcium channel-blocking actions (activity significantly higher than verapamil); calcium-antagonistic (monitoring the suppression of depolarization-induced Ca2+ uptake in rat pituitary GH4C1 cells) | rat pituitary GH4C1 cell | 2, 6, and 20 µg/mL | [35] |
| Angelica archangelica | roots/twenty different extracts | calcium-antagonistic (monitoring the suppression of depolarization-induced Ca2+ uptake in rat pituitary GH4C1 cells) | clonal rat pituitary GH4C1 cells | 2, 6, and 20 µg/mL | [36] |
| Angelica dahurica | roots/ 70% methanol extract | vasorelaxant (endothelium-independent pathway that involves blocking extracellular calcium influx through receptor-mediated Ca2+ channels and voltage-dependent calcium channel pathways) | male Sprague Dawley rats | 0.03–1.0 mg/mL | [37] |
| Angelica furcijuga | roots/methanol extract | vasorelaxant | male Wistar rats | 3–100 µM | [38] |
| Angelica keiskei | isolated xanthoangelol E | hypotensive | rabbits | 0.05–1.0 mM | [39] |
| Angelica keiskei | roots/ 50% ethanol extract (ethyl acetate-soluble fraction) | vasorelaxant (suppresses phenylephrine-induced vasoconstriction in rat aortic rings) | male Wistar rats | 100 µg/mL | [40] |
| Angelica pubescens | osthole | vasorelaxant | male Wistar rats | 40–200 μM | [41] |
| Angelica sinensis | polysaccharides | cardioprotective (alleviates myocardial ischemia–reperfusion injury in rats through deceleration TLR4/NF-κβ) | male Sprague Dawley rats | 50–100 mg/kg intragastric administration for 4 weeks | [42] |
| Apium graveolens | aerial parts/aqueous and ethanol extract | hypotensive, negative chronotropic, and inotropic effect | Wistar albino rats and rabbits | 0.5–15 mg/kg | [43] |
| Apium graveolens | whole plant/ aqueous extract | vasorelaxant (endothelium dependent vasorelaxation) | male Sprague Dawley rats | not given | [44] |
| Apium graveolens | isolated apigenin | vasorelaxant (by inhibiting Ca2+ influx, leading to blocked aortic ring contractions) | Wistar rats | not given | [45] |
| Apium graveolens | seeds/hexan, methanol, water-ethanol extracts | hypotensive | male Wistar rats | 300 mg/kg intraperitoneally | [46] |
| Apium graveolens | seeds/ 80% ethanol extract | hypotensive | human | 1.34 g/day for 4 weeks | [47,48] |
| Apium graveolens | stems and leaves/ aqueous extract | hypolipidemic (cholesterol-lowering properties) | Wistar rats | 10 g/day for 4 weeks | [49] |
| Carum carvi | seeds/aqueous extract | diuretic (Carum carvi extracts boosted urinary Na+ and K+ levels, while furosemide only raised Na+ levels and diminished urinary K+ levels) | male Wistar rats | not given | [50] |
| Centella asiatica | whole plant/ aqueous extract | cardioprotective (reduces increased serum levels of myocardial marker enzymes in rats with cardiomyopathy) | male albino Wistar rats | 200 mg/kg b.w. orally | [51] |
| Centella asiatica | aerial parts/methanol extract (EtOAc, n-BuOH, and water soluble phases) | anti-thrombotic (prevent blood coagulation) | male Wistar ST rats | orally twice a day at intervals of 6 h for 14 days (4 mg/4 mL water/kg/day) | [52] |
| Centella asiatica | whole plant/ ethanol extract | hypolipidemic (cholesterol-lowering properties) | male KM mice and male Golden Syrian hamsters | 1000–1500 mg/kg intragastrically | [53] |
| Coriandrum sativum | leaves/Soxhlet extraction with multiple solvents | hypotensive | rabbits | 100 μg/mL | [54] |
| Coriandrum sativum | fruits/ 70% methanol extract | hypotensive (cholinergic, Ca2+ antagonist), diuretic | Balb-C mice, Sprague Dawley rats and isolated guinea pig ileum, rabbit jejunum | 1–30 mg/kg; 0.3–5.0 mg/mL; 100 mg/kg | [55] |
| Coriandrum sativum | seeds/methanol extract | reduce lipid peroxidation, hypolipidemic, cardioprotective (reduce markers of cardiac damage and myofibrillar failure), enhance endogenous antioxidants and ATPases | male Wistar rats | 100, 200, and 300 mg/kg/day orally for 30 days | [56] |
