Abstract
Seventeen adamantane derivatives were synthesized according to facile condensation reaction protocols. Spectral analysis (1H NMR and 13C NMR) was applied to confirm the chemical structure of the obtained substances. The synthesized compounds were tested for in vitro antimicrobial activity against a panel of Gram-positive and Gram-negative bacterial strains and towards fungi from Candida spp. Among them, four derivatives numbered 9, 14, 15 and 19 showed the highest antibacterial potential with MIC = 62.5–1000 µg/mL with respect to all Gram-positive bacteria. S. epidermidis ATCC 12228 was the most susceptible among the tested bacterial strains and C. albicans ATCC 10231 among fungi. Additionally, the cytotoxicity for three derivatives was measured with the use of the MTT test on A549, T47D, L929 and HeLa cell lines. Our cytotoxicity studies confirmed that the tested substances did not cause statistically significant changes in cell proliferation within the range of the tested doses.
1. Introduction
Adamantane is an organic chemical compound belonging to polycyclic hydrocarbons. According to the review of the scientific literature, it can be stated that searching for new adamantane derivatives is currently a course of research adopted by many research groups around the world. The main reason for this state of affairs is the documented biological activity of many derivatives of this compound [1]. It is also very important that the introduction of the adamantane system to novel compounds usually results in an increase in lipophilicity. In turn, this may modify the bioavailability of synthesized substances and enlarge their therapeutic effect [1].
Adamantane derivatives display significant biological activity, including antiviral [1,2,3,4,5,6], antidiabetic [1,7,8,9,10,11], antibacterial [1,12,13,14,15], antimalarial [1,16,17], anticancer [1,18,19,20,21] activities and anti-inflammatory properties [1,22,23].
Many currently used drugs contain the adamantane system. Amantadine, rimantadine and tromantadine are examples of amine derivatives of adamantane with antiviral activity (Figure 1). Some adamantane derivatives, like vildagliptin or saxagliptin, show antidiabetic activity and are used to treat type 2 diabetes mellitus (T2DM) (Figure 2), whereas adapalene is known for its anti-inflammatory activity, and it is used in the treatment of acne (Figure 3) [1,11,22].
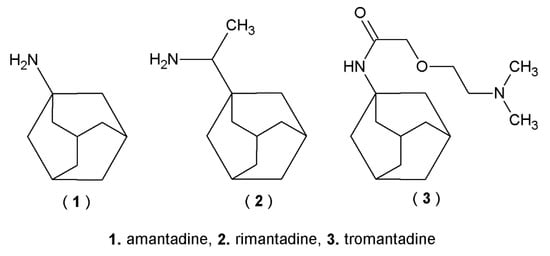
Figure 1.
Amine derivatives of adamantane with antiviral activity.
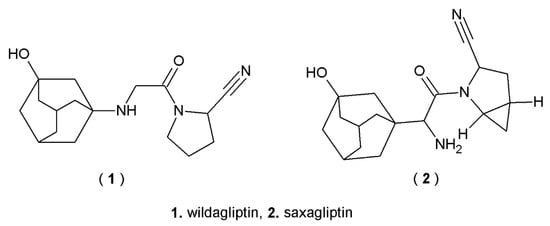
Figure 2.
Adamantane derivatives with antidiabetic activity.
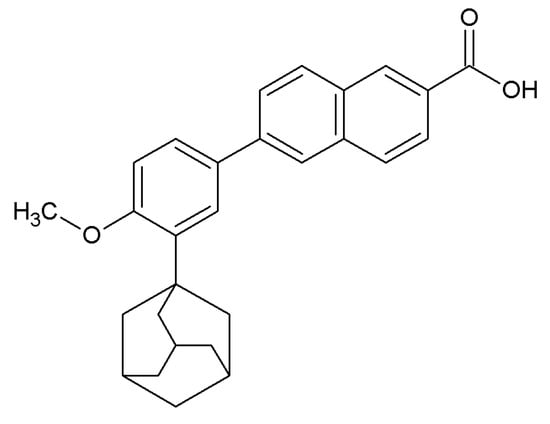
Figure 3.
Chemical structure of adapalene.
In recent years, scientists have also paid attention to the antimicrobial properties of adamantane derivatives [1] since bacterial resistance to commonly used antibacterial agents is rising, making the treatment of infections much more challenging than before [24,25].
Al-Wahaibi et al. [14] synthesized interesting derivatives of isothiourea that contained the adamantane system. The obtained compounds showed significant antibacterial activity towards Gram-positive and Gram-negative bacteria. Additionally, synthesized derivatives highly decreased glucose levels in blood serum in comparison to gliclazide [14]. Pham et al. [15] obtained novel hydrazide–hydrazones with the 1-adamantane carbonyl moiety and tested the synthesized substances on some Gram-negative and Gram-positive bacteria, as well as the fungus Candida albicans. The screening results revealed that four compounds possess good antibacterial properties against Gram-positive bacteria and C. albicans in comparison with known antimicrobial reference substances [15]. According to the latest research, derivatives of adamantane are reported to also influence the biofilm formation of E. faecalis, P. aeruginosa and methicillin-resistant S. aureus, and their antimicrobial activity may be attributed to membranotropic activity [26,27,28].
For many years now, our research team has focused on the synthesis of novel compounds with a hydrazide–hydrazone moiety that display significant antimicrobial and anticancer activity [29,30]. According to our previous studies, hydrazide–hydrazones are promising antibacterial agents, especially against Gram-positive bacterial strains, and antifungal against the yeasts from Candida spp. [31,32,33].
Based on the facts presented above, which point to the interesting and broad biological activity of adamantane derivatives, we decided to synthesize novel Schiff bases and hydrazide–hydrazones containing the adamantane system and then establish their in vitro antimicrobial activity and test their cytotoxicity. Our goal was to investigate whether the connection of the adamantane system with a Schiff base or hydrazide–hydrazone moiety would enhance or decrease its antibacterial and antifungal activity in comparison with our previously obtained results for other compounds from the same group.
2. Materials and Methods
2.1. Chemistry
Sigma-Aldrich (Munich, Germany) or Merck Co. (Darmstadt, Germany) were the manufacturers from whom all reagents and solvents were purchased. In order to confirm the purity of synthesized derivatives and to monitor the progress of the conducted reactions, we applied TLC chromatography on aluminum plates covered with silica gel (aluminum oxide 60 F-254, Merck Co.) with the chloroform–ethanol mixture 10:1 (v/v) used as the mobile phase. Spots on the chromatograms were detected by irradiation with UV light at λ = 254 nm. The Bruker Avance 300 and 600 apparatus (Bruker BioSpin GmbH, Ettlingen, Germany) were used to register the 1H NMR and 13C NMR spectra. The Fisher–Johns apparatus (Fisher Scientific, Dreieich, Germany) was used to establish melting temperatures of the novel derivatives of adamantane. The Perkin Elmer 2400 series II CHNS/O analyzer (Waltham, MA, USA) was used to perform elemental analysis of the obtained substances. The results were within ±0.4% of the theoretical value.
2.2. Experimental
2.2.1. General Method for the Synthesis of 3-Aminotricyclo[3.3.1.13,7]decan-1-ol Derivatives (2, 3), Tricyclo[3.3.1.13,7]decan-1-amine Derivatives (5–11), 1-(Tricyclo[3.3.1.13,7]dec-1-yl)methanamine Derivatives (13–17)
A total of 0.2 g (1.2 mmol) of 3-aminotricyclo[3.3.1.13,7]decan-1-ol (1) or 0.2 g (1.3 mmol) of 1-adamantanylamine (4) or 0.2 g (1.2 mmol) of 1-adamantanemethylamine (12) was placed in a round-bottomed flask and dissolved in ethanol (5 mL, 96%) by heating under reflux. Then, appropriate amounts of various aldehydes or ketones (1.3 mmol) were added, and the mixture in the flask was heated under reflux for 3 h. After that, the solution was allowed to cool and was put into the refrigerator for 24 h. Subsequently, a precipitate appeared, and it was filtered off under reduced pressure, then dried and re-crystalized from ethanol (96%).
- 3-[(2,3-dimethoxybenzylidene)amino]tricyclo[3.3.1.13,7]decan-1-ol (2)
Cream solid; M.p.: 116 °C; Yield: 87%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.53–1.55 (d, J = 6.0 Hz, 2H, CH2-adamantane), 1.58–1.61 (m, 8H, 4×CH2-adamantane), 1.64–1.66 (d, J = 6.0 Hz, 2H, CH2-adamantane), 2.24–2.26 (t, 2H, 2×CHadamantane), 3.77 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.56 (s, 1H, OH), 7.08–7.10 (t, J = 6.0, 1H, ArH), 7.12–7.13 (d, J = 3.0 Hz, 1H, ArH), 7.41–7.43 (d, J = 6.0 Hz, 1H, ArH), 8.54 (s, 1H, =CH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 30.74 (2×CHadamantane), 35.30 (CH2-adamantane), 42.33 (2×CH2-adamantane), 44.73 (2×CH2-adamantane), 50.99 (CH2-adamantane), 56.26 (Cadamantane), 60.98 (OCH3), 61.66 (OCH3), 68.30 (Cadamantane), 114.97, 118.21, 124.42, 130.40 (4×Car), 149.26 (=CH), 150.45, 153.05 (2×Car).
- 3-[(2,4-dimethoxybenzylidene)amino]tricyclo[3.3.1.13,7]decan-1-ol (3)
Yellow solid; M.p.: 86 °C; Yield: 96%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.51–1.53 (m, 4H, 2×CH2-adamantane), 1.57–1.58 (d, J = 3.0 Hz, 6H, 3×CH2-adamantane), 1.60–1.62 (d, J = 6.0 Hz, 2H, CH2-adamantane), 2.22–2.24 (t, J = 3.0 Hz, 2H, 2×CH-adamantane), 3.80 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 4.53 (s, 1H, OH), 6.55–6.56 (d, J = 3.0 Hz, 1H, ArH), 6.60–6.61 (d, J = 3.0 Hz, 1H, ArH), 7.76–7.78 (d, J = 3.0 Hz, 1H, ArH), 8.51 (s, 1H, =CH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 30.74 (2×CHadamantane), 35.31 (CH2-adamantane), 42.57 (2×CH2-adamantane), 44.73 (2×CH2-adamantane), 51.22 (CH2-adamantane), 56.06 (Cadamantane), 60.55 (OCH3), 61.66 (OCH3), 68.34 (Cadamantane), 98.35, 106.37, 118.07, 127.75 (4×Car), 149.53 (=CH), 160.17, 162.95 (2×Car).
- 2-ethoxy-6-[(tricyclo[3.3.1.13,7]dec-1-ylimine)methyl]phenol (5)
Yellow solid; M.p.: 74 °C; Yield: 90%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.30–1.32 (t, J = 3.0 Hz, 3H, CH3), 1.67–1.73 (m, 6H, 3×CH2-adamantane), 1.81–1.82 (d, J = 3.0 Hz, 6H, 3×CH2-adamantane), 2.14–2.16 (t, J = 3.0 Hz, 3H, 3×CHadamantane), 3.99–4.03 (q, J = 3.0 Hz, J = 6.0 Hz, 2H, CH2), 6.69–6.72 (t, J = 3.0 Hz, J = 6.0 Hz, 1H, ArH,), 6.95–6.97 (d, J = 6.0 Hz, 1H, ArH), 7.02–7.04 (d, J = 6.0 Hz, 1H, ArH), 8.53 (s, 1H, =CH), 14.76 (s, 1H, OH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 15.30 (CH3), 29.26 (3×CHadamantane), 36.12 (3×CH2-adamantane), 42.82 (3×CH2-adamantane), 56.84 (Cadamantane), 64.39 (CH2), 116.51, 117.11, 118.61, 124.22 (4×Car), 148.02 (=CH), 154.61, 161.33 (2×Car).
- 2-ethoxy-4-[(tricyclo[3.3.1.13,7]dec-1-ylimine)methyl]phenol (6)
Yellow solid; M.p.: 118 °C; Yield: 21%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.05–1.07 (t, J = 3.0 Hz, 3H, CH3), 1.61–1.69 (m, 6H, 3×CH2-adamantane), 1.71–1.72 (d, J = 3.0 Hz, 6H, 3×CH2-adamantane), 2.10–2.12 (t, J = 3.0 Hz, 3H, 3×CHadamantane), 4.01–4.05 (q, J = 3.0 Hz, 2H, CH2), 6.91–6.92 (d, J = 3.0 Hz, 1H, ArH), 7.11–7.13 (d, J = 3.0 Hz, 1H, ArH), 7.31 (s, 1H, ArH), 8.16 (s, 1H, =CH), 9.72 (s, 1H, OH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 15.23 (CH3), 29.47 (3×CHadamantane), 36.52 (3×CH2-adamantane), 43.39 (3×CH2-adamantane), 56.96 (Cadamantane), 64.19 (CH2), 111.60, 115.76, 123.02, 128.01, 147.48 (5×Car), 148.10 (=CH), 154.74 (Car).
- 1-(2-nitrophenyl)-N-(tricyclo[3.3.1.13,7]dec-1-yl)methanimine (7)
Yellow solid; M.p.: 128 °C; Yield: 63%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.65–1.73 (m, 6H, 3×CH2-adamantane), 1.74–1.75 (d, 6H, 3×CH2-adamantane), 2.13 (m, 3H, 3×CHadamantane), 7.67–7.70 (t, J = 3.0 Hz, 1H, ArH), 7.77–7.80 (t, J = 3.0 Hz, J = 6.0 Hz, 1H, ArH), 7.93–7.95 (d, J = 6.0 Hz, 1H, ArH), 8.02–8.03 (d, J = 3.0 Hz, 1H, ArH), 8.53 (s, 1H, =CH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 29.34 (3×CHadamantane), 36.43 (3×CH2-adamantane), 42.88 (3×CH2-adamantane), 58.48 (Cadamantane), 124.60, 129.61, 131.27, 131.58, 133.89 (5×Car), 149.35 (=CH), 151.96 (Car).
- 1-(3-nitrophenyl)-N-(tricyclo[3.3.1.13,7]dec-1-yl)methanimine (8)
Yellow solid; M.p.: 110 °C; Yield: 47%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.66–1.68 (m, 3H, CH2-adamantane), 1.72–1.74 (m, 3H, CH2-adamantane), 1.78 (d, J = 3.0 Hz, 6H, 3×CH2-adamantane), 2.14 (t, J = 3.0 Hz, 3H, 3×CHadamantane), 7.73–7.76 (t, J = 3.0 Hz, J = 6.0 Hz, 1H, ArH), 8.18–8.19 (d, J = 3.0 Hz, 1H, ArH), 8.27–8.29 (m, 1H, ArH), 8.48 (s, 1H, =CH), 8.58–8.59 (m, 1H, ArH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 29.36 (3×CHadamantane), 36.42 (3×CH2-adamantane), 43.07 (3×CH2-adamantane), 58.19 (Cadamantane), 121.88, 125.12, 130.73, 134.56, 138.98 (5×Car), 148.59 (=CH), 153.93 (Car).
- 1-(4-nitrophenyl)-N-(tricyclo[3.3.1.13,7]dec-1-yl)methanimine (9)
Orange solid; M.p.: 100 °C; Yield: 84%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.63–1.67 (m, 3H, CH2-adamantane), 1.71–1.73 (m, 3H, CH2-adamantane), 1.76–1.77 (d, J = 3.0 Hz, 6H, 3×CH2-adamantane), 2.12–2.14 (t, J = 3.0 Hz, 3H, 3×CHadamantane), 8.00–8.02 (d, J = 6.0 Hz, 2H, ArH), 8.28–8.30 (d, J = 6.0 Hz, 2H, ArH), 8.46 (s, 1H, =CH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 29.36 (3×CHadamantane), 36.42 (3×CH2-adamantane), 43.03 (3×CH2-adamantane), 58.52 (Cadamantane), 124.30, 129.16, 142.99 (5×Car), 148.78 (=CH), 154.17 (Car).