| Coriandrum sativum | leaves/ 80% methanol extract | cardioprotective properties towards HL-1 cell lines | MTT assay, cell lines | 50 μg/mL | [57] |
| Coriandrum sativum | seeds/ 80% methanol extract | hypotensive (reduces arsenic-induced hypertension by protecting the endothelium) | Wistar albino rats | 500 mg/kg b.w. | [58] |
| Coriandrum sativum | seeds/ 70 % methanol extract | hypolipidemic (cholesterol-lowering properties) | rabbits | 250 mg/kg b.w./day | [59] |
| Coriandrum sativum | seeds powder | hypotensive, hypolipidemic (cholesterol-lowering properties) | human | 2 g/day | [60] |
| Coriandrum sativum | seeds/methanol extract | cardioprotective | rats | 80, 110, 140, 170, and 200 mg/kg b.w. | [61] |
| Coriandrum sativum | seeds/aqueous extract | cardioprotective, hypolipidemic | Meriones shawi rats | 20 mg/kg | [62] |
| Coriandrum sativum | seed oil | hypotensive, antioxidant | determination of ACE inhibition activity | 20–100 µg/mL | [63] |
| Coriandrum sativum | seeds | hypolipidemic | Sprague Dawley rats | 10% of diet during a period of 75 days | [64] |
| Coriandrum sativum | seeds/aqueous extract | cardioprotective, hypolipidemic, improvement of left ventricle functions | Wistar albino rats | 1 g/kg b.w. | [65] |
| Coriandrum sativum | coriander powder suspended in water | hypolipidemic (cholesterol-lowering properties) | male Wistar rats | 1 g/kg b.w. | [66] |
| Coriandrum sativum | seeds/aqueous extract | antiarrhythmic | male albino rats | 300 mg/kg b.w. orally for 16 days | [67] |
| Coriandrum sativum | whole plant | hypotensive | male Sprague Dawley rats | 1.0–2.0 g/kg b.w. orally | [68] |
| Cuminum cyminum | seeds/methanol extract | hypolipidemic (cholesterol-lowering properties) | Sprague Dawley rats | 2 mL/kg dose volume (two divided doses) | [69] |
| Daucus carota | whole plant/ aqueous extract | vasorelaxant (endothelium dependent vasorelaxation) | male Sprague Dawley rats | not given | [44] |
| Daucus carota | aerial parts/ethanol extract (ethyl acetate and water fractions) | Calcium channel-blocking actions, hypotensive | Sprague Dawley rats; rabbit aorta and guinea pig atria | 1–10 mg/kg intravenous administration in rats; in vitro: 10–200 µg/mL guinea pig atria and rabbit aorta | [70] |
| Daucus carota | aerial parts/ethanol extract | hypotensive (demonstrate similar direct relaxant effects on cardiac muscle and smooth muscle as blocking Ca2+ channels) | rabbit thoracic aorta, guinea pig paired atria, rats | 10–100 mg/kg in rats; 0.3–5 mg/mL in guinea pig paired atria | [71] |
| Daucus carota | whole plant | hypolipidemic (cholesterol-lowering properties); increase in antioxidant status | male Wistar rats | a 3-week supplementation of the diet with carrot (15% dry matter) | [72] |
| Eryngium carlinae | aerial parts/ ethanol extract | hypolipidemic | Wistar albino rats | 30 mg/kg b.w. orally for 40 days | [73] |
| Foeniculum vulgare | leaves/aqueous extract | hypotensive | male albino Sprague Dawley rats | 2–20 mg/kg i.v. | [74] |
| Foeniculum vulgare | essential oils | anti-thrombotic | guinea pig plasma | 30 mg/kg per day for 5 days | [75] |
| Ligusticum wullichii | tetramethylpyrazine | hypotensive, anti-thrombotic (prevent blood coagulation) | adult mongrel dogs | 2–15 mg/kg i.v. | [76] |
| Petroselinum crispum | aerial parts/ aqueous extract | hypotensive | rats, isolated thoracic aortic rings | rats: 160 mg/kg during 6 h for 7 days; rat isolated aortic rings: 0.02–2.5 μg/mL | [77] |
| Petroselinum crispum | leaves/aqueous and ethanol extracts | hypotensive (ethanol extract); strong inhibitory effect on the rate and amplitude of contraction | rats | 0.33–10 mg/kg intravenous administration | [78] |
| Petroselinum crispum | leaves/aqueous extract | anti-thrombotic (prevent blood coagulation) | human blood, inhibitory effect of extract and isolated flavonoids on clotting formation and ADP-induced platelet aggregation | not given | [79] |