- 1-(2-chloro-5-nitrophenyl)-N-(tricyclo[3.3.1.13,7]dec-1-yl)methanimine (10)
Yellow solid; M.p.: 100 °C; Yield: 95%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.68–1.70 (m, 3H, CH2-adamantane), 1.72–1.74 (m, 3H, CH2-adamantane), 1.80–1.81 (d, J = 3.0 Hz, 6H, 3×CH2-adamantane), 2.14–2.16 (t, 3H, 3×CHadamantane), 7.84–7.85 (d, J = 3.0 Hz, 1H, ArH), 8.27–8.29 (d, J = 3.0 Hz, 1H, ArH), 8.62 (s, 1H, =CH), 8.67–8.68 (d, J = 3.0 Hz, 1H, ArH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 29.33 (3×CHadamantane), 36.33 (3×CH2-adamantane), 42.91 (3×CH2-adamantane), 59.05 (Cadamantane), 122.51, 126.28, 132.03, 135.01, 140.73 (5×Car), 147.15 (=CH), 150.19 (Car).
- N-(tricyclo[3.3.1.13,7]dec-1-yl)tricyclo[3.3.1.13,7]decan-2-imine (11)
White solid; M.p.: 110 °C; Yield: 11%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.49–1.60 (m, 22H, 11×CH2-adamantane), 1.96–1.98 (m, 5H, 5×CHadamantane), 2.15–2.17 (m, 2H, 2×CHadamantane).
- 1-(2-nitrophenyl)-N-(tricyclo[3.3.1.13,7]dec-1-ylmethyl)methanimine (13)
Orange solid; M.p.: 44 °C; Yield: 33%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.68–1.71 (m, 12H, 6×CH2-adamantane), 2.02–2.04 (t, J = 3.0 Hz, 3H, 3×CHadamantane), 3.41 (s, 2H, CH2), 7.58–7.60 (t, J = 3.0 Hz, 1H, ArH), 7.68–7.70 (t, J = 3.0 Hz, 1H, ArH), 7.83–7.85 (d, J = 6.0 Hz, 1H, ArH), 8.25–8.27 (d, J = 6.0 Hz, 1H, ArH), 8.58 (s, 1H, =CH).
- 1-(3-nitrophenyl)-N-(tricyclo[3.3.1.13,7]dec-1-ylmethyl)methanimine (14)
Yellow solid; M.p.: 60 °C; Yield: 4%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.53–1.55 (d, J = 6.0 Hz, 6H, 3×CH2-adamantane), 1.59–1.61 (m, 4H, 2×CH2-adamantane), 1.66–1.68 (m, 2H, CH2-adamantane), 1.93–1.95 (t, 3H, 3×CH-adamantane), 3.27 (s, 2H, CH2), 7.88–7.91 (t, J = 6.0 Hz, J = 3.0 Hz, 1H, ArH), 8.32–8.34 (d, J = 6.0 Hz, 1H, ArH), 8.51–8.53 (m, 1H, ArH), 8.68–8.70 (m, 1H, ArH), 10.14 (s, 1H, =CH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 28.35 (3×CHadamantane), 34.58 (Cadamantane), 37.12 (3×CH2-adamantane), 41.07 (3×CH2-adamantane), 73.50 (CH2), 124.53, 129.01, 131.42, 135.36, 137.64 (5×Car), 148.75 (=CH), 159.59 (Car).
- 1-(4-nitrophenyl)-N-(tricyclo[3.3.1.13,7]dec-1-ylmethyl)methanimine (15)
Yellow solid; M.p.: 192 °C; Yield: 11%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.56 (d, J = 3.0 Hz, 6H, 3×CH2-adamantane), 1.61–1.63 (m, 3H, CH2-adamantane), 1.68–1.70 (m, 3H, CH2-adamantane), 1.96 (m, 3H, 3xCHadamantane), 3.31 (s, 2H, CH2), 8.00–8.01 (d, J = 3.0 Hz, 2H, ArH), 8.30–8.32 (d, J = 6.0 Hz, 2H, ArH), 8.43 (s, 1H, =CH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 28.35 (3×CHadamantane), 34.66 (Cadamantane), 37.11 (3×CH2-adamantane), 41.07 (3×CH2-adamantane), 73.73 (CH2), 124.41, 129.29, 142.15 (5×Car), 148.92 (=CH), 159.87 (Car).
- 5-bromo-N-(tricyclo[3.3.1.13,7]dec-1-ylmethyl)tricyclo[3.3.1.13,7]decan-2-imine (16)
White solid; M.p.: 158 °C; Yield: 54%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.50–1.52 (d, J = 6.0 Hz, 6H, 3×CH2-adamantane), 1.60–1.69 (m, 10H, 5×CH2-adamantane), 1.93–1.95 (m, 6H, 3×CH2-adamantane), 2.16–2.17 (m, 1H, CHadamantane), 2.22–2.24 (m, 1H, CHadamantane), 2.34–2.39 (m, 1H, CHadamantane), 2.44–2.46 (m, 3H, 3×CHadamantane), 3.21 (s, 2H, CH2); 13C NMR (150 MHz, DMSO-d6): 28.39 (CHadamantane), 31.51 (3×CHadamantane), 32.18 (Cadamantane), 34.13 (2×CH2-adamantane), 36.07 (CHadamantane), 37.24 (3×CH2-adamantane), 42.49 (CHadamantane), 46.82 (3×CH2-adamantane), 48.83 (2×CH2-adamantane), 50.03 (CH2-adamantane), 61.94 (Cadamantane), 65.85 (CH2), 173.61 (=Cadamantane).
- 5-hydroxy-N-(tricyclo[3.3.1.13,7]dec-1-ylmethyl)tricyclo[3.3.1.13,7]decan-2-imine (17)
White solid; M.p.: 266 °C; Yield: 95%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.50–1.52 (d, J = 6.0 Hz, 6H, 3×CH2-adamantane), 1.55–1.61 (m, 4H, 2×CH2-adamantane), 1.61–1.65 (m, 2H, CH2-adamantane), 1.65–1.74 (m, 10H, 5×CH2-adamantane), 1.93–1.95 (m, 3H, 3×CHadamantane), 2.15–2.17 (m, 3H, 3×CHadamantane), 3.15 (s, 2H, CH2), 4.56 (s, 1H, OH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 28.42 (CHadamantane), 30.28 (3×CHadamantane), 32.88 (Cadamantane), 34.13 (2×CH2-adamantane), 37.29 (CHadamantane), 38.23 (3×CH2-adamantane), 41.00 (CHadamantane), 44.74 (3×CH2-adamantane), 45.14 (2×CH2-adamantane), 46.20 (CH2-adamantane), 62.05 (Cadamantane), 66.31 (CH2), 175.89 (=Cadamantane).
2.2.2. Synthesis of Hydrazide of 1-Adamantanecarboxylic Acid (19)
The tricyclo[3.3.1.13,7]decane-1-carbonyl chloride (compound 18, 1.0 mmol, 0.2g, CAS number: 2094-72-6) was placed in a round-bottomed flask and dissolved in ethanol (5 mL, 96%) by heating under reflux. Then, 1.1 mmol of 100% hydrazine hydrate was added, and the mixture was heated under reflux for 3 h. After that, the mixture was cooled and left in the refrigerator for 24 h. The formed precipitate was filtered off under reduced pressure, dried and re-crystallized from ethanol (96%).
- tricyclo[3.3.1.13,7]decane-1-carbohydrazide (19)
CAS Number: 81375-05-5; White powder; M.p.: 224 °C; Yield: 25%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.63–1.69 (m, 6H, 3×CH2-adamantane), 1.80–1.82 (d, J = 6.0 Hz, 6H, 3×CH2-adamantane), 1.95–1.97 (m, 3H, 3×CHadamantane), 4.04 (s, 2H, NH2), 9.13 (s, 1H, NH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 27.84 (3×CHadamantane), 36.50 (3×CH2-adamantane), 38.79 (3×CH2-adamantane), 38.96 (Cadamantane), 178.85 (C=O).
Synthesis of Hydrazide–Hydrazones of 1-Adamantanecarbarboxylic Acid (20–23)
A total of 1.0 mmol (0.19 g) of hydrazide of 1-adamantanecarboxylic acid (19) was dissolved in a round-bottomed flask in ethanol (5 mL, 96%). Then 1.1 mmol of appropriate aldehyde or ketone was added, and the flask was heated under reflux for 3 h. The content of the flask was allowed to cool and was placed in the refrigerator for 24 h. The precipitate obtained was filtered off, dried and re-crystallized from ethanol (96%).
- N-[(4-hydroxy-3-ethoxyphenyl)methylidene]tricyclo[3.3.1.13,7]decane-1-carbohydrazide (20)
Yellow solid; M.p.: 246 °C; Yield: 29%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.36–1.38 (t, J = 3.0 Hz, 3H, CH3), 1.63–1.69 (m, 6H, 3×CH2-adamantane), 1.81–1.82 (d, 6H, 3×CH2-adamantane), 1.95–1.97 (t, 3H, 3CHadamantane), 4.06–4.09 (q, J = 3.0 Hz, 2H, CH2), 6.88–6.90 (d, J = 6.0 Hz, 1H, ArH), 7.23–7.25 (d, J = 9.0 Hz, 1H, ArH), 7.43 (s, 1H, ArH), 8.55 (s, 1H, =CH), 9.12 (s, 1H, OH), 9.66 (s, 1H, NH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 15.19 (CH3), 28.03 (3×CHadamantane), 36.58 (3×CH2-adamantane), 38.98 (3×CH2-adamantane), 56.48 (Cadamantane), 64.29 (CH2), 111.90, 116.04, 123.75, 125.94 (4×Car), 147.56 (=CH), 150.57, 161.05 (2×Car), 176.38 (C=O).
- N-[(2-chloro-5-nitrophenyl)methylidene]tricyclo[3.3.1.13,7]decane-1-carbohydrazide (21)
Orange solid; M.p.: 196 °C; Yield: 5%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.71–1.73 (m, 3H, CH2-adamantane), 1.75–1.77 (m, 4H, 2×CH2-adamantane), 1.90–1.91 (d, J = 3.0 Hz, 6H, 3×CH2-adamantane), 2.06–2.08 (t, J = 3.0 Hz, 3H, 3×CHadamantane), 7.60–7.61 (d, J = 3.0 Hz, 1H, ArH), 8.15–8.16 (d, J = 3.0 Hz, 1H, ArH), 8.33 (s, 1H, ArH), 8.54 (s, 1H, =CH), 10.34 (s, 1H, NH).
- N-(5-bromotricyclo[3.3.1.13,7]dec-2-ylidene)tricyclo[3.3.1.13,7]decane-1-carbohydrazide (22)
Cream solid; M.p.: 160 °C; Yield: 7%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.57–1.69 (m, 8H, 4×CH2-adamantane), 1.81–1.83 (d, J = 6.0 Hz, 6H, 3×CH2-adamantane), 1.97–2.01 (m, 8H, 4×CH2-adamantane), 2.13–2.17 (m, 1H, CHadamantane), 2.22–2.26 (m, 1H, CHadamantane), 2.36–2.39 (m, 1H, CHadamantane), 2.44–2.47 (m, 3H, 3×CHadamantane), 10.84 (s, 1H, NH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 28.03 (CHadamantane), 32.03 (3×CHadamantane), 34.77 (Cadamantane), 35.93 (2×CH2-adamantane), 36.58 (CHadamantane), 37.24 (3×CH2-adamantane), 38.97 (CHadamantane), 42.49 (3×CH2-adamantane), 48.00 (2×CH2-adamantane), 49.76 (CH2-adamantane), 65.25 (Cadamantane), 168.24 (=Cadamantane), 176.39 (C=O).
- N-(5-hydroxytricyclo[3.3.1.13,7]dec-2-ylidene)tricyclo[3.3.1.13,7]decane-1-carbohydrazide (23)
White solid; M.p.: 238 °C; Yield: 4%; 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.63–1.69 (m, 16H, 8×CH2-adamantane), 1.80–1.82 (d, J = 6.0 Hz, 6H, 3×CH2-adamantane), 1.96–1.98 (m, 6H, 6×CHadamantane), 5.45 (s, 1H, OH), 9.12 (s, 1H, NH); 13C NMR (150 MHz, DMSO-d6) δ (ppm): 29.93 (CHadamantane), 33.46 (3×CHadamantane), 37.05 (Cadamantane), 38.09 (2×CH2-adamantane), 38.42 (CHadamantane), 44.30 (3×CH2-adamantane), 44.83 (CHadamantane), 45.30 (3×CH2-adamantane), 45.90 (2×CH2-adamantane), 47.07 (CH2-adamantane), 65.98 (Cadamantane), 167.86 (=Cadamantane), 176.54 (C=O).
2.3. Antimicrobial Activity Assays
Microbiological Material and In Vitro Screening Method
The novel adamantane derivatives were screened for antimicrobial activity according to guidelines provided by EUCAST (European Committee on Antimicrobial Susceptibility Testing) [34] and CLSI (Clinical and Laboratory Standards Institute) [35] against a panel of reference microorganisms from ATCC (American Type Culture Collection). The detailed assay procedures were described earlier by our research team [31,32,33].
2.4. Cytotoxicity
2.4.1. Cell Lines
The A549 (ECACC 86012804) and the T47D (ECACC 85102201) cell lines were purchased from ECACC (European Collection of Authenticated Cell Cultures) and maintained in DMEM growth medium (PAN Biotech GmbH, Aidenbach, Bayern, Germany), supplemented with 2 mM of glutamine, 10% Fetal Bovine Serum and antibiotics (1% of 100 U/L penicillin, 100 mg/mL streptomycin). The L929 (NCTC clone 929, ATCC® CCL-1™) and HeLa (ATCC® CCL-2™) cell lines came from ATCC (American Type Culture Collection) and were maintained in EMEM growth medium (PAN Biotech GmbH, Aidenbach, Bayern, Germany) supplemented with antibiotics (1% of 100 U/L penicillin, 100 mg/mL streptomycin) and with 5% and 10% FBS (Foetal bovine serum, (PAN Biotech GmbH, Aidenbach, Bayern, Germany), respectively. The cell lines were grown in tissue culture flasks (75 cm2) and kept in a humidified atmosphere of 5% CO2 at 37 °C. The examined cell lines were tested against mycoplasma contamination by microbiological assays.
2.4.2. MTT Analysis
In order to establish the cytotoxicity of the tested compounds (7, 10, 21), an indirect method was applied, i.e., the spectrophotometric measurement of the product concentration resulting from the reduction in the chemical compound by living cells (with functional mitochondria). The amount of colored product was directly proportional to the metabolic activity of the cell.
The principle of the examination relies on the ability of live cells, with intact mitochondrial membranes, to reduce water-insoluble yellow 3-(4,5-dimethyl-1,3-thiazol-2-yl)-2,5-diphenyl-2H-tetrazole bromide (MTT) to purple formazan, also insoluble in water. Therefore, L929, A549, HeLa and T47D cell lines were cultured for 24 h in 96-well plates so that they could adhere well to the basis. Then, 20 μL of the tested compounds marked as 7, 10 and 21 (range 5–200 μM) were added to the cells. After 24 and 48 h of incubation, 10 µL of MTT was added to the cells. Then after 3 h, 100 µL of medium was withdrawn from each well and quenched with the same amount of DMSO (dimethyl sulfoxide). After 5 min of incubation, the absorbance at 570 nm was read off a BioTek model EPOCH ELISA plate reader. The absorbance values of the samples ranged from about 0.3 to about 1.2 and therefore were within the range of linearity of the Lambert–Beer law.
3. Results
3.1. Chemistry
As a starting compound for the synthesis in this research, we used the following adamantane derivatives: 3-aminotricyclo[3.3.1.13,7]decan-1-ol (1), tricyclo[3.3.1.13,7]decan-1-amine (4), 1-(tricyclo[3.3.1.13,7]dec-1-yl)methanamine (12) and tricyclo[3.3.1.13,7]decane-1-carbonyl chloride (18).
Two novel 3-aminotricyclo[3.3.1.13,7]decan-1-ol derivatives (2, 3) were synthesized with the condensation reaction of 3-amineadamantane-1-ol (1) with substituted benzaldehydes. The reactions were carried out with the use of ethanol (96%) as a solvent (Scheme 1).