| Petroselinum crispum | seeds/methanol extract | hypolipidemic and antioxidant activity | male albino rats | 20% w/w for 8 weeks | [80] |
| Petroselinum crispum | genins isolated from leaves | anti-thrombotic (prevent blood coagulation) | in vitro on human platelet aggregation and adhesion to a collagen-coated surface under physiologic flow conditions | 0.3 mg/mL | [81] |
| Petroselinum crispum | leaves/aqueous extract | anti-thrombotic (influence on aggregation induced by thrombin, ADP, collagen, and epinephrine (in vitro) and on rat bleeding time and aggregation ex vivo | anti-platelet activity in rats, on platelet aggregation in vitro and ex vivo | 3 g/kg orally | [82] |
| Petroselinum crispum | seeds/aqueous extract | diuretic | male Sprague Dawley rats | 20 % w/v | [83] |
| Plant Species | Tested Material | Cardiovascular Activity | Experimental Model | Concentration Used | References |
|---|---|---|---|---|---|
| Agrimonia eupatoria | aerial parts/ aqueous infusion, EtOAc fraction | vasorelaxant, vasoprotective | human distal segments of internal thoracic arteries harvested from patients undergoing coronary revascularization | 0.002–0.2 mg/mL | [84] |
| Agrimonia eupatoria | aerial parts/extract | hypotensive | cats | 0.25–1.00 mL/kg i.v. | [85] |
| Alchemilla vulgaris | aerial parts/aqueous and methanol extracts | microvascular and blood pressure lowering | male Wistar albino rats | 0.01–10 mg/mL | [86] |
| Alchemilla vulgaris | extract | arterial hypertension | male SHR and Wistar rats | 300 mg/kg for 10 days | [87] |
| Crataegus | StragolTM herbal heart drop (standardized to 0.060 mg vitexin-2-rhamnoside) | hypolipidemic (LDL, total cholesterol, triglyceride lowering properties, reduction in atherosclerosis), cardioprotective | human | 60 drops daily for 4 weeks | [88] |
| Crataegus spp. | aqueous extract | cardioprotective | isolated, perfused working rat heart during ischemia and reperfusion | 0.01 and 0.05% | [89] |
| Crataegus spp. | isolated flavonoids (luteolin-7-glucoside, hyperoside, rutin) | cardioprotective (tonic action on cardiac myocytes), positive inotropic effect, positive chronotropic effect, growth of coronary blood flow | Langendorff-perfused isolated guinea pig hearts | 10−7 to 5 × 10−4 mol/L | [90] |
| Crataegus aronia | whole plant/ aqueous extract | antiplatelet effect (preventing proliferation after vessel injury, modified the bleeding and the closure time, defined by the level of PFA-100 and thromboxane B2) | male albino Wistar rats | 100, 200, 500, 1000, and 2000 mg/kg orally once a day for 7 days | [91] |
| Crataegus curvisepala | leaves and flowers/ ethanol and aqueous extract | hypotensive | human | not given | [92] |
| Crataegus laevigata | micronized flower and leaf preparation | hypolipidemic (total cholesterol, LDL, non-HDL lowering properties), ↓ neutrophil elastase | human | 400 mg three times a day | [93] |
| Crataegus laevigata | powdered whole plant | hypolipidemic (intravascular cholesterol-lowering properties) | Zebrafish | not given | [94] |
| Crataegus laevigata | flowering tops (Faros® 600 [LI 132, Lichtwer Pharma, Berlin] extract 3:1, standardized to 2.2% flavonoids) | hypotensive (decrease diastolic blood pressure) | human | 1200 mg of extract orally once a day for 16 weeks | [95] |
| Crataegus mexicana | leaves/methanol; 60% ethanol; acetone, aqueous, acetic acid (AWAc, 80:18.5:1.5), and acetone, methanol, aqueous, acid acetic (AMWAc, 40:40:18.5:1.5) extracts | vasorelaxant | male Wistar rats | not given | [96] |
| Crataegus meyeri | flowering tops/ chloroform, ethylacetate and methanol (70%) extracts | hypotensive, antiarrhythmic (decrease in the incidence and severity of ischemia-related arrhythmias) | male Wistar rats | 1 mg/kg/min | [97] |
| Crataegus microphylla | leaves/methanol extract | vasorelaxant (prevents vasospasm, iNOS expression), anti-inflammatory (Plasma levels of TNF-α, IL-6) | male Sprague Dawley rats | 100 mg/kg orally | [98] |