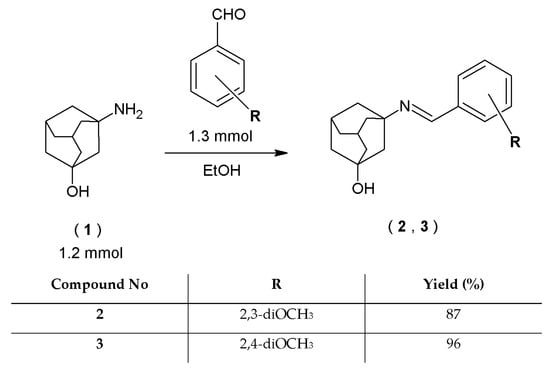
Scheme 1.
Synthesis of 3–amineadamantane–1–ol derivatives.
Novel 1-adamantanylamine derivatives (5–11) were synthesized with the condensation reaction of tricyclo[3.3.1.13,7]decan-1-amine (4) with six different appropriate substituted aromatic aldehydes and one ketone. As previously, the reactions were carried out with the use of ethanol (96%) as a solvent (Scheme 2).
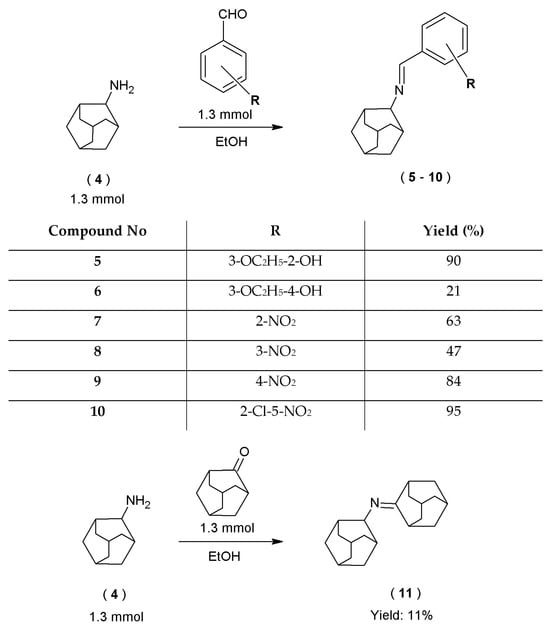
Scheme 2.
Synthetic route to new 1–adamantylamine derivatives.
In the same synthetic way, but with the use of a different starting compound, novel 1-adamantylmethylamine derivatives (13–17) were synthesized. The 1-(tricyclo [3.3.1.13,7]dec-1-yl)methanamine (12) was dissolved in ethanol (96%) and subjected to the condensation reaction with three substituted benzaldehydes and two ketones (Scheme 3).
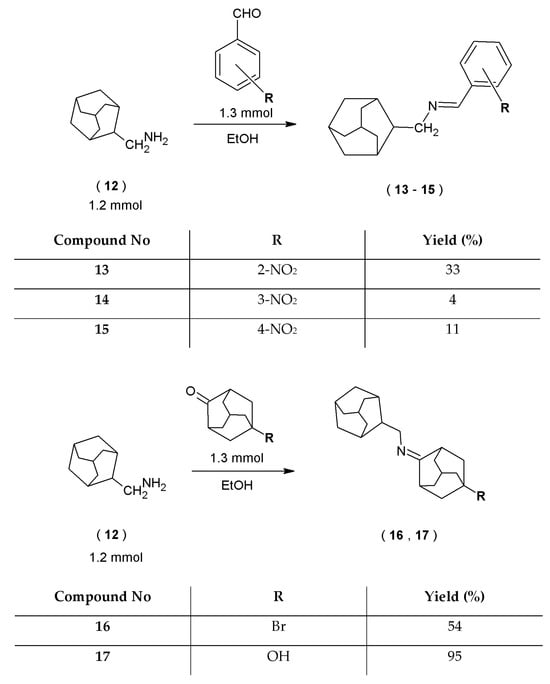
Scheme 3.
Synthesis scheme of 1-adamantylmethyl amine derivatives.
Novel hydrazide–hydrazones of 1-adamantanecarbarboxylic acid (20–23) were obtained with a two-stage synthetic method. Firstly, hydrazide of 1-adamantanylcarboxylic acid (19) was obtained with the reaction of tricyclo[3.3.1.13,7]decane-1-carbonyl chloride (18) with 100% hydrazine hydrate (Scheme 4). Subsequently, hydrazide of 1-adamantanecarboxylic acid (19) was subjected to the condensation reaction with two aromatic aldehydes and two ketones to obtain four novel hydrazide–hydrazones of 1-adamantanecarbarboxylic acid (20–23) (Scheme 5).
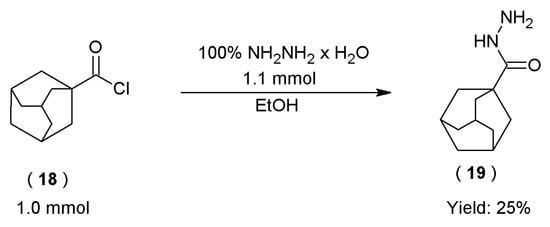
Scheme 4.
The course of synthesis of the hydrazide of 1-adamantanecarboxylic acid.
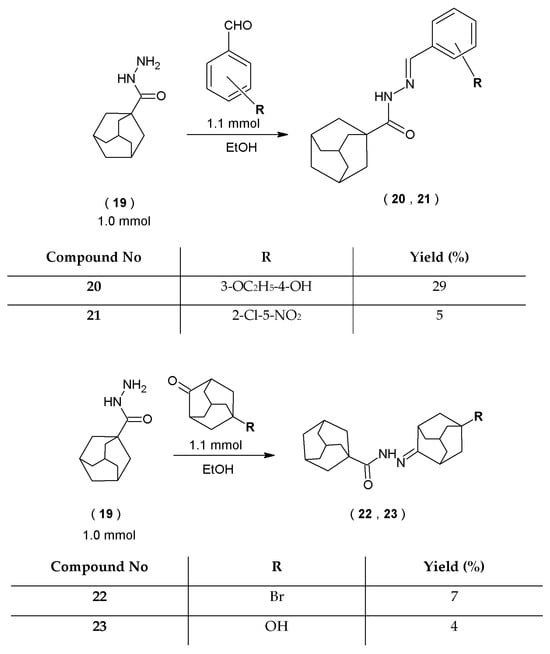
Scheme 5.
Route to the synthesis of hydrazide–hydrazones of 1-adamantanecarboxylic acid.
The chemical structure of all synthesized adamantane derivatives was confirmed with the analysis of the 1H NMR and 13C NMR spectra.
3.2. Antimicrobial Activity Assays
The screening results obtained in vitro indicated that few of the analyzed compounds displayed antimicrobial activity (Table 1). The majority of the compounds exhibited an antibacterial effect towards Gram-positive bacteria, except substances numbered 3, 11, 16 and 23, which were inactive towards them. The sensitivity of the other substances was varied. In the case of active compounds, the Minimal Inhibitory Concentrations (MICs) and Minimal Bactericidal Concentrations (MBCs) against these bacteria were within the scope of 62.5 µg/mL to 1000 µg/mL and from 250 µg/mL to >1000 µg/mL, respectively. Among them, derivatives 9, 14, 15 and 19 showed the highest antibacterial potential with MIC = 62.5–1000 µg/mL towards all Gram-positive bacteria. S. epidermidis ATCC 12228 was the most susceptible to compound 9 (MIC = 62.5 µg/mL and MBC = 250 µg/mL, MBC/MIC = 4) with a bactericidal effect. Other compounds displayed a moderate or mild effect (MIC = 250–1000 µg/mL) or were inactive towards these bacteria.

Table 1.
The results of adamantane derivatives in vitro antimicrobial activity screening.
Schiff bases numbered as 9 substituted with 4-nitrophenyl and 14 with 3-nitrophenyl moiety as well as hydrazide 19 also inhibited growth of all Gram-negative bacterial strains (MIC = 125–1000 µg/mL, MBC = 250 – > 1000 µg/mL). Among them, hydrazide of 1-adamantanecarboxylic acid 19 possessed the highest activity with a moderate effect (MIC = 125–500 µg/mL and similar MBC = 250–1000 µg/mL). This hydrazide showed a bactericidal effect towards all reference bacteria from this group (MBC/MIC = 1–4). The activity of other compounds was lower. Among these microorganisms, B. bronchiseptica ATCC 4617 was the most sensitive to the tested substances (MIC = 125–1000 µg/mL, MBC = 500 – > 1000 µg/mL), except to derivatives 2, 3, 10, 11, 22 and 23 (which had no activity). Moreover, Schiff base 5 substituted with 3-ethoxy-2-hydroxyphenyl and hydrazide 19 were slightly active against some rods from the Enterobacteriaceae family and Pseudomonas aeruginosa ATCC 9027, respectively.
The results included in Table 1 also indicated some antifungal effects of the tested compounds against yeasts from Candida spp. Most of these substances were active at MIC and MFC (Minimal Fungicidal Concentration), ranging from 62.5 µg/mL to 1000 µg/mL and from 125 µg/mL to >1000 µg/mL, respectively. The compounds showed mainly moderate or mild effects. The activity of one of them—the Schiff base numbered 5, which was substituted with 3-ethoxy-2-hydroxyphenyl—was good (MIC = 62.5 µg/mL and MFC = 125 µg/mL, MFC/MIC = 2) towards C. albicans ATCC 10231 with a fungicidal effect. Compounds 5, 9, 14 and 15 exhibited a moderate fungicidal effect against all fungi (MIC = 62.5–500 µg/mL, MFC = 125–1000 µg/mL and MFC/MIC = 1–4). Compounds 2, 8 and 10 had a slightly weaker effect. Moreover, derivatives 7, 19 and 23 showed activity only towards some of yeasts. Compounds 16, 20 and 22 had no anticandidal activity.
3.3. Cytotoxicity
The 24 and 48 h cultures of L929 cells with the compounds marked 7, 10 and 21 showed that the most cytotoxic compounds were 10 at a dose of 100 µM during 24 h and 48 h of culture and 21—at a dose of 100 µM during 48 h of culture. However, none of the tested compounds caused a cytotoxic effect below 50% (Table 2 and Table 3, Figures S1–S6 in Supplementary Materials).

Table 2.
The cell proliferation in % after 24 h exposition on studied compounds in L929 cell line.

Table 3.
The cell proliferation in % after 48 h exposition on studied compounds in L929 cell line.
The 48 h culture of A549 with the tested compounds (7, 10, 21) showed that the best effect in promoting cytotoxicity was obtained with the use of compound 10 at a concentration of 25 and 150 µM and derivative 21 at a concentration of 100 µM but with no compound could be given the IC50. On the other hand, during the 24 h culture of the A549 cell line, these compounds caused a slight increase in cell proliferation (Table 4 and Table 5, Figures S7–S12 in Supplementary Materials).

Table 4.
The cell proliferation in % after 24 h exposition on studied compounds in A549 cell line.

Table 5.
The cell proliferation in % after 48 h exposition on studied compounds in A549 cell line.
The 24 h culture of T47D cells with the examined compounds (7, 10, 21) showed that the main antiproliferative effect was observed with compounds 7 at a dose of 25–50 µM and 21 at a dose of 25 µM. During the 48 h cell culture, only compound 21 at a dose of 25 µM caused such an effect. However, none of the tested compounds caused a cytotoxic effect below 50% (Table 6 and Table 7, Figures S13–S18 in Supplementary Materials).

Table 6.
The cell proliferation in % after 24 h exposition on studied compounds in T47D cell line.

Table 7.
The cell proliferation in % after 48 h exposition on studied compounds in T47D cell line.
During the 24 and 48 h of cultures of the HeLa cell line, it was shown that all tested compounds did not significantly inhibit cell proliferation. The only exception was compound 10 at a dose of 25 µM during 48 h culture, which resulted in a decrease in proliferation of approximately 60% (Table 8 and Table 9, Figures S19–S24 in Supplementary Materials).

Table 8.
The cell proliferation in % after 24 h exposition on studied compounds in HeLa cell line.

Table 9.
The cell proliferation in % after 48h exposition on studied compounds in HeLa cell line.
The above study indicates that the compounds designated as 7, 10 and 21 do not have strong antiproliferative properties against cancer cells.
4. Discussion
4.1. Chemistry
The chemical syntheses described in the current research enabled us to obtain seventeen novel adamantane derivatives not described in the scientific literature so far, i.e., two derivatives of 3-aminotricyclo[3.3.1.13,7]decan-1-ol (2,3), seven derivatives of tricyclo [3.3.1.13,7]decan-1-amine (5–11), five derivatives of 1-(tricyclo[3.3.1.13,7]dec-1-yl)methanamine (13–17) and four adamantanecarboxylic acid derivatives with a hydrazide–hydrazone moiety (20–23). Compounds 2, 3, 5–11, 13–17 and 20–23 were synthesized based on a typical condensation reaction between the amine group of one compound and the carbonyl group of another substance. For the synthesis of the above-mentioned derivatives, we used the following starting materials: 1.2 mmol of 3-aminotricyclo[3.3.1.13,7]decan-1-ol (1) or 1.3 mmol of 1-adamantanylamine (4) or 1.2 mmol of 1-adamantanemethylamine (12) or 1.0 mmol of hydrazide of 1-adamantanecarboxylic acid (19) and an appropriate amount of aldehydes or ketones.
The yields of the condensation reaction were different for each of the obtained derivatives, and they varied significantly. The yields of the synthesis depended on the aldehydes or ketones used for the synthesis. The lack of a catalyst used in our research may also have contributed to the low efficiency of the reactions performed. A review of the literature shows that the addition of a few drops of glacial acetic acid is usually used to obtain substances based on condensation reactions [36].
The yields of the performed reactions were within the scope of 4–96%. The highest yield of 96% was found in the synthesis of 3-[(2,4-dimethoxybenzylidene)amino]tricyclo[3.3.1.13,7]decan-1-ol (3), whereas the lowest yield of 4% was found for 1-(3-nitrophenyl)-N-(tricyclo[3.3.1.13,7]dec-1-ylmethyl)methanimine (14) and N-(5-hydroxytricyclo[3.3.1.13,7]dec-2-ylidene)tricyclo[3.3.1.13,7]decane-1-carbohydrazide (23).
4.2. NMR Spectra Analysis
The analysis of the 1H NMR and 13C NMR spectra made it possible to identify and confirm the chemical structure of the obtained substances and the correctness of their synthesis.
The derivatives of 3-aminotricyclo[3.3.1.13,7]decan-1-ol (2,3) on the 1H NMR spectra possessed a characteristic singlet signal for the proton of the =CH group at δ 8.51–8.54 ppm. On the 13C NMR spectra, the signal for the carbon atom of the =CH group appeared at δ 149.26 ppm (2) and 149.53 ppm (3). The presence of this signal both on the 1H NMR and 13C NMR confirmed the correctness of the synthesis. For the derivatives (2,3), on the 1H NMR spectra, a singlet signal for the proton of hydroxyl group was found at δ 4.53–4.56 ppm.
In the case of the derivatives of tricyclo[3.3.1.13,7]decan-1-amine (5–10), on the 1H NMR spectra, a singlet signal for the proton of the =CH group was present in the range of δ 8.16–8.62 ppm. The carbon atom of =CH on the 13C NMR spectra for these substances (5–10) appeared in the range of δ 147.15–149.35 ppm.
The correctness of the synthesis of the novel derivatives of 1-(tricyclo[3.3.1.13,7]dec-1-yl)methanamine (13–15) was confirmed with the 1H NMR spectroscopy based on the presence of singlet signals for a proton of CH2 and the =CH group, which appeared at δ 3.15–3.41 ppm and δ 8.43–10.14 ppm, respectively. The carbon atoms of CH2 and the =CH group of compounds 13–15 gave signals on the 13C NMR spectra in the range of δ 65.85–73.73 ppm (CH2) and δ 148.75–148.92 ppm (=CH). For compound 16, we found a characteristic signal for =Cadamantane at δ 173.61 ppm because this condensation reaction was carried out with ketone.
On the 1H NMR spectra of the hydrazide of 1-adamantanecarboxylic acid (19), we found characteristic singlet signals for protons that corresponded to the NH (δ 9.13 ppm) and NH2 groups (δ 4.04 ppm). On the 13C NMR spectra for this compound, signals for carbon atoms were found in the expected range of chemical shift.