| Crataegus monogyna | leaves and stems/50% ethanol extract, and tablet-derived extract prepared from Heartcare® tablets standardized to 18.75% oligomeric procyanidins (Nature’s Way Products, Inc., Springville, UT, USA) | negative chronotropic activity (decrease in heart rate through muscarinic receptor triggering) | female adult mouses | 0.2 mg/mL | [99] |
| Crataegus orientalis | leaves/ 50% ethanol extract | anti-thrombotic | Swiss albino mice | 100, 200, and 300 mg/kg | [100] |
| Crataegus oxyacantha | flowers | improved heart working capacity | human | not given | [101] |
| Crataegus oxyacantha | HeartCare hawthorn extract tablets (Nature’s Way, Springville, UT, USA)/50% ethanol extract | cardioprotective (reduces apoptotic incidence in myocardial ischemia– reperfusion injury by regulating Akt and Hif-1 signaling pathways) | male Sprague Dawley rats | 100 mg/kg b.w. | [102] |
| Crataegus oxyacantha | fruits/ethanol extract | antioxidant (diminish LPO damage, boost activities of antioxidant enzymes), inhibit ADP-stimulated oxygen uptake rate and respiratory coupling coefficient; cardioprotective | male albino Wistar rats | 0.5 mL/100 g b.w. orally for 30 days | [103] |
| Crataegus oxyacantha | fruits/ethanol extract | antioxidant (diminish LPO damage and enzymes of Kreb’s cycle, support mitochondrial antioxidant status) | male albino Wistar rats | 0.5 mL/100 g b.w. orally for 30 days | [104] |
| Crataegus oxyacantha | leaves and stems or dried berries/ 50% ethanol extract | antiarrhythmic | neonatal mice | 30–300 μg/mL | [105] |
| Crataegus oxyacantha | standardized hawthorn extract WS 1442 | hypotensive, antiarrhythmic, improved heart working capacity, improvements in clinical symptoms (fatigue, palpitations, exercise dyspnea) and in exercise tolerance test | human | one tablet of the extract (84.3 mg of procyanidin per tablet) twice daily for 24 weeks | [106] |
| Crataegus oxyacantha | fruits/ethanol extract | reduce histological and enzymes changes in the liver of isoproterenol- induced myocardially infarcted rats | male albino Wistar rats | 0.5 mL/100 g b.w. orally for 30 days | [107] |
| Crataegus oxyacantha | fruits/50% ethanol extract | hypolipidemic (LDL lowering properties), antioxidant, reduction in creatine kinase and LPO | male albino Wistar rats | 0.5 mL/100 g b.w. orally for 60 days | [108] |
| Crataegus oxyacantha (Hawthorn, two species) | leaves, berries, and flowers/12% ethanol extract | positive inotropic effect (increase Ca2þ transport and impact on the Naþ/Kþ-ATPase in cardiomyocytes), initiation of robust calcium transients and calcium overload | rats | not given | [109] |
| Crataegus pinnatifida | not given | hypotensive, vasorelaxant (resulting from nitrous oxide stimulation) | rabbits | not given | [110] |
| Crataegus pinnatifida | leaves/70% ethanol extract | hypolipidemic (triglyceride and free fatty acid lowering properties) | male Wistar rats and ddY mice | 125, 250, and 500 mg/kg | [111] |
| Crataegus spp. | leaves and flowers/70% methanol extract (LI 132) | antiarrhythmic | male Wistar rats | 3-month oral pretreatment with 2% Crataegus extract | [112] |
| Crataegus spp. | leaves and flowers/50% ethanol extract WS® 1442 | vasorelaxant (elicit endothelium- dependent relaxation of coronary artery rings via Src/PI3-kinase/Akt-dependent eNOS phosphorylation) | porcine coronary artery rings | 300 μg/mL | [113] |
| Crataegus spp. | leaves and flowers/70% methanol extract (LI 132) | improved heart working capacity (clinical assessment; symptom score) | human | 3 × 100 mg per day | [114] |
| Crataegus spp. | leaves and flowers/ WS® 1442 extract | vasorelaxant (induces endothelium- dependent vasodilation mediated through NO phosphorylation of eNOS at serine 1177) | male Wistar rats and isolated human vessel preparations | 5–100 μg/mL | [115] |
| Crataegus spp. | leaves and flowers/ WS® 1442 extract | hypotensive, negative chronotropic effect, an increase in the ejection fraction of the heart | human | not given | [116] |
| Crataegus spp. | leaves and flowers/ 95% ethanol, methanol, and 70% acetone extracts of WS® 1442 | activated a decrease in endothelial hyperpermeability | human | not given | [117] |
| Crataegus spp. | leaves and flowers/ 45% ethanol extract of WS® 1442 | decelerates intimal hyperplasia induced by balloon catheter in the rat carotid artery (direct impact on PDGFR-β) | male Sprague Dawley rats | 300 mg/kg | [118] |
| Crataegus spp. | leaves and flowers/ 45% ethanol extract of WS® 1442 | cardioprotective | human | not given | [119] |
| Crataegus spp. | leaves and flowers/ WS® 1442 extract | reduction in sudden cardiac death | murine and human embryonic stem cells | 5–200 µg/mL | [120] |
| Crataegus spp. | leaves and flowers/ WS® 1442 extract | cardioprotective—reduces the incidence of cardiac mortality and echocardiographic parameters | human | two film-coated tablets of 450 mg of WS 1442® per day for 24 months | [121] |
| Crataegus spp. | leaves and flowers/ WS® 1442 extract | remodels left ventricular and prevents myocardial dysfunction in early cardiac hypertrophy caused by pressure overload | male Sprague Dawley rats | 130 mg/kg/day for 4 weeks | [122] |
| Crataegus spp. | leaves and flowers/ WS® 1442 extract | negative chronotropic activity, hypotensive | human | 1 capsule twice a day for 8 weeks | [123] |
| Crataegus spp. | leaves and flowers/ 70% methanol extract (LI 132) | antiarrhythmic (extends the duration of action potential and postpones the return to Vmax) | male guinea pigs | 10 mg/L | [124] |
| Crataegus spp. | leaves and flowers/ WS® 1442 extract | vasoprotective | mouses | 10 µg/mL | [125] |
| Crataegus spp. | leaves and flowers/ 70 % methanol extract (LI 132) | positive inotropic effect on the amplitude of contraction comparable to isoprenaline and ouabain | rats | 30–180 µg/mL | [126] |
| Crataegus spp. | leaves and flowers/ 70% methanol extract (LI 132) | hypotensive, negative chronotropic effect, improved heart working capacity | human | 1 capsule (200 mg) three times a day for 8 weeks | [127] |
| Crataegus spp. | leaves and flowers/ 45% ethanol extract WS® 1442 | positive inotropic effect (cAMP- independent mechanism; sodium pump; intracellular Ca2+ concentration), improves force-frequency relationship in failing human heart muscle | human | 0.1 and 100 μg/mL | [128] |
| Crataegus spp. | leaves and flowers/ WS® 1442 extract | improved heart working capacity of patients with heart failure | human | 450 mg or 900 mg WS 1442 once a day for 16 weeks | [129] |
| Crataegus spp. | leaves and flowers/ WS® 1442 extract | vasorelaxant | male Wistar rats | 10 or 100 mg/kg | [130] |
| Crataegus spp. | leaves and flowers/ WS® 1442 extract | improved heart working capacity | human | 900 mg WS 1442 for 8 weeks | [131] |
| Crataegus spp. | leaves and flowers (5:1) WS® 1442 extract | hypotensive, negative chronotropic effect (proven clinical efficacy in patients with congestive heart failure (NYHA class II). | human | 240 mg/day | [132] |
| Crataegus spp. | leaves and flowers/ WS® 1442 extract (Crategutt forte, Willmar Schwabe Pharmaceuticals, Karlsruhe, Germany) | lack of evidence supporting symptomatic or functional benefits in patients with heart failure | human | 450 mg orally twice a day | [133] |
| Crataegus spp. (Hawthorn, C. oxyacantha) | leaves and flowers/ ethanol and aqueous extract (standardized to 50 mg oligomeric procyanidin per 250 mg extract) | unproven effectiveness on brachial artery flow mediated dilation | human | 1000, 1500, and 2500 mg orally twice a day | [134] |
| Crataegus tanacetifolia | leaves/aqueous extract | hypotensive | male Wistar albino rats | 100 mg/kg/day | [135] |
| Eriobotrya japonica | leaves/aqueous extract | cardioprotective (impaired cardiac hypertrophy and myocardial function) | age-matched spontaneously hypertensive rats and normotensive control Wistar–Kyoto rats | 100 and 300 mg/kg twice/week | [136] |