The hydrazide–hydrazones of 1-adamantanecarboxylic acid (20–21) obtained in the reaction with aldehydes possessed two typical signals for this class of compounds on the 1H NMR spectra. One singlet signal for the proton of the =CH group was found in the range of δ 7.94–9.13 ppm, and the other singlet signal for the proton of the NH group was found at δ 9.12–10.84 ppm. On the 13C NMR spectra for compounds 20–21, a signal for the carbon atom of the =CH group appeared around δ 147 ppm, whereas for derivatives 22–23, signals for =Cadamantane were found at δ 168.24 ppm (compound 22) and δ 167.86 ppm (compound 23).
Signals for other aliphatic and aromatic fragments of obtained substances on the 1H NMR and 13C NMR spectra were found at the expected values of chemical shift.
The examples of the 1H NMR spectra of the synthesized adamantane derivatives are presented in Supplementary Materials (Figures S25–S27).
4.3. Antimicrobial Activity Assays
Our research goal was to investigate the effect of the incorporation of the adamantane system into novel Schiff bases and hydrazide–hydrazone compounds. Unfortunately, the combination of adamantane and Schiff bases or a hydrazide-hydrazone moiety did not provide the expected significant enhancement in antibacterial activity against Gram-positive bacterial strains in comparison with previously reported hydrazone compounds [31,32,33]. Only selected adamantane derivatives synthesized by our research group were active against all tested Gram-positive bacteria (Schiff bases 9, 14, 15 and hydrazide 19).
However, the antibacterial activity towards Gram-negative bacterial strains shown by the newly synthesized adamantane derivatives described in this research is not so common in the scientific literature devoted to the antibacterial activity profile of hydrazide–hydrazones [31,32,33].
Although, in the case of antifungal activity, the results were better than expected even though they were much lower than for reference substances. For the activity of hydrazide–hydrazones that were previously reported by our research team, the antifungal activity was moderate, whereas in this research, it is worth underlining, especially compound 5 with a 3-ethoxy-2-hydroxyphenyl substituent, which showed the lowest value of MIC towards C. albicans ATCC 10231 (MIC = 62.5 μg/mL, MBC = 125 μg/mL, MBC/MIC = 2). Other synthesized adamantane derivatives also influence the growth of yeasts from Candida spp.
According to literature findings, the adamantane derivatives synthesized by Orzeszko et al. [12,13] and Pham et al. [15], similar to the compounds synthesized by our research group, also displayed interesting activity toward Candida albicans and a few species of Gram-positive bacteria.
After the analysis of the structure–antibacterial and structure–antifungal activity relationships of the obtained derivatives, it is clear that among hydrazide–hydrazones (20–23), the substitution of the NH2 group in hydrazide 19 resulted in a decrease in both antibacterial and antifungal activity. The free amino group of hydrazide 19 promoted activity against Gram-positive bacterial strains. Among Schiff bases (2, 3, 5–11, 13–17), the substitution of the phenyl ring with the electron-withdrawing group—the nitro group at positions 3 (compound 14) and 4 (compound 9, 15)—was the most beneficial substitution for an increase in antibacterial activity. The presence of two electron donating groups in the phenyl ring—two methoxy groups at positions 2 and 3 (compound 2), 2 and 4 (compound 3), as well as the adamantanyl (11) moiety—decreased the antibacterial potential of the synthesized adamantane derivatives. In the case of antifungal activity, there was no direct connection between the presence of electron-donating or electron-withdrawing groups at the phenyl ring and the bioactivity. The activity increased when in the structure of the Schiff base there was a 3-ethoxy-2-hydroxyphenyl (5) or 4-nitrophenyl (9) substituent. Staphylococcus epidermidis ATCC 12228 and Candida albicans ATCC 10231 were the most sensitive microorganisms towards tested adamantane derivatives.
4.4. Cytotoxicity
In our study, the cytotoxicity results of the tested compounds (7, 10, 21) showed that the newly synthesized derivatives of adamantane did not cause statistical changes in cell proliferation within the range of the tested doses.
Our results were like those obtained by Pham et al. [36] in both experiments; the A549 and HeLa cell lines showed similar cell viability with the use of adamantane derivatives [36]. The lack of a negative effect on fibroblasts (L929 line) means that these compounds can be considered for further tests in the antiviral or antibacterial direction, and this negative effect does not prejudge their potential as antiproliferative compounds in relation to other types of cancer.
In our study, we selected only the three most popular types of cancer in Poland (cervical cancer, breast cancer and lung cancer). Therefore, further research is needed to check the exact cytotoxicity of these compounds in relation to other types of cancer. Hassan et al. [37], for instance, have shown that some adamantane derivatives were effective for hepatocellular carcinoma [37]. Turk-Erbul et al. [38] showed the effectiveness of adamantane derivatives against lung cancer lines, but these were completely different derivatives than those used in our experiment [38]. Therefore, further research in this direction should be performed.
5. Conclusions
In this research, we managed to synthesize and establish the chemical structure of novel adamantane derivatives. The obtained substances were subjected to antimicrobial activity screening. Only a few of the synthesized derivatives showed some antimicrobial activity, especially derivatives 9 and 14, towards all reference microorganisms. Additionally, all Gram-positive and Gram-negative bacteria were sensitive to substance 19. In turn, compound 15 was active towards all Gram-positive bacteria. Moreover, reference yeasts belonging to Candida spp. were sensitive to the majority of the tested adamantane derivatives. The bacteria and yeasts included in these studies constitute a natural, opportunistic or pathogenic microflora of the human body. Therefore, it seems practical to use these compounds, with some future structure modification, in the prevention and treatment of infections caused by the selected microorganisms, especially fungi. Additionally, cytotoxicity studies confirmed that the tested substances did not cause statistically significant changes in cell proliferation within the range of the tested doses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14093700/s1. Cytotoxicity—List of figures: Figures S1–S24. Examples of the 1H NMR spectra of the synthesized adamantane derivatives—Figures S25–S27.
Author Contributions
Conceptualization, Ł.P.; methodology, Ł.P., W.J., A.H. and A.B.; validation, Ł.P., W.J., A.H. and A.B.; formal analysis, Ł.P., W.J., A.H. and A.B.; investigation, Ł.P., W.J., A.H. and A.B.; resources, Ł.P.; data curation, Ł.P.; writing—original draft preparation, Ł.P., A.H. and A.B.; writing—review and editing, Ł.P., A.H. and A.B.; visualization, Ł.P.; supervision, Ł.P.; project administration, Ł.P.; funding acquisition, Ł.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lis-Cieplak, A. Adamantane derivatives—Variety of biological activities. A review of medications approved in Poland and potential drugs. Biul. Wydz. Farm. WUM 2012, 3, 18–25. [Google Scholar]