| Fragaria ananassa | fruits/freeze-dried strawberry (FDS) | anti-inflammatory (reduce CRP), antioxidant (reduce MDA), diminish glycosylated hemoglobin (HbA1c) | human | 2 cups of FDS beverage (50 g of FDS is equivalent to 500 g of fresh strawberries) daily for 6 weeks | [137] |
| Fragaria vesca | leaves/aqueous extract | vasorelaxant | male guinea pigs and male Wistar rats | 0.06, 0.6, 6, 60 mg/100 mL | [138] |
| Malus domestica | fruits/aqueous extract | anti-thrombotic, calcium channel blocking activity | guinea pig ileum and human blood | 1, 5, and 10 mg/mL | [139] |
| Malus sylvestris | fruits | hypolipidemic | female Wistar albino rats | 2 g/day | [140] |
| Prunus amygdalus | fruits | hypolipidemic (total cholesterol, LDL, LDL/HDL lowering properties), diminish apolipoprotein B/A1 ratio (indicator of atherosclerotic cardiovascular disease) | human | 56 g of almonds a day | [141] |
| Prunus amygdalus | fruits | hypolipidemic (oxidized LDL-C lowering properties), anti-inflammatory (reduction IL-6, TNF-α, and CRP), no significant change in ICAM-1 (intracellular adhesion molecule) and VCAM-1 (vascular adhesion molecule) | human | 56 g of almonds a day | [142] |
| Rosa damascena | flowers/ 70% ethanol extract | hypotensive | male Wistar rats | 250, 500, and 1000 mg/kg | [143] |
| Rubus idaeus | fruits/methanol extract | vasorelaxant | male New Zealand rabbits | 25 μL | [144] |
| Rubus idaeus | whole red raspberry | vasorelaxant | obese Zucker rats (OZR) | 8% w/w for 8 weeks | [145] |
| Rubus idaeus (European red raspberry) | raspberry ketone— red raspberry constituent | cardioprotective (respecting PPAR alpha) | male Wistar albino rats | 50, 100, and 200 mg/kg | [146] |
| Rubus idaeus (Xinjiang red raspberry) | fruits/95% ethanol extract and its fractions (petroleum ether, ethyl acetate, butanol) | hypotensive (dose-dependent lower elevated blood pressure action in SHR, related to increased NO activation), cardioprotective (correct vascular endothelial dysfunction) | male spontaneously hypertensive rats and Wistar–Kyoto rats | 100 mg/kg/day for 5 weeks | [147] |
| Plant Species | Country | Vernacular/Local Name | Application | References |
|---|---|---|---|---|
| Alepidea amatymbica | sub-Saharan Africa | Igwili/Umvuthuza, Ikhathazo | hypotensive (powder of rhizome and fruits), obesity | [148] |
| Ammi visnaga | Marocco (north-central region) | Bachnikha | diabetes | [149] |
| Ammodaucus leucotrichus | Morocco | not given | hypotensive cardiac diseases (fruits and seeds) | [150] |
| Ammodaucus leucotrichus | Marocco (north-central region) | Kamoun soouf | cardiac diseases | [149] |
| Anethum graveolens | Iran | dillweed | hypolipidemic (aerial parts) | [34] |
| Angelica pubescens | China | not given | hypotensive (tinctures or decoctions of this plant cause a short-lived antihypertensive response) | [41] |
| Apium graveolens | Marocco (north-central region) | Kraffess | cardiotonic, renal diseases | [149] |
| Apium graveolens | Mauritius | not given | hypotensive (decoction of leaves) | [151] |
| Apium graveolens | Lebanon | Krafs | hypotensive (fresh juice of shoots and leaves, 1 cup twice/week) | [152] |
| Apium graveolens | India | not given | anti-thrombotic (prevent blood coagulation), cardioprotective | [153] |
| Centella asiatica | India | not given | anti-thrombotic (prevent blood coagulation) | [154] |
| Centella asiatica | India | not given | hypotensive | [155] |
| Coriandrum sativum | India | not given | diuretic | [154] |
| Coriandrum sativum | India | Kasbour, Coriander, Cilantro | hypotensive, cardiac diseases | [156] |
| Coriandrum sativum | Thailand, Songkhla province | Phak chi la | hypotensive (boil fresh materials/drink 1–2 tablespoons after breakfast and dinner a day) | [157] |
| Coriandrum tordylium | India | Kasbour, Coriander, Cilantro | hypotensive, cardiac diseases | [156] |
| Cuminum cyminum | India | not given | anti-thrombotic (prevent blood coagulation), hypotensive | [154] |
| Cuminum cyminum | Marocco (north-central region) | Kamoun | cardiac diseases | [149] |
| Daucus carota | Mexico | Zanahoria | cardiotonic | [158] |
| Eryngium carlinae | Mexico | Chichicahoaztic | heart pain | [158] |
| Eryngium creticum | Lebanon | Kers Aanni | hypotensive (juice of young shoots and leaves, 1 or 2 cup/day) | [152] |
| Ferula narthex | Mexico | Asafetida | cardiotonic | [158] |
| Foeniculum vulgare | Marocco (north-central region) | Nafaa | renal diseases, diabetes | [149] |
| Foeniculum vulgare | Lebanon | Choumar | hypotensive (decoction of seeds, 2 cups/day) | [151] |
| Lichtensteinia lacera | South Africa | iQwili, Kaalmoes, Kalmiswortel | hypotensive (boiling and infusions obtained from leaves, bulbs, stems) | [148] |
| Ligusticum wallich | China | not given | hypotensive, sedative | [159] |
| Ligusticum wullichii | China | not given | cardiac diseases (treating vascular disorders) | [160] |
| Ligustium wollichii | China | not given | hypotensive | [161] |
| Pastinaca sativa | Marocco (north-central region) | Maâdanous | hypotensive | [149] |
| Petroselinum crispum | India | not given | hypotensive | [154] |
| Petroselinum crispum | Mauritius | Persil | hypolipidemic (cholesterol-lowering properties; decoction and juice of leaves) | [162] |
| Peucedanum galbanum | sub-Saharan Africa | Droedas | hypotensive (infusion of leaves) | [163] |
| Centella asiatica | India | Brahmi | blood purifier (whole plant) | [164] |
| Coriandrum sativum | Marocco | not given | diuretic (oral administration of plant parts) | [165] |
| Coriandrum sativum | Iran | not given | diuretic (the whole plant parts) | [165] |
| Plant Species | Country | Vernacular/Local Name | Application | References |
|---|---|---|---|---|
| Cerasus vulgaris | Marocco (north-central region) | Sferjel | diabetes | [149] |
| Crataegus orientalis | Turkey | Alıç, Kırmızı Alıç, Koyun Alıcı, Deli Alıç | cardiac diseases, vasorelaxant, cardiotonic (decoction of fruits) | [166] |
| Crataegus laevigata | England | not given | hypotensive (herbal practitioners in the England use leaves, flowers and berries in combination with prescribed medications) | [95] |
| Crataegus laevigata | sub-Saharan Africa | Aubepine | hypotensive, hypolipidemic (cholesterol-lowering properties)—infusions obtained from leaves and flowers | [148] |
| Crataegus mexicana | Mexico | Tejocote | cardiotonic | [158] |
| Crataegus monogyna | Serbia | not given | hypotensive and cardiac sedative (tea); regulation of heartbeat (tincture from crashed fruits—20 mg of fruits in 100 mg of alcohol) | [167] |
| Crataegus monogyna | Spain | not given | hypotensive, cardiac diseases | [168] |
| Crataegus oxyacantha | European and Chinese medicine | not given | cardiotonic, hypotensive, antiarrhythmic, congestive heart failure | [169] |
| Crataegus pinnatifida | China | not given | cardioprotective (reducing the risk of CVD), hypolipidemic (anti-atherosclerosis) | [15] |
| Crataegus pinnatifida | China | not given | xardiotonic, enhance blood circulation (consumed raw or cooked) | [111] |
| Eriobotrya japonica | sub-Saharan Africa | not given | hypotensive (infusion of leaves) | [148] |
| Filipendula ulmaria | not given | not given | diuretic, hypolipidemic (anti-atherosclerosis), anti-thrombotic (prevent blood coagulation) | [170] |
| Fragaria vesca | Marocco (north-central region) | Fraiz berri | renal diseases, diabetes | [149] |
| Hawthorn (Crataegus spp.) | Germany | not given | cardiac diseases (hydroalcoholic extracts, leaves with flowers) | [130] |
| Leucosidea sericea | South Africa | Umtshitshi | hypotensive | [148] |
| Leucosidea sericea | Lesotho | Cheche | hypotensive | [171] |
| Malus domestica | Indian traditional medicine | not given | hypotensive, cardiac diseases | [154] |
| Malus domestica | sub-Saharan Africa | Pomme | hypolipidemic (cholesterol-lowering properties of fruits and juice) | [148] |