- Ickes, D.E.; Venetta, T.M.; Phonphok, Y.; Rosenthal, K.S. Tromantadine inhibits a late step in herpes simplex virus type 1 replication and syncytium formation. Antivir. Res. 1990, 14, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, G.; Kolocouri, N.; Naesens, L.; De Clercq, E. Design and synthesis of 1,2-annulated adamantane piperidines with anti-influenza virus activity. Bioorg. Med. Chem. 2009, 17, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Zarubaev, V.V.; Golod, E.L.; Anfimov, P.M.; Shtro, A.A.; Saraev, V.V.; Gavrilov, A.S.; Logvinov, A.V.; Kiselev, O.I. Synthesis and anti-viral activity of azolo-adamantanes against influenza A virus. Bioorg. Med. Chem. 2010, 18, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Shibnev, V.A.; Garaev, T.M.; Deryabin, P.G.; Finogenova, M.P.; Mishin, D.V. Synthesis and antiviral activity of adamantylpeptides against hepatitis C virus. Pharm. Chem. J. 2015, 49, 449–454. [Google Scholar] [CrossRef]
- Suslov, E.; Zarubaev, V.V.; Slita, A.V.; Ponomarev, K.; Korchagina, D.; Ayine-Tora, D.M.; Reynisson, J.; Volcho, K.; Salakhutdinov, N. Anti-influenza activity of diazaadamantanes combined with monoterpene moieties. Bioorg. Med. Chem. Lett. 2017, 27, 4531–4535. [Google Scholar] [CrossRef] [PubMed]
- Pagire, S.H.; Pagire, H.S.; Lee, G.B.; Han, S.-J.; Kwak, H.J.; Kim, J.Y.; Kim, K.Y.; Rhee, S.D.; Ryu, J.I.; Song, J.S.; et al. Discovery and optimization of adamantane carboxylic acid derivatives as potent diacylglycerol acyltransferase 1 inhibitors for the potential treatment of obesity and diabetes. Eur. J. Med. Chem. 2015, 101, 716–735. [Google Scholar] [CrossRef]
- Koyanagawa, N.; Miyoshi, H.; Ono, K.; Nakamura, A.; Cho, K.Y.; Yamamoto, K.; Takano, Y.; Dan-noura, M.; Atsumi, T. Comparative effects of vildagliptin and sitagliptin determined by continuous glucose monitoring in patients with type 2 diabetes mellitus. Endocr. J. 2016, 63, 747–753. [Google Scholar] [CrossRef]
- Amblee, A.; Lious, D.; Fogelfeld, L. Combination of Saxagliptin and Metformin Is Effective as Initial Therapy in New-Onset Type 2 Diabetes Mellitus With Severe Hyperglycemia. J. Clin. Endocrinol. Metab. 2016, 101, 2528–2535. [Google Scholar] [CrossRef] [PubMed]
- Uchii, M.; Kimoto, N.; Sakai, M.; Kitayama, T.; Kunori, S. Glucose-independent renoprotective mechanisms of the tissue dipeptidyl peptidase-4inhibitor, saxagliptin, in Dahl salt-sensitive hypertensive rats. Eur. J. Pharmacol. 2016, 783, 56–63. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhang, F.; Diao, H.; Wu, R. Covalent Inhibition Mechanism of Antidiabetic Drugs-Vildagliptin vs. Saxagliptin. ACS Catal. 2019, 9, 2292–2302. [Google Scholar] [CrossRef]
- Orzeszko, A.; Kamińska, B.; Orzeszko, G.; Starościak, B.J. Synthesis and antimicrobial activity of new adamantane derivatives II. Il Farm. 2000, 55, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Orzeszko, A.; Kamińska, B.; Starościak, B.J. Synthesis and antimicrobial activity of new adamantane derivatives III. Il Farm. 2002, 57, 61–624. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, L.H.; Hassan, H.M.; Abo-Kamar, A.M.; Ghabbour, H.A.; El-Emam, A.A. Adamantane-Isothiourea Hybrid Derivatives: Synthesis, Characterization, In Vitro Antimicrobial, and In Vivo Hypoglycemic Activities. Molecules 2017, 22, 710. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Phan, T.P.D.; Phan, D.C.; Vu, B.D. Synthesis and Bioactivity of Hydrazide-Hydrazones with the 1-Adamantyl-Carbonyl Moiety. Molecules 2019, 24, 4000. [Google Scholar] [CrossRef] [PubMed]
- Chinnapattu, M.; Sathiyanarayanan, K.I.; Iyer, P.S. Synthesis and biological evaluation of adamantane-based aminophenols as a novel class of antiplasmodial agents. Bioorg. Med. Chem. Lett. 2015, 25, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Srbljanović, J.; Štajner, T.; Konstantinović, J.; Terzić-Jovanović, N.; Uzelac, A.; Bobić, B.; Šolaja, B.A.; Djurković-Djaković, O. Examination of the antimalarial potential of experimental aminoquinolines: Poor in vitro effect does not preclude in vivo efficacy. Inter. J. Antimicrob. Agents 2017, 50, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Fytas, C.; Zoidis, G.; Tsotinis, A.; Fytas, G.; Khan, M.A.; Akhtar, S.; Rahman, K.M.; Thurston, D.E. Novel 1-(2-aryl-2-adamantyl)piperazine derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 93, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Pandey, V.; Mohan, C.D.; Chia, Y.T.; Rangappa, S.; Mathai, J.; Baburajeev, C.P.; Paricharak, S.; Mervin, L.H.; Bulusu, K.C.; et al. Novel Adamantanyl-Based Thiadiazolyl Pyrazoles Targeting EGFR in Triple-Negative Breast Cancer. ACS Omega 2016, 1, 1412–1424. [Google Scholar] [CrossRef]
- Ali, A.G.; Mohamed, M.F.; Abdelhamid, A.O.; Mohamed, M.S. A novel adamantane thiadiazole derivative induces mitochondria-mediated apoptosis in lung carcinoma cell line. Bioorg. Med. Chem. 2017, 25, 241–253. [Google Scholar] [CrossRef]
- Bao, X.; Sun, Y.; Bao, C.; Zhang, J.; Zou, S.; Yang, J.; Wu, C.; Wang, L.; Chen, G. Design, synthesis and evaluation of N-hydroxypropenamides based on adamantane to overcome resistance in NSCLC. Bioorg. Chem. 2019, 86, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Piérard, G.E.; Piérard-Franchimont, C.; Paquet, P.; Quatresooz, P. Spotlight on adapalene. Expert Opin. Drug Metab. Toxicol. 2009, 5, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Kouatly, O.; Geronikaki, A.; Kamoutsis, C.; Hadjipavlou-Litina, D.; Eleftheriou, P. Adamantane derivatives of thiazolyl-N-substituted amide, as possible non-steroidal anti-inflammatory agents. Eur. J. Med. Chem. 2009, 44, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Geddes-McAlister, J.; Shapiro, R.S. New pathogens, new tricks: Emerging, drug-resistant fungal pathogens and future prospects for antifungal therapeutics. Ann. N. Y. Acad. Sci. 2019, 1435, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Humeniuk, N.; Zelena, L.; Vrynchanu, N.; Ishchenko, L.; Bukhtiarova, T.; Korotkij, Y.; Vazhnichaya, E. Effect of adamantane derivative on expression of biofilm-associated genes in methicillin-resistant Staphylococcus aureus. Med. Drug Discov. 2023, 18, 100155. [Google Scholar] [CrossRef]
- Dudikova, D.M.; Vrynchanu, N.O.; Nosar, V.I. Alteration of Pseudomonas aeruginosa respiration by 4-(1-adamantyl)-phenol derivative. Biologija 2018, 64, 228–234. [Google Scholar] [CrossRef]
- Tan, F.; She, P.; Zhou, L.; Liu, Y.; Chen, L.; Luo, Z.; Wu, Y. Bactericidal and Anti-biofilm Activity of the Retinoid Compound CD437 against Enterococcus faecalis. Front. Microbiol. 2019, 10, 2301. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef]
- Popiołek, Ł. Updated Information on Antimicrobial Activity of Hydrazide–Hydrazones. Int. J. Mol. Sci. 2021, 22, 9389. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Rysz, B.; Biernasiuk, A.; Wujec, M. Synthesis of promising antimicrobial agents: Hydrazide-hydrazones of 5-nitrofuran-2-carboxylic acid. Chem. Biol. Drug. Des. 2020, 95, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł.; Tuszyńska, K.; Biernasiuk, A. Searching for novel antimicrobial agents among hydrazide-hydrazones of 4-iodosalicylic acid. Biomed. Pharmacother. 2022, 153, 113302. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł.; Gawrońska-Grzywacz, M.; Dziduch, A.; Biernasiuk, A.; Piątkowska-Chmiel, I.; Herbet, M. Design, Synthesis, and In Vitro and In Vivo Bioactivity Studies of Hydrazide–Hydrazones of 2,4-Dihydroxybenzoic Acid. Int. J. Mol. Sci. 2023, 24, 17481. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. EUCAST discussion document E. Dis 5.1. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; M27-S4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Pham, V.H.; Phan, T.P.D.; Phan, D.C.; Vu, B.D. Synthesis and Bioactivity of Thiosemicarbazones Containing Adamantane Skeletons. Molecules 2020, 25, 324. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Al-Wahaibi, L.H.; Shehatou, G.S.G.; El-Emam, A.A. Adamantane-linked isothiourea derivatives suppress the growth of experimental hepatocellular carcinoma via inhibition of TLR4-MyD88-NF-κB signaling. Am. J. Cancer. Res. 2021, 11, 350–369. [Google Scholar]
- Turk-Erbul, B.; Karaman, E.F.; Duran, G.N.; Ozbil, M.; Ozden, S.; Goktas, F. Synthesis, in vitro cytotoxic and apoptotic effects, and molecular docking study of novel adamantane derivatives. Arch. Pharm. 2021, 354, e2000256. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).