| Malus sylvestris | Spain | not given | cardiac diseases, strengthen blood vessels | [172] |
| Prunus spinosa | Central and Eastern Europe | not given | diuretic, hypolipidemic (anti-atherosclerosis) | [173] |
| Rosa damascena | Bangladesh (Barendra and Shamatat region) | Golap | cardiac diseases (leaves and fruits) | [174] |
| Rubus idaeus | China (Xinjiang region, the Tianshan and the Altai Mountains) | not given | hypotensive (local Mongolian herdsmen use roots for elevated blood pressure reduction) | [147] |
| Apigenin (1) | Catechin (2) | Cosmosiin (3) |
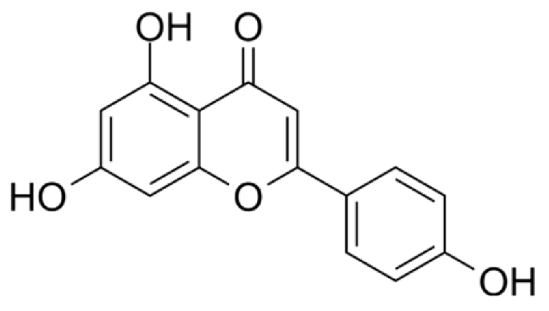 | 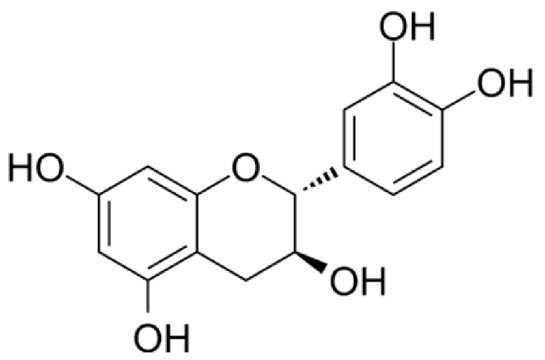 | 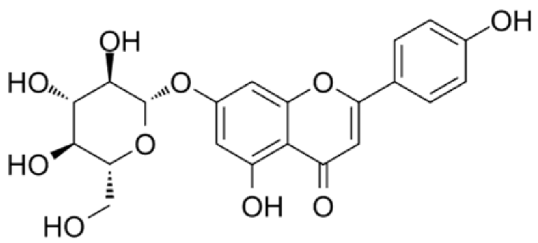 |
| Epicatechin (4) | Hesperidin (5) | Hyperoside (6) |
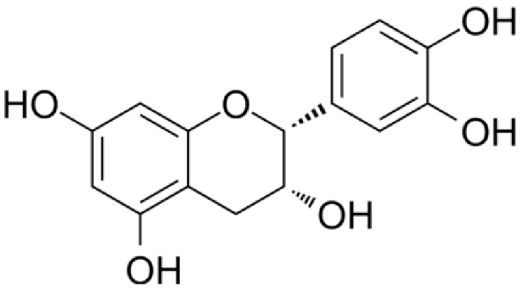 | 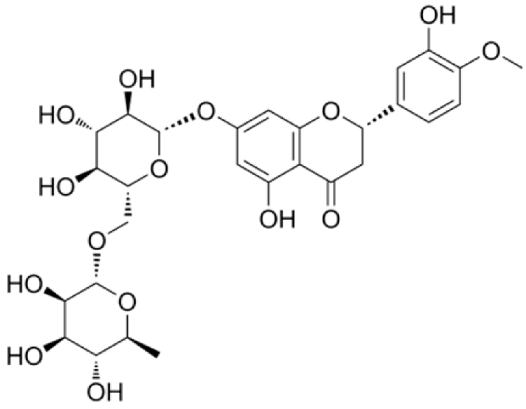 | 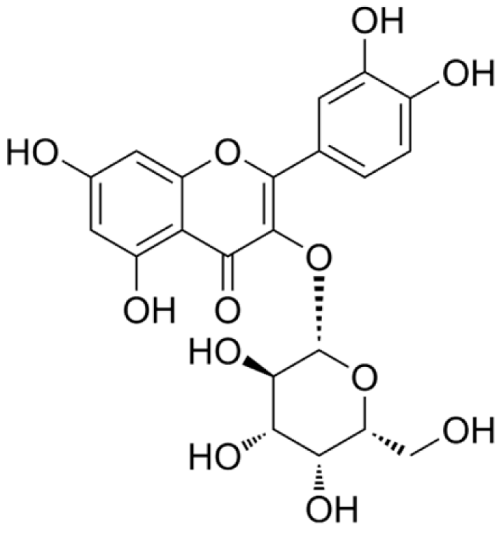 |
| Kaempferol (7) | Luteolin (8) | Phloretin (9) |
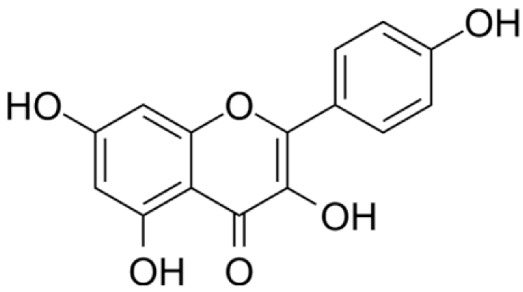 | 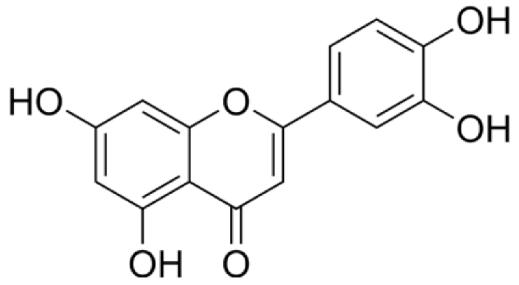 | 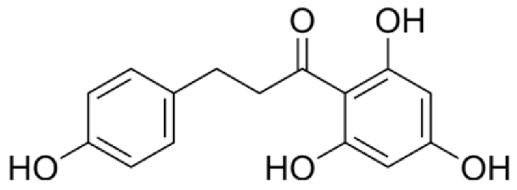 |
| Quercetin (10) | Rutin (11) | Vitexin (12) |
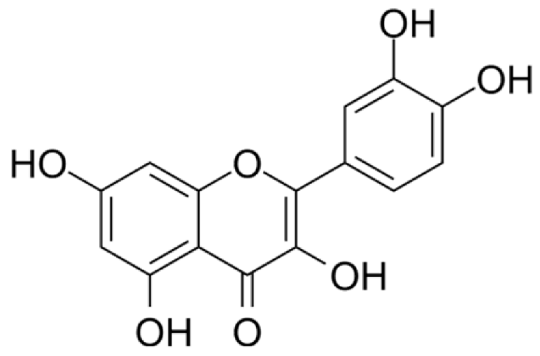 | 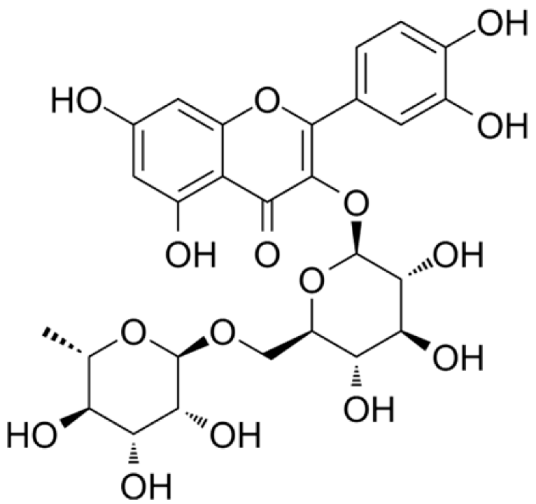 |  |
| Silibinin (13) | Cyanidin diglucoside (14) | Pelargonidin-3-rutinoside (15) |
 |  |  |
| Caffeic acid (16) | Chebulagic acid (17) | Chebulinic acid (18) |
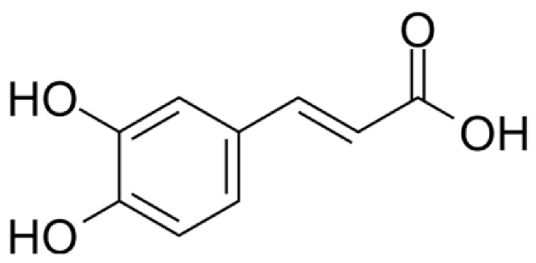 |  |  |
| Chlorogenic acid (19) | Cinnamic acid (20) | Ellagic acid (21) |
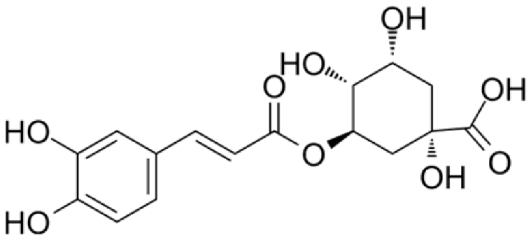 | 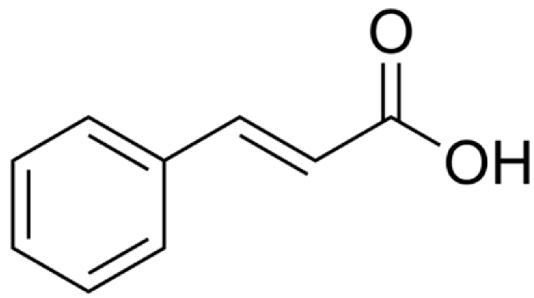 | 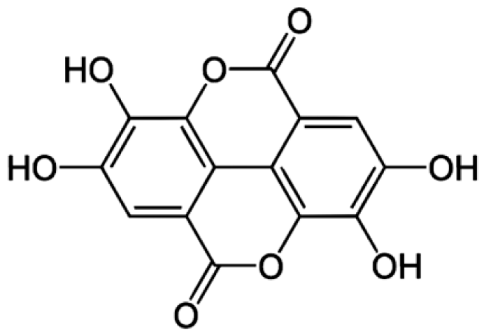 |
| Ferulic acid (22) | Feruloylquinic acid (23) | Gallic acid (24) |
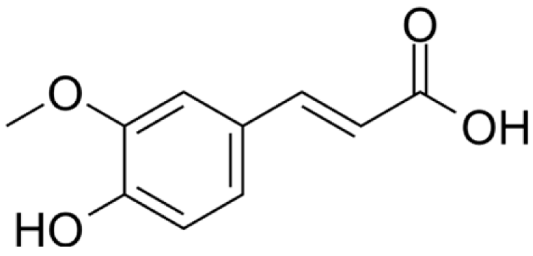 |  | 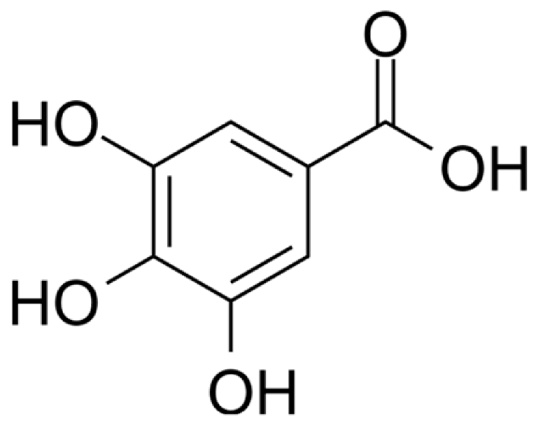 |
| Neochlorogenic acid (25) | p-coumaric acid (26) | Syringic acid (27) |
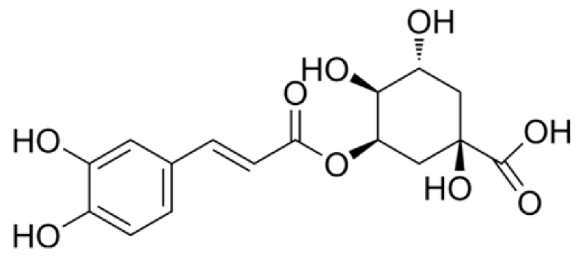 | 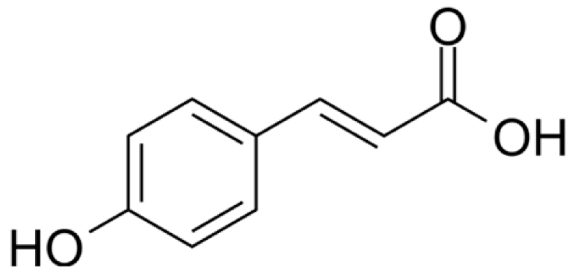 | 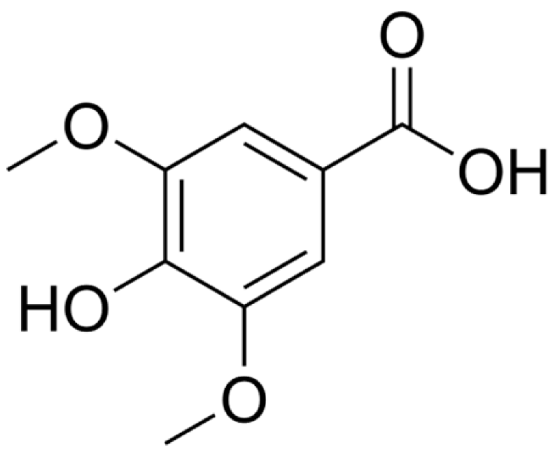 |
| Vanillic acid (28) | Linalool (29) | Anethole (30) |
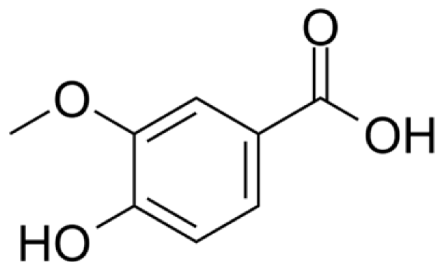 |  | 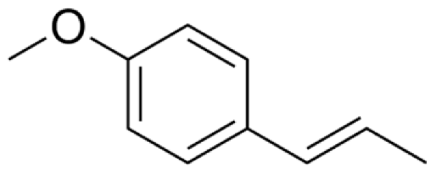 |
| 3-n-butylphthalide (31) | Tocopherol (32) | Petroselinic acid (33) |
 |  |  |
| Visnadin (34) | Samidin (35) | Khellin (36) |
 | 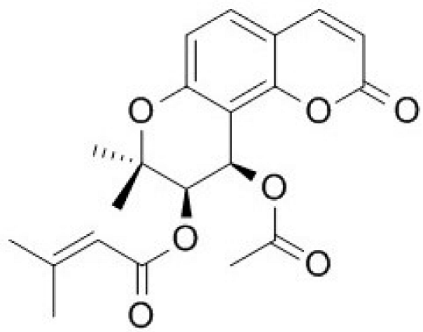 | 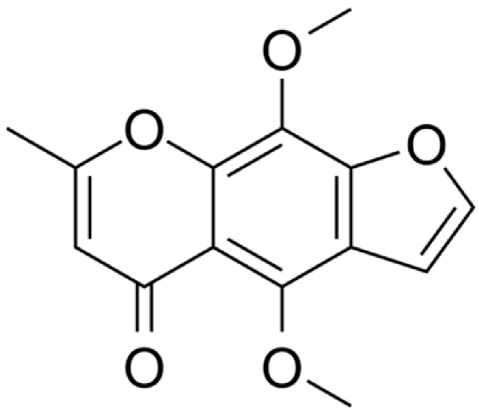 |
| Visnagin (37) | Ursolic acid (38) | Oleanolic acid (39) |
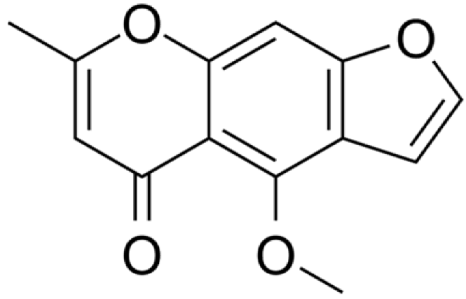 | 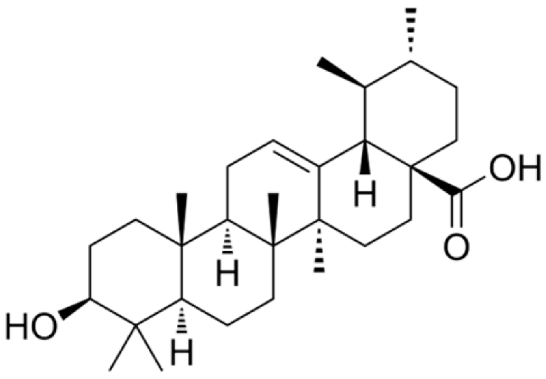 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celiński, R.; Krzemińska, B.; Grzywa-Celińska, A.; Szewczyk, G.; Szewczyk, K.D.S. A Review on the Potential Use of Medicinal Plants from the Apiaceae and the Rosaceae Families in Cardiovascular Diseases—Experimental Evidence and Traditional Applications. Appl. Sci. 2024, 14, 3728. https://doi.org/10.3390/app14093728
Celiński R, Krzemińska B, Grzywa-Celińska A, Szewczyk G, Szewczyk KDS. A Review on the Potential Use of Medicinal Plants from the Apiaceae and the Rosaceae Families in Cardiovascular Diseases—Experimental Evidence and Traditional Applications. Applied Sciences. 2024; 14(9):3728. https://doi.org/10.3390/app14093728
Chicago/Turabian StyleCeliński, Rafał, Barbara Krzemińska, Anna Grzywa-Celińska, Gabriela Szewczyk, and Katarzyna Dos Santos Szewczyk. 2024. "A Review on the Potential Use of Medicinal Plants from the Apiaceae and the Rosaceae Families in Cardiovascular Diseases—Experimental Evidence and Traditional Applications" Applied Sciences 14, no. 9: 3728. https://doi.org/10.3390/app14093728
APA StyleCeliński, R., Krzemińska, B., Grzywa-Celińska, A., Szewczyk, G., & Szewczyk, K. D. S. (2024). A Review on the Potential Use of Medicinal Plants from the Apiaceae and the Rosaceae Families in Cardiovascular Diseases—Experimental Evidence and Traditional Applications. Applied Sciences, 14(9), 3728. https://doi.org/10.3390/app14093728